Abstract
The autoimmune disease which affects MRL/MpJ-faslpr mice results in cerebral leucocyte recruitment and cognitive dysfunction. We have previously observed increased leucocyte trafficking in the cerebral microcirculation of these mice; however, the types of leucocytes recruited have not been analysed thoroughly, and the roles of key endothelial adhesion molecules in recruitment of these leucocytes have not been investigated. Therefore the aim of this study was to classify the phenotypes of leucocytes present in inflamed brains of MRL/MpJ-faslpr mice, and dissect the roles of endothelial adhesion molecules in their accumulation in the brain. Immunohistochemical analysis revealed significant leucocyte infiltration in the brains of 16- and 20-week-old MRL/MpJ-faslpr mice, affecting predominantly the choroid plexus. Isolation of brain-infiltrating leucocytes revealed that lymphocytes and neutrophils were the main populations present. The CD3+ lymphocytes in the brain consisted of similar proportions of CD4+, CD8+ and CD4–/CD8–[double negative (DN)] populations. Assessment of MRL/MpJ-faslpr mice deficient in endothelial adhesion molecules intercellular adhesion molecule-1 (ICAM-1) or P-selectin indicated that cerebral leucocyte recruitment persisted in the absence of these molecules, with only minor changes in the phenotypes of infiltrating cells. Together these data indicate that the brains of MRL/MpJ-faslpr mice are affected by a mixed leucocyte infiltrate, of which the unusual DN lymphocyte phenotype contributes a substantial proportion. In addition, endothelial adhesion molecules ICAM-1 and P-selectin, which modulate survival of MRL/MpJ-faslpr mice, do not markedly inhibit leucocyte entry into the central nervous system.
Keywords: brain, leucocyte recruitment, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a debilitating autoimmune disease in which a range of organs are targeted by a damaging inflammatory response. One of the organs affected most commonly in SLE patients is the central nervous system (CNS) [1]. Indeed, SLE patients can exhibit symptoms ranging from mild cognitive dysfunction to seizures and strokes [2,3]. There is a growing body of evidence that the local inflammatory response contributes to the development of these symptoms. Pathological assessment of brains from SLE patients show evidence of inflammation, with widespread microinfarcts and perivascular lymphocytic cuffing being common post mortem findings [1,4,5]. However, the mechanisms of the cerebral inflammatory response in SLE patients are poorly understood.
Much information about the inflammatory mechanisms of systemic autoimmune disease has come from the analysis of a number of spontaneous murine models, in particular the MRL/MpJ-faslpr mouse. Indeed, this mouse strain is used routinely as a model of SLE. MRL/MpJ-faslpr mice develop a severe autoimmune disease with many similarities to that seen in SLE patients, including autoantibody production, immune complex formation and systemic inflammation [6]. Furthermore, multiple lines of evidence indicate that the CNS in these animals is also affected by this disease. Behavioural studies in MRL/MpJ-faslpr mice have demonstrated cognitive impairments which increase in severity as the disease progresses [7–9]. In addition, the brains of these animals display extensive leucocyte infiltration and evidence of leakage of plasma proteins into the parenchyma of the brain [10]. In order to examine the mechanisms responsible for the accumulation of leucocytes in the brains of lupus-prone mice, we recently used intravital microscopy to examine the intact cerebral (pial) microvasculature of MRL/MpJ-faslpr mice [11]. Post-capillary venules in the cerebral microvasculature of these mice readily supported rolling and adhesive interactions with blood-borne leucocytes during the active phase of disease, in marked contrast to the rarity of these interactions in similarly aged, non-diseased MRL+/+ mice [11]. These findings indicate that the cerebral microvasculature is activated as part of the disease process in these mice. However, this study did not examine the types of leucocytes which enter the brain, and the molecular requirements for leucocyte accumulation in the CNS. Furthermore, the biology of the circulating leucocytes in MRL/MpJ-faslpr mice is highly unusual with many CD3+ T cells lacking expression of both CD4 and CD8 [termed double negative (DN) T cells] [12]. The contribution of these leucocytes to the cerebral leucocyte infiltrate is unclear.
Examination of MRL/MpJ-faslpr mice lacking key adhesion molecules has provided strong evidence for the important roles of adhesion molecules in disease-associated pathology. Deletion of intercellular adhesion molecule-1 (ICAM-1) has been shown to increase survival and reduce leucocyte infiltration into inflamed organs such as the kidney [13,14]. In addition, absence of the ICAM-1 ligand, lymphocyte function-associated antigen-1 (LFA-1/CD11a), resulted in comparable improvements in survival and reductions in disease severity [15]. Together these findings demonstrate that interactions mediated by ICAM-1/LFA-1 play central roles in the development of inflammatory disease in MRL/MpJ-faslpr mice. However, it is noteworthy that this does not extend to all adhesion molecule pathways. MRL/MpJ-faslpr mice lacking P-selectin are not protected from early lethality and leucocyte recruitment to organs such as the kidney proceeds unabated in these animals [16,17]. Despite these studies, the roles of these adhesion molecules in promoting leucocyte entry into the brain have not been investigated. Therefore, the aims of this study were to characterize fully the phenotype of the leucocytes which enter the brains of lupus-prone MRL/MpJ-faslpr mice, and examine the contribution of ICAM-1 and P-selectin to the accumulation of leucocytes in the CNS.
Materials and methods
Animals
MRL/MpJ-faslpr mice and MRL/MpJ (MRL+/+) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). ICAM-1–/– MRL/MpJ-faslpr mice were generated by back-crossing a gene-targeted mutation on to the MRL/MpJ-faslpr strain background, as described previously [13]. A similar approach was used to generate the P-selectin–/– MRL/MpJ-faslpr mice [16,17]. Mice were used at 8, 16 and 20–25 weeks of age and weighed 30–50 g.
Antibodies
The antibodies used for flow cytometry were purchased from BD Biosciences (San Diego, CA, USA) and directed against murine antigens: 30-F11 − anti-CD45, conjugated to allophycocyanin (APC); 17A2 − anti-CD3, conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE); GK1·5 − anti-CD4, conjugated to PE; 53–6·7 − anti-CD8a, conjugated to APC; RB6–8C5 − anti-Ly-6G (Gr-1), conjugated to PE; RA3–6B2 − anti-B220, conjugated to FITC; KT3 − anti-CD3 and M1/70 − anti-Mac-1, both purified from hybridoma supernatant and conjugated to Alexa 488 (Molecular Probes, Eugene, OR, USA).
