Abstract
β-cell replacement is the only way to restore euglycaemia in patients with type-1 diabetes. Pancreatic tissue, processed for subsequent clinical islet transplantation, is exposed to ischaemia causing injury and death in a large number of islets before and after transplantation. In this review we summarize what is known on the sources of environmental stress for pancreatic islets, such as insufficient oxygen supply during pancreas procurement and in culture prior to intraportal transplantation, nutritional and oxygen deprivation during the isolation process, and the consequences of hyperglycaemia. An increasingly recognized role in the modulation of β-cell function and these environmental stress factors plays the vascular network of the pancreatic islets. Islet revascularization by angiogenesis is relevant for the survival of the graft subsequent to transplantation. Potential strategies offered by therapeutic induction of revascularization to ameliorate the detrimental impact of these factors on the quality of islet transplants are discussed.
Keywords: environmental stress, ischaemia, hypoxia, angiogenesis, apoptosis, inflammation
Introduction
Clinical diabetes is associated with progressive loss of the insulin producing β-cells of the endocrine pancreas. Type-1 diabetes is an autoimmune disease characterized by activation of the Th1-phenotype, lymphocytic infiltration of pancreatic islets, and programmed cell death of β-cells. Death of the pancreatic β-cells is associated with hyperglycaemia which is the main determinant of chronic vascular complications in people with diabetes. Intensive insulin therapies reduce the onset and progression of diabetic complications but they are related to an increased risk of life-threatening hypoglycaemic episodes. β-cell replacement is the only way to restore euglycaemia and ameliorate the progression of diabetic complications. Pancreas or pancreatic islet transplantation are such strategies to treat patients with type-1 diabetes, since if successful could provide a cure for the disease. The transplantation of isolated islets represents a minimal invasive approach for β-cell replacement and recently developed protocols enhanced the short-term success rate of islet transplantation [1]. However, there is still a lack of metabolic capacity in islet transplants in the long run which cannot even be compensated by transplantation of a massive amount of islets. This phenomenon must be mainly attributed to detrimental nutritional and inflammatory conditions against which pancreatic islets possess no significant means of protection. In particular, delayed and insufficient islet revasculariziation can deprive newly transplanted islets of oxygen, resulting in permanent cell death and contributing to early graft failure.
Inflammatory molecules, such as FasL and cytokines like IL-1β, TNF-α and IFN-gamma have the potential to damage β-cells [2,3]. They promote insulitis and β-cell destruction in autoimmune diabetes together with nonspecific toxic molecules, such as reactive oxygen species (ROS). ROS will cause DNA damage in β-cells which by activation of repair enzymes will compromise ATP production in the cells [4].
Glucose in chronic excess causes toxic effects on structure and function of organs, including the pancreatic islet. Multiple biochemical pathways and mechanisms of action for glucose toxicity have been suggested including protein kinase C activation, hexosamine metabolism, and oxidative phosphorylation. Glucose metabolites traveling along these pathways were found in relation to β-cell damage. These pathways have in common the formation of ROS that over time, result in chronic oxidative stress, which in turn causes defective insulin gene expression, reduced insulin secretion, and increased apoptosis of β-cells.
Pancreatic insulitis was described to be linked to increased blood flow in islets [5]. Inflammatory tissue is prone to induce new vessel formation in different diseases, such as bacterial infection of skin wounds, rheumatoid arthritis, and cancer [6–8]. On the other hand, islets transplanted into a syngenic environment do not induce sufficient angiogenesis to ensure optimal oxygen supply [9]. Transplanted islets seem to suffer from chronic oxygen deficiency [10]. Hypoxia and inflammation both are inhibiting factors for the success of pancreatic islet transplantation. They are also mechanisms driving angiogenesis of pancreatic islet grafts.
In this review we want to present links between the different stress factors, inflammatory, nutritional and hypoxic stress of pancreatic β-cells and endothelial cells with a particular focus on the consequences for islet transplantation.
Sources of nutritional and inflammatory stress
In response to short periods of hypoxia, cells adapt to low oxygen by different strategies. Cell activities demanding high amount of ATP are decreased to conserve energy for maintenance of essential elements for survival. Oxygen delivery is increased and glycolysis is enhanced by induction of genes that are associated with angiogenesis and glucose metabolism. Hypoxia activates survival pathways if cells are threatened by damage and death [11]