Immunohistochemical analysis of brain leucocyte infiltration
The location of leucocytes present in the brains of lupus-prone mice was determined by immunohistochemical staining for the pan-leucocyte marker CD45. Briefly, anaesthetized mice were killed via intracardiac perfusion with phosphate-buffered saline (PBS), then fixed by perfusion with cold paraformaldehyde/lysine/periodate (PLP) fixative. Brains were removed and cryoprotected in 20% sucrose and embedded in optimum cutting temperature (OCT) embedding compound. Brains were frozen in a bath of isopentane suspended over liquid nitrogen (LN2) then submerged in LN2. Coronal cryostat sections (10 µm) were cut from the anterior end of the brain and 10 sections were collected at 200 µm intervals, taking the first section at the most anterior position of the ventricles. CD45+ leucocytes in brain sections were identified using a modified three-layer immunohistochemistry technique [18]. Briefly, sections were blocked with normal swine serum then incubated with anti-CD45 (clone 30F-11, BD Biosciences, 1 µg/ml, overnight, 4°C). Sections were then treated with secondary antibody (rabbit anti-rat IgG), tertiary antibody [horseradish peroxidase (HRP)-conjugated sheep anti-rabbit IgG] and developed with diaminobenzidine and counterstained with haematoxylin, as described previously [18].
Analysis of anti-CD45-stained brain sections
In accord with previous data, leucocyte infiltration was located predominantly in the choroid plexus [10]. Therefore, two aspects of this region were examined in detail: (i) the area of the choroid plexus and (ii) the number of CD45+ leucocytes present within the choroid plexus. The area of the choroid plexus was measured in five sections of the brain between 1000 and 2000 µm from the anterior aspect of the ventricles. Sections were examined on a Leica BMLB laboratory microscope. Images were captured at 50× magnification using a Leica DC 300F digital camera and Photoshop CS software (Adobe Systems, San Jose, CA, USA). The area of the choroid plexus was measured using Scion Image image analysis software, following calibration using a stage micrometer, and expressed as mean area of each choroid plexus villus. The number of CD45+ leucocytes in each villus (examined at 400× magnification) was also counted, and expressed relative to the villus area (cells/mm2). The CNS parenchyma was also assessed for leucocyte infiltration in multiple high-power fields.
Isolation and identification of leucocytes in the CNS
Leucocytes present in the brain and spinal cords of MRL+/+, MRL/MpJ-faslpr, P-selectin–/– MRL/MpJ-faslpr and ICAM-1–/– MRL/MpJ-faslpr mice were isolated and analysed by flow cytometry using a modification of a previously described technique [19,20]. Briefly, anaesthetized mice were killed by intracardiac perfusion of 15 ml cold PBS. The brain and spinal cord were then removed, and dissociated by passing through wire mesh. The resultant cell suspension was separated on a 90%/37%/30% Percoll (Amersham Biosciences AB, Uppsala, Sweden) gradient, and the leucocyte fraction isolated from the 90%/37% interface. Leucocytes were washed in PBS/1·0% bovine serum albumin (BSA) three times. Aliquots (100 µl) of the resultant cell suspension were incubated in one of the three following antibody combinations: (1) CD45-APC, Gr-1-PE, M1/70-FITC; (2) CD45-APC, CD3-PE, B220-FITC; (3) CD3-FITC, CD4-PE and CD8-APC. Cells were incubated for 20 min at room temperature, washed and then fixed in 4% neutral buffered formalin. Labelled cells were analysed using a MoFlo flow cytometer (Cytomation, Fort Collins, CO, USA).
To identify leucocytes from within the cell fraction isolated from the brain, cells were gated on positive staining for the leucocyte common antigen, CD45. CD45+ subpopulations were classified subsequently as follows: granulocytes, Gr-1hi/M1/70hi; monocytes, Gr-1int/M1/70hi; T cells, CD3+; B cells B220+/CD3–, in accord with previous observations in mice [21]. The number of cells of each subpopulation was expressed as a percentage of CD45+ cells in the sample. CD3+ T cells were defined further as CD4+, CD8+ or CD4–/CD8– (DN), a leucocyte population observed routinely in MRL/MpJ-faslpr mice [12], and expressed as a percentage of CD3+ cells.
Flow cytometric analysis of circulating leucocytes
To identify the leucocyte populations present in the blood of lupus-prone mice, 100 µl samples of whole blood were incubated with the same combinations of antibodies used for the brain samples. Samples underwent subsequent erythrocyte lysis and fixation using a Q-prep Workstation (Beckman Coulter, Miami, FL, USA) and flow cytometric analysis using identical conditions to the brain samples. The numbers of each cell type present in the blood were determined by reference to the circulating leucocyte counts of each animal.
Statistics
Comparisons between mouse strains were performed using Student's t-tests, using Bonferroni's calculation to correct for multiple comparisons. P < 0·05 was deemed significant.
Results
Comparison of the phenotypes of circulating leucocytes in MRL+/+ and MRL/MpJ-faslpr mice revealed that at 8 weeks, the numbers of neutrophils, monocytes, CD4 and CD8 T cells and B cells were significantly lower in MRL/MpJ-faslpr mice (Tables 1 and 2). In MRL+/+ mice, leucocyte counts remained relatively stable to 20 weeks. In contrast, in MRL/MpJ-faslpr mice at 16 weeks, there was a marked increase in the number of circulating leucocytes. Much of this change was due to a striking elevation in circulating T cells of almost 10 × 106/ml, with an increase in circulating DN T cells accounting for the majority of this difference. In MRL+/+ mice, DN T cells represented a very minor population. Small but significant increases in neutrophils and monocytes were also observed in 16-week MRL/MpJ-faslpr mice (Tables 1 and 2). By 20 weeks, the leucocyte counts in MRL/MpJ-faslpr mice had undergone a further increase to nearly 30 × 106/ml, with more than 14 × 106/ml being DN T cells.
Table 1.
Circulating leucocyte counts and leucocyte subsets in MRL+/+, MRL/MpJ-faslpr, P-selectin–/– -MRL/MpJ-faslpr, and intercellular adhesion molecule-1 (ICAM-1)–/– -MRL/MpJ-faslpr mice at 8, 16 and 20–25 weeks of age.*
| M1/70hi/Gr-1hi | M1/70 hi/Gr-1int | CD3+ | B220+/CD3– | ||
|---|---|---|---|---|---|
| Total | (PMNs) | (monocyte lineage) | (T cells) | (B cells) | |
| MRL+/+ | |||||
| 8 weeks | 6·8 ± 0·5 (6) | 1·5 ± 0·1 (6) | 0·20 ± 0·28 (6) | 3·2 ± 0·3 (6) | 2·1 ± 0·2 (6) |
| 16 weeks | 7·4 ± 0·5 (6) | 1·6 ± 0·1 (6) | 0·20 ± 0·01 (6) | 3·2 ± 0·2 (6) | 1·7 ± 0·1 (6) |
| 20–25 weeks | 4·9 ± 0·6 (7) | 1·3 ± 0·2 (7) | 0·14 ± 0·05 (7) | 1·9 ± 0·2 (7) | 0·9 ± 0·2 (7) |
| MRL/MpJ-faslpr | |||||
| 8 weeks | 5·3 ± 0·4 (6) | 0·9 ± 0·1 (6)† | 0·07 ± 0·01 (6)† | 2·0 ± 0·1 (9)† | 1·0 ± 0·1 (9)† |
| 16 weeks | 19·4 ± 3·8 (6) | 4·5 ± 0·9 (6)† | 0·77 ± 0·17 (6)† | 12·8 ± 1·3 (6)† | 1·4 ± 0·1 (6) |
| 20–25 weeks | 29·6 ± 4·1 (7) | 5·3 ± 1·8 (6)† | 0·72 ± 0·18 (6)† | 20·8 ± 1·6 (6)† | 2·0 ± 0·2 (6)† |
| P-selectin–/–-MRL/MpJ-faslpr | |||||
| 8 weeks | 10·9 ± 0·5 (9) | 4·4 ± 0·3 (8)** | 0·56 ± 0·07 (8)** | 3·1 ± 0·4 (6)** | 1·7 ± 0·3 (6)** |
| 16 weeks | 20·2 ± 4·9 (5) | 5·7 ± 0·7 (6) | 0·79 ± 0·14 (6) | 10·7 ± 0·8 (8) | 2·8 ± 0·2 (8)** |
| 20–25 weeks | 27·8 ± 4·9 (6) | 6·5 ± 1·6 (6) | 0·72 ± 0·16 (6) | 14·6 ± 1·6 (7)** | 3·1 ± 0·4 (7)** |
| ICAM-1–/–-MRL/MpJ-faslpr | |||||
| 16 weeks | 16·1 ± 4·4 (5) | 3·2 ± 0·8 (6) | 0·50 ± 0·13 (6) | 7·3 ± 0·7 (8)** | 2·0 ± 0·2 (8)** |
| 20–25 weeks | 28·7 ± 6·7 (5) | 4·3 ± 0·8 (6) | 0·51 ± 0·09 (6) | 18·1 ± 0·8 (6) | 4·7 ± 0·7 (6)** |
Data are expressed as cells × 106/ml ± s.e.m. The number of mice sampled is shown in parentheses.
P < 0·05 versus MRL+/+ at same age.
P < 0·05 versus MRL/MpJ-faslpr at same age.
Table 2.
Phenotypes of circulating CD3+ leucocytes in MRL+/+, MRL/MpJ-faslpr, P-selectin–/– MRL/MpJ-faslpr and intercellular adhesion molecule-1 (ICAM-1)–/– MRL/MpJ-faslpr mice at 8, 16 and 20–25 weeks of age.*
| CD4+ | CD8+ | CD4–/CD8– (DN) | B220+ | |
|---|---|---|---|---|
| MRL+/+ | ||||
| 8 weeks (6) | 1·75 ± 0·05 (6) | 1·30 ± 0·03 (6) | 0·09 ± 0·02 (6) | 0·98 ± 0·43 (6) |
| 16 weeks (7) | 1·50 ± 0·10 (7) | 1·40 ± 0·10 (7) | 0·22 ± 0·08 (7) | 0·35 ± 0·11 (6) |
| 20–25 weeks (7) | 1·04 ± 0·04 (7) | 0·82 ± 0·05 (7) | 0·06 ± 0·02 (7) | 0·05 ± 0·01 (7) |
| MRL/MpJ-faslpr | ||||
| 8 weeks (9) | 0·86 ± 0·04 (9)† | 0·91 ± 0·03 (9)† | 0·18 ± 0·01 (9)† | 0·38 ± 0·03 (9) |
| 16 weeks (6) | 1·75 ± 0·17 (6) | 2·50 ± 0·39 (6)† | 8·46 ± 0·55 (6)† | 9·20 ± 1·32 (6)† |
| 20–25 weeks (6) | 2·71 ± 0·18 (6)†c | 3·56 ± 0·24 (6)† | 14·42 ± 0·34 (6)† | 14·33 ± 0·67 (6)† |
| P-selectin–/–-MRL/MpJ-faslpr | ||||
| 8 weeks (8) | 1·44 ± 0·03 (8)** | 1·44 ± 0·03 (8)** | 0·24 ± 0·02 (8)** | 0·54 ± 0·09 (6)** |
| 16 weeks (6) | 1·69 ± 0·23 (6) | 1·52 ± 0·29 (6)** | 7·43 ± 0·41 (6) | 7·50 ± 0·93 (8) |
| 20–25 weeks (7) | 2·35 ± 0·18 (7) | 2·06 ± 0·44 (7)** | 10·17 ± 0·43 (7)** | 10·76 ± 1·36 (7)** |
| ICAM-1–/–-MRL/MpJ-faslpr | ||||
| 16 weeks (6) | 1·43 ± 0·11 (6) | 1·50 ± 0·19 (6)** | 4·29 ± 0·30 (6)** | 3·30 ± 0·77 (8)** |
| 20–25 weeks (6) | 4·14 ± 0·23 (6)** | 3·41 ± 0·12 (6) | 10·49 ± 0·30 (6)** | 11·73 ± 0·77 (6)** |
CD4+, CD8+, CD4–/CD8– and B220+data are expressed as cells × 106/ml ± s.e.m. based on the total CD3+ cell count, and percentage data for each subclass. The number of mice sampled is shown in parentheses.
P < 0·05 versus MRL+/+ at same age.
P < 0·05 versus MRL/MpJ-faslpr at same age.
Leucocyte infiltration is elevated in the choroid plexus of MRL/MpJ-faslpr mice
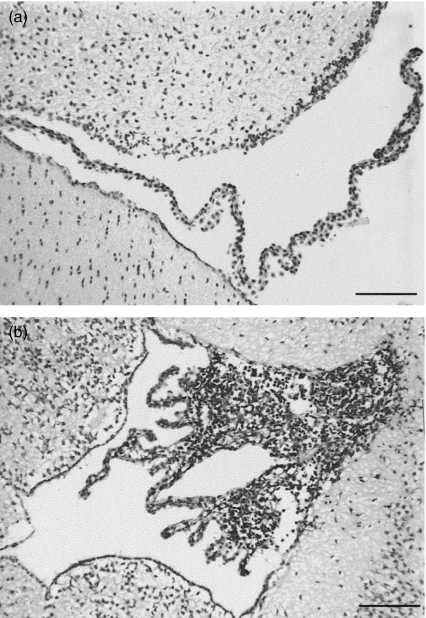
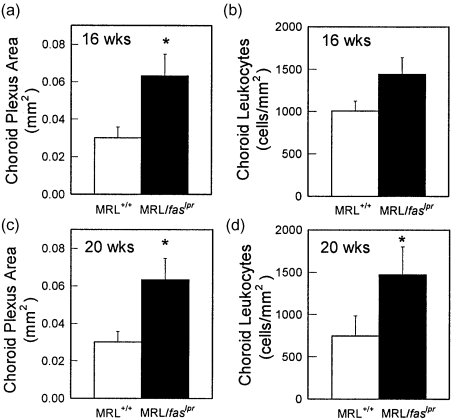
Histological examination of leucocyte infiltration in brains of MRL/MpJ-faslpr mice revealed that, in accord with previous observations, the choroid plexus was the primary site of leucocyte infiltration (Fig. 1), with leucocytes rarely detectable within the parenchyma [10]. Therefore, detailed comparison of MRL+/+ and MRL/MpJ-faslpr mice was based on analysis of the choroid plexus. In MRL+/+ mice, the villi of the choroid plexus were thin and contained diffuse areas of CD45+ cell infiltration (Fig. 1a). In contrast, the intensity of infiltration in the choroid plexus of MRL/MpJ-faslpr mice was such that the size of the villi appeared increased relative to MRL+/+ mice (Fig. 1b). We therefore quantified the size of the choroid plexus villi and intensity of infiltration of the choroid in MRL+/+ and MRL/MpJ-faslpr mice. At 16 weeks, the mean area of the choroid plexus in MRL/MpJ-faslpr mice was greater than twice that in MRL+/+ mice (P< 0·05) (Fig. 2a). Moreover, by 20 weeks, both the area of the choroid villi and the number of leucocytes per mm2 of choroid villus area were significantly greater in MRL/MpJ-faslpr mice relative to MRL+/+ mice (Fig. 2c,d).
Fig. 1.
Cerebral leucocyte recruitment in the brains of MRL+/+ (a) and MRL/MpJ-faslpr (b) mice at 20 weeks. Intense leucocyte infiltrate is present in choroid plexus villi of MRL/MpJ-faslpr mice but not in MRL+/+ mice. Bar denotes 100 µm.
Fig. 2.
Quantification of leucocyte recruitment in the choroid plexus of MRL+/+ and MRL/MpJ-faslpr mice at 16 and 20 weeks. Brains were sectioned at regular intervals and leucocyte recruitment identified using immunohistochemistry for CD45. (a,c) Area of the choroid plexus in MRL+/+ and MRL/MpJ-faslpr mice at 16 (a)and 20 (c)weeks. (b,d) Density of CD45+ leucocyte infiltrate present in the choroid plexus of both mouse strains at 16 (b) and 20 (d)weeks. n = 6–8 mice per group. Data are shown as mean ± s.e.m. *P < 0·05 versus MRL+/+ mice.
Neutrophils and T cells are the dominant leucocytes recruited to the brains of MRL/MpJ-faslpr mice
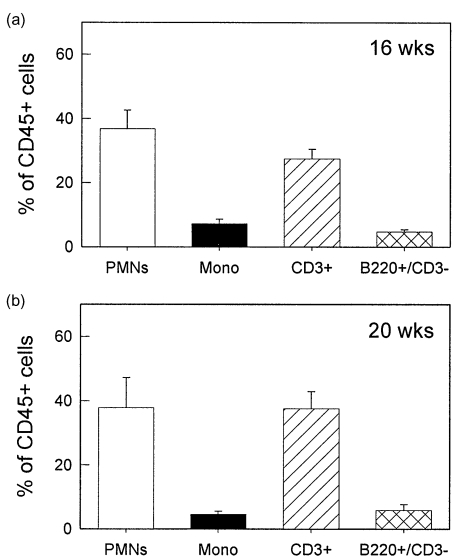
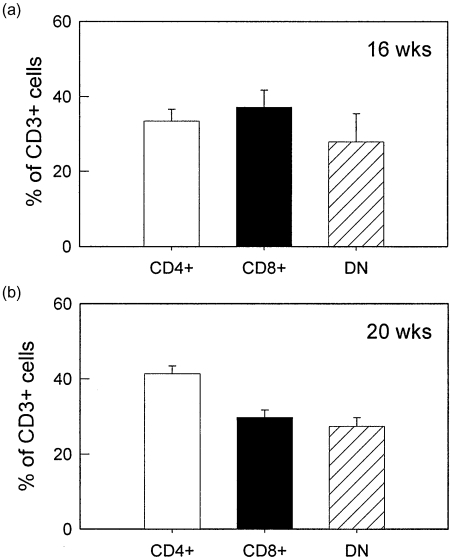
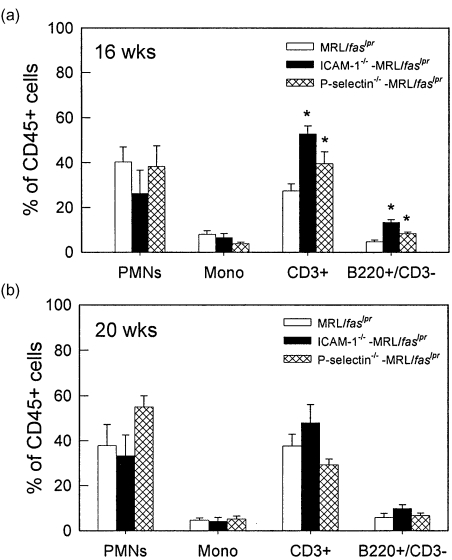
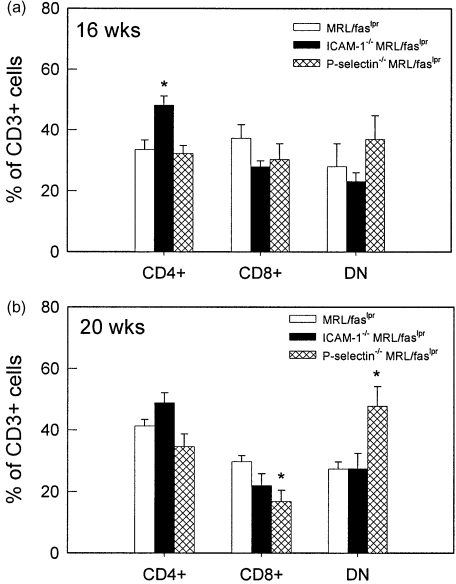
The infiltrating leucocytes in brains of MRL/MpJ-faslpr mice were then purified from the brain via density gradient centrifugation and identified using flow cytometry. At both 16 and 20 weeks, the dominant cell types present in the brains were neutrophils and lymphocytes, together comprising 70–80% of the CD45+ leucocyte population (Fig. 3). Monocytes and B cells each comprised < 10% of the infiltrated leucocytes. It must be noted that, in many cases, the total leucocyte percentages did not reach 100%. Given that this was not observed when leucocytes were isolated from blood, this suggests that the process of brain dissociation and leucocyte isolation induced loss of one of the surface markers used for leucocyte identification. We next examined the T lymphocyte populations present in the brain (Fig. 4). At 16 weeks, the numbers of CD4+, CD8+ and DN cells were similar (Fig. 4a). At 20 weeks the number of CD4+ cells was higher than the other two populations; however, both CD8 and DN populations were present in substantial numbers (Fig. 4b). These data indicate that DN T cells are able to exit the cerebral vasculature and enter the brain to a similar extent as the other T cell phenotypes.
Fig. 3.
Leucocyte phenotypes present in brains of MRL/MpJ-faslpr mice at 16 and 20 weeks. Leucocytes were isolated from brains using density centrifugation and classified using flow cytometry as follows: PMNs − Gr-1hi/M1/70hi; monocytes − Gr-1int/M1/70hi; T cells – CD3+; B cells − B220+/CD3–. n = 7–13 mice per group. Data are shown as mean ± s.e.m.
Fig. 4.
T cell phenotypes present in brains of MRL/MpJ-faslpr mice at 16 and 20 weeks. Leucocytes were isolated from brains using density centrifugation and CD3+ leucocytes were classified using flow cytometry as CD4+, CD8+ or CD4–/CD8– (DN). n = 6 mice per group. Data are shown as mean ± s.e.m.
Effect of ICAM-1 and P-selectin deficiency on infiltration
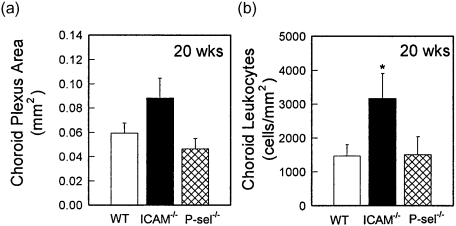
We next examined leucocyte infiltration in MRL/MpJ-faslpr mice deficient in either ICAM-1 or P-selectin. In 20-week-old mice, neither adhesion molecule deficiency resulted in a reduction in choroid leucocyte infiltration (Fig. 5). In contrast, ICAM-1–/– MRL/MpJ-faslpr mice displayed increased leucocyte recruitment, apparent as a significant increase in the density of leucocyte infiltrate within the affected area (Fig. 5b). P-selectin–/– mice did not differ from wild-type mice.
Fig. 5.
Quantification of leucocyte recruitment in the choroid plexus of wild-type (WT) MRL/MpJ-faslpr mice and intercellular adhesion molecule-1 (ICAM-1)–/– and P-selectin–/– MRL/MpJ-faslpr mice at 20 weeks. Brains were sectioned at regular intervals and leucocyte recruitment identified using immunohistochemistry for CD45. (a) Area of choroid plexus. (b) Density of CD45+ leucocyte infiltrate present in the choroid plexus of each of the mouse strains. n = 7 for WT mice and 4 for ICAM-1–/– and P-selectin–/– mice. Data are shown as ± s.e.m. *P < 0·05 versus MRL/MpJ-faslpr mice.
Assessment of the types of leucocytes present in the brains of these mice showed that at 16 weeks, a greater proportion of both T and B cells were present in brains of both ICAM-1–/– and P-selectin–/– MRL/MpJ-faslpr mice, relative to wild-type MRL/MpJ-faslpr mice (Fig. 6a). However, by 20 weeks, no differences were observed in the broad phenotypes of leucocytes in the brains of these mice (Fig. 6b). In addition, investigation of the types of T cells present revealed only minor differences in the T cell populations present (Fig. 7). At 16 weeks, ICAM-1–/– mice had a significantly increased proportion of CD4+ cells, whereas at 20 weeks, DN T cells were significantly increased in P-selectin–/– MRL/MpJ-faslpr mice, relative to wild-type MRL/MpJ-faslpr mice.
Fig. 6.
Leucocyte phenotypes present in brains of wild-type, ICAM-1–/– and P-selectin–/–MRL/MpJ-faslpr mice at 16 and 20 weeks. Leucocytes were isolated from brains using density centrifugation and classified using flow cytometry as described for Fig. 3. Data are shown as mean ± s.e.m. of 6–13 mice per group. *P < 0·05 versus MRL/MpJ-faslpr mice.
Fig. 7.
T cell phenotypes present in brains of wild-type, ICAM-1–/– and P-selectin–/–MRL/MpJ-faslpr mice at 16 and 20 weeks. Leucocytes were isolated from brains using density centrifugation and CD3+ leucocytes were classified using flow cytometry as CD4+, CD8+ or CD4–/CD8– (DN). Data are shown as mean ± s.e.m. of 5–10 mice per group. *P < 0·05 versus MRL/MpJ-faslpr mice.
Effect of deficiency of ICAM-1 or P-selectin on phenotype of circulating leucocytes in MRL/MpJ-faslpr mice
Mice deficient in ICAM-1 and P-selectin on conventional backgrounds have been described previously to have increased circulating leucocyte counts [22,23]. Therefore, we examined circulating leucocyte phenotypes of these mice on the MRL/MpJ-faslpr background (Tables 1 and 2). At 8 weeks, P-selectin–/– MRL/MpJ-faslpr mice displayed significant elevations in all cell types, but most prominently in neutrophils. At 16 and 20 weeks, the number of circulating leucocytes in both strains showed comparable elevations (relative to MRL+/+ mice) to those seen in wild-type MRL/MpJ-faslpr mice. However, ICAM-1–/– mice showed evidence of a delay in the increase in CD3+ cells, as at 16 weeks the number of CD3+ cells was lower than that seen in wild-type MRL/MpJ-faslpr mice, due to reduced numbers of CD8 and DN T cells. However, by 20 weeks, ICAM-1–/– mice had become similar to wild-type MRL/MpJ-faslpr mice, apart from an alteration in the proportions of T cells such that CD4 cells were increased significantly and DN T cells were decreased significantly (Tables 1 and 2). In P-selectin–/– mice at 20 weeks, the number of CD3+ T cells was reduced relative to the extremely high levels in wild-type MRL/MpJ-faslpr mice, due to lower levels of CD8 and DN T cells in P-selectin–/– mice (Tables 1 and 2). Finally, DN T cells in MRL/MpJ-faslpr mice have been observed previously to express B220 [24]. Examination of B220 expression on CD3+ cells in the present study revealed that the number of B220+ CD3+ lymphocytes was very similar to the number of DN T cells, suggesting that all circulating DN T cells in all strains of MRL/MpJ-faslpr mice examined expressed B220 (Table 2).
Discussion
It has been assumed that leucocytes rarely enter the brain, due to the nature of the blood–brain barrier. However, it is clear that in response to local inflammatory stimuli leucocytes can gain access readily to the brain [20,25–27]. This also applies in the systemic disease which affects the MRL/MpJ-faslpr mouse [10,28]. Moreover, the finding that immunosuppressive treatments which reduce cerebral leucocyte infiltration protect MRL/MpJ-faslpr mice from cognitive dysfunction provides support for the concept that leucocytes are key contributors to cerebral pathology in these lupus-prone mice [29]. However, the mechanisms responsible for this leucocyte recruitment are unknown. We have demonstrated previously that the cerebral microvasculature of MRL/MpJ-faslpr mice shows evidence of aberrant activation, such that leucocyte–endothelial cell interactions are increased relative to non-diseased mice [11]. Due to the extremely low rate of leucocyte trafficking in the uninflamed brain, it is probable that in diseased MRL/MpJ-faslpr mice this endothelial activation is critical to allowing large numbers of leucocytes to attach to the cerebral endothelium and exit the vasculature. Despite these studies, the endothelial adhesion molecules required for leucocyte entry into the brain remain unclear. Furthermore, the complex immunology of these mice has meant that the types of leucocytes which are recruited to these animals have not been identified clearly. Therefore, the two aims of this study were to classify the types of leucocytes recruited to the brains of these mice, and investigate the roles of two candidate adhesion molecules in their recruitment. The data show that the leucocyte infiltrate consists predominantly of a mixture of T cells and neutrophils, and that DN T cells comprise approximately one-third of all T cells. In addition, the absence of either ICAM-1 or P-selectin did not affect markedly the recruitment of the key leucocyte populations to the inflamed brains of MRL/MpJ-faslpr mice.
DN T cells are generated in large numbers in MRL/MpJ-faslpr mice [6]. Moreover, evidence indicates that they contribute significantly to tissue injury in the kidney [30]. However, DN T cells have been difficult to identify definitively using immunohistochemistry, as the main marker used to identify them (B220) is also expressed on B cells. Therefore, to investigate their contribution to cerebral leucocyte infiltration, we isolated leucocytes from the brains and used flow cytometry to examine multiple cell markers on individual cells. This allowed the unequivocal identification of CD3+ DN T cells in brains of lupus mice, in addition to CD4 and CD8 single-positive cells. This demonstrated that DN T cells were able to enter the brain effectively, to the extent that they comprised approximately one-third of all T cells in the brain. Infiltration of CD4+/CD8+‘double-positive’ cells was also investigated, as it has been suggested that CD4+ cells accumulate in the brain only because they are dysfunctional and display aberrant expression of both CD4 and CD8 [10,31]. However, in the present study double-positive cells were observed rarely in the inflamed brains of lupus mice.
The conventional paradigm used to explain the movement of leucocytes from the mainstream of blood flow into tissues requires that leucocytes first roll then adhere onto the endothelial surface, and subsequently exit the vasculature [32]. In many cases, the initial rolling interaction is selectin-mediated, whereas the subsequent adhesion step requires interaction between leucocyte integrins and their endothelial ligands, including ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) [33]. We have observed previously a key role for the α4-integrin/VCAM-1 pathway in mediating leucocyte interactions in pial post-capillary venules in MRL/MpJ-faslpr mice [11]. Moreover, although P-selectin mediated some rolling in cerebral microvessels, in its absence the number of leucocytes which became adherent was unaltered. However, this study examined only intravascular rolling and adhesive interactions. The approach used did not allow assessment of the molecular basis for the subsequent step of leucocyte emigration from the vasculature. In addition, the area of the most intense cerebral leucocyte recruitment in these animals, the choroid plexus, was not examined. The molecular basis of rolling and adhesion is characterized readily, as inhibitory antibodies can block these interactions rapidly. In contrast, emigration and leucocyte accumulation in the brain is a much slower process [34]. Under these circumstances, use of MRL/MpJ-faslpr mice deficient in specific adhesion molecules provides an excellent tool for the assessment of the molecular basis for leucocyte accumulation in the brain. These experiments have demonstrated clearly that ICAM-1 and P-selectin are not required for leucocyte recruitment to the CNS in MRL/MpJ-faslpr mice.
It was noteworthy that in 20-week ICAM-1–/– MRL/MpJ-faslpr mice, leucocyte infiltration was elevated significantly relative to wild-type MRL/MpJ-faslpr mice, suggesting that ICAM-1 acts to reduce the numbers of leucocytes which accumulate in the CNS. The mechanisms whereby ICAM-1 may mediate this effect are not clear. Given the established role of ICAM-1 as a lymphocyte co-stimulatory molecule, the elevation in recruitment may be a consequence of altered lymphocyte activation in ICAM-1-deficient cells [35,36]. Alternatively ICAM-1 may be involved in leucocyte elimination from the inflamed brain via processes such as apoptosis or recirculation.
Deficiency in ICAM-1 has been shown previously to have a specific effect on neutrophil migration, as shown by a fourfold increase in circulating neutrophils and a concomitant decrease in neutrophil migration to sites of inflammation [22]. Similarly, deficiency in P-selectin has been reported to result in a 2·4-fold increase in circulating neutrophil numbers, and an inhibition, but not elimination, of neutrophil entry into the inflamed peritoneum [23]. The effect of deficiency of P-selectin on leucocyte recruitment is variable depending on the model investigated. In some models, the absence of P-selectin results in almost complete absence of leucocyte recruitment [37], whereas in others the absence of P-selectin does not reduce recruitment [38,39]. Studies with mice lacking both P-selectin and E-selectin have shown that additional absence of E-selectin results in a much more profound deficiency in recruitment, suggesting that in the absence of P-selectin, E-selectin can mediate critical leucocyte rolling interactions required for recruitment induced by inflammatory stimuli [40,41]. Given these findings, it is arguably not surprising that in the present experiments, leucocyte entry into the brain was not affected by either deletion. This suggests that alternative adhesion molecules may assume the roles played by P-selectin and ICAM-1 in supporting leucocyte entry into the brain. This possibility is supported by the fact that the inflammation which affects the MRL/MpJ-faslpr mouse occurs over several months, allowing time for expression of alternative adhesion molecule pathways.
The reason the choroid plexus is a preferential target of cerebral leucocyte recruitment in MRL/MpJ-faslpr mice is unclear. It is possible that the fenestrated endothelium of the choroid plexus allows leucocyte entry to occur more readily, and the preferential deposition of immune complexes in this site may also act to attract and retain leucocytes [28]. In addition, investigation of adhesion molecule expression in the choroid has demonstrated that the choroid epithelial cells, but not the associated endothelial cells, constitutively express ICAM-1 and VCAM-1, and increase expression of these molecules during inflammatory responses [42]. This finding raises the possibility that following leucocyte exit from the vasculature, epithelial cell-expressed molecules are important in retaining leucocytes at this site. It is clear from the present findings that ICAM-1 does not serve this function, as large numbers of leucocytes accumulate in the choroid plexus of ICAM-1–/– MRL/MpJ-faslpr mice. However, epithelial cell-expressed VCAM-1 could act in this fashion as many of the leucocyte types present in the choroid, including DN T cells (data not shown), express VCAM-1 ligands such as the α4-integrin, making them capable of adhering to VCAM-1-expressing cells [43,44].
Recent observations have shown that in some tissues the necessity for a rolling interaction prior to leucocyte adhesion to the endothelium is bypassed [45–47]. Furthermore, in CNS microvessels, adhesion of activated lymphoblasts has been observed to occur without prior rolling [34]. Given these findings, it is conceivable that in the choroid plexus vasculature the conventional multi-step paradigm of leucocyte recruitment does not apply. Under these circumstances it is plausible that neither P-selectin nor ICAM-1 are required for leucocyte adhesion within the choroidal vasculature. Further experiments with MRL/MpJ-faslpr mice lacking alternative adhesion molecules would aid in clarifying this issue.
Acknowledgments
These studies were supported by a programme grant (no. 334067) from the National Health and Medical Research Council (NHMRC), Australia and an NIH RO1 grant (no. AR51807-01), both held by M. Hickey, and an NIH grant (no. RR-017009) to D. Bullard. M. Hickey is an NHMRC R. D. Wright Fellow.
References
- 1.Lahita RG. Systemic lupus erythematosus. 3. San Diego: Academic Press; 1999. [Google Scholar]
- 2.van Dam AP. Diagnosis and pathogenesis of CNS lupus. Rheumatol Int. 1991;11:1–11. doi: 10.1007/BF00290244. [DOI] [PubMed] [Google Scholar]
- 3.Mitsias P, Levine SR. Large cerebral vessel occlusive disease in systemic lupus erythematosus. Neurology. 1994;44:385–93. doi: 10.1212/wnl.44.3_part_1.385. [DOI] [PubMed] [Google Scholar]
- 4.Hanly JG, Walsh NM, Sangalang V. Brain pathology in systemic lupus erythematosus. J Rheumatol. 1992;19:732–41. [PubMed] [Google Scholar]
- 5.Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–11. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 6.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 7.Hess DC, Taormina M, Thompson J, et al. Cognitive and neurologic deficits in the MRL/lpr mouse: a clinicopathologic study. J Rheumatol. 1993;20:610–7. [PubMed] [Google Scholar]
- 8.Sakic B, Szechtman H, Talangbayan H, et al. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol Behav. 1994;56:609–17. doi: 10.1016/0031-9384(94)90309-3. [DOI] [PubMed] [Google Scholar]
- 9.Walker SE, Wright DC, O'Sullivan FX, et al. Memory, learning ability, and neuropathologic status of mice with systemic lupus erythematosus. Ann NY Acad Sci. 1997;823:107–15. doi: 10.1111/j.1749-6632.1997.tb48383.x. [DOI] [PubMed] [Google Scholar]
- 10.Vogelweid CM, Johnson GC, Besch-Williford CL, et al. Inflammatory central nervous system disease in lupus-prone MRL/lpr mice: comparative histologic and immunohistochemical findings. J Neuroimmunol. 1991;35:89–99. doi: 10.1016/0165-5728(91)90164-3. [DOI] [PubMed] [Google Scholar]
- 11.James WG, Bullard DC, Hickey MJ. Critical role of the alpha 4 integrin/VCAM-1 pathway in cerebral leukocyte trafficking in lupus-prone MRL/fas(lpr) mice. J Immunol. 2003;170:520–7. doi: 10.4049/jimmunol.170.1.520. [DOI] [PubMed] [Google Scholar]
- 12.Hammond DM, Nagarkatti PS, Gote LR, et al. Double-negative T cells from MRL-lpr/lpr mice mediate cytolytic activity when triggered through adhesion molecules and constitutively express perforin gene. J Exp Med. 1993;178:2225–30. doi: 10.1084/jem.178.6.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullard DC, King PD, Hicks MJ, et al. Intercellular adhesion molecule-1 deficiency protects MRL/MpJ-Faslpr mice from early lethality. J Immunol. 1997;159:2058–67. [PubMed] [Google Scholar]
- 14.Lloyd CM, Gonzalo J-A, Salant DA, et al. Intercellular adhesion molecule-1 deficiency prolongs survival and protects against the development of pulmonary inflammation during murine lupus. J Clin Invest. 1997;100:963–71. doi: 10.1172/JCI119647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kevil CG, Hicks MJ, He X, et al. Loss of LFA-1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am J Pathol. 2004;165:609–16. doi: 10.1016/S0002-9440(10)63325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X, Panoskaltsis-Mortari A, Samuel S, et al. P-selectin deficiency accelerates development of autoimmune disease in MRL/MpJ-Faslpr mice. FASEB J. 2004;18:A554. [Google Scholar]
- 17.Hickey MJ, Bullard DC, Issekutz A, et al. Leukocyte–endothelial cell interactions are enhanced in dermal postcapillary venules of MRL/fas lpr (lupus-prone) mice: roles of P- and E-selectin. J Immunol. 2002;168:4728–36. doi: 10.4049/jimmunol.168.9.4728. [DOI] [PubMed] [Google Scholar]
- 18.Huang XR, Holdsworth SR, Tipping PG. Evidence for delayed-type hypersensitivity mechanisms in glomerular crescent formation. Kidney Int. 1994;46:69–78. doi: 10.1038/ki.1994.245. [DOI] [PubMed] [Google Scholar]
- 19.Krakowski ML, Owens T. The central nervous system environment controls effector CD4+ T cell cytokine profile in experimental allergic encephalomyelitis. Eur J Immunol. 1997;27:2840–7. doi: 10.1002/eji.1830271115. [DOI] [PubMed] [Google Scholar]
- 20.Kerfoot SM, Kubes P. Overlapping roles of P-selectin and alpha(4) integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1000–6. doi: 10.4049/jimmunol.169.2.1000. [DOI] [PubMed] [Google Scholar]
- 21.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Meth. 1996;197:139–50. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 22.Sligh JE, Jr, Ballantyne CM, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–33. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayadas TN, Johnson RC, Rayburn H, et al. Leukocyte rolling and extravasation are severely compromised in P-selectin-deficient mice. Cell. 1993;74:541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 24.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt B, Vestweber D, Hallmann R, et al. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood–brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–72. [PubMed] [Google Scholar]
- 26.Engelhardt B, Laschinger M, Schulz M, et al. The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin. J Clin Invest. 1998;102:2096–105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitagawa K, Matsumoto M, Mabuchi T, et al. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18:1336–45. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Alexander EL, Murphy ED, Roths JB, et al. Congenic autoimmune models of CNS disease in connective tissue disorders. Ann Neurobiol. 1983;14:242–8. doi: 10.1002/ana.410140211. [DOI] [PubMed] [Google Scholar]
- 29.Farrell M, Sakic B, Szechtman H, et al. Effect of cyclophosphamide on leukocytic infiltration in the brain of MRL/lpr mice. Lupus. 1997;6:268–74. doi: 10.1177/096120339700600310. [DOI] [PubMed] [Google Scholar]
- 30.Wada T, Schwarting A, Chesnutt MS, et al. Nephritogenic cytokines and disease in MRL-Fas (lpr) kidneys are dependent on multiple T-cell subsets. Kidney Int. 2001;59:565–78. doi: 10.1046/j.1523-1755.2001.059002565.x. [DOI] [PubMed] [Google Scholar]
- 31.Via CS, Shearer GM. Functional heterogeneity of L3T4+ T cells in MRL-lpr/lpr mice. L3T4+ T cells suppress major histocompatibility complex-self-restricted L3T4+ T helper cell function in association with autoimmunity. J Exp Med. 1988;168:2165–81. doi: 10.1084/jem.168.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer TA. Traffic signals of lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 33.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 34.Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–65. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhlman P, Moy VT, Lollo BA, et al. The accessory function of murine intercellular adhesion molecule-1 in T lymphocyte activation. Contributions of adhesion and co-activation. J Immunol. 1991;146:1773–82. [PubMed] [Google Scholar]
- 36.Van Seventer GA, Shimizu Y, Horgan KJ, et al. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–86. [PubMed] [Google Scholar]
- 37.Kanwar S, Smith CW, Kubes P. An absolute requirement for P-selectin in ischemia/reperfusion-induced leukocyte recruitment in cremaster muscle. Microcirculation. 1998;5:281–7. [PubMed] [Google Scholar]
- 38.Bullard DC, Mobley JM, Justen JM, et al. Acceleration and increased severity of collagen-induced arthritis in P-selectin mutant mice. J Immunol. 1999;163:2844–9. [PubMed] [Google Scholar]
- 39.Rosenkranz AR, Mendrick DL, Cotran RS, et al. P-selectin deficiency exacerbates experimental glomerulonephritis: a protective role for endothelial P-selectin in inflammation. J Clin Invest. 1999;103:649–59. doi: 10.1172/JCI5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frenette PS, Mayadas TN, Rayburn H, et al. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–74. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 41.Staite ND, Justen JM, Sly LM, et al. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–9. [PubMed] [Google Scholar]
- 42.Wolburg K, Gerhardt H, Schulz M, et al. Ultrastructural localization of adhesion molecules in the healthy and inflamed choroid plexus of the mouse. Cell Tissue Res. 1999;296:259–69. doi: 10.1007/s004410051287. [DOI] [PubMed] [Google Scholar]
- 43.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25:215–33. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 44.Poon BY, Ward CA, Giles WR, et al. Emigrated neutrophils regulate ventricular contractility via alpha4 integrin. Circ Res. 1999;84:1245–51. doi: 10.1161/01.res.84.11.1245. [DOI] [PubMed] [Google Scholar]
- 45.Wong J, Johnston B, Lee SS, et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–90. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Downey GP, Worthen GS, Henson PM, et al. Neutrophil sequestration and migration in localized pulmonary inflammation. Capillary localization and migration across the interalveolar septum. Am Rev Respir Dis. 1993;147:168–76. doi: 10.1164/ajrccm/147.1.168. [DOI] [PubMed] [Google Scholar]
- 47.De Vriese AS, Endlich K, Elger M, et al. The role of selectins in glomerular leukocyte recruitment in rat anti-glomerular basement membrane glomerulonephritis. J Am Soc Nephrol. 1999;10:2510–7. doi: 10.1681/ASN.V10122510. [DOI] [PubMed] [Google Scholar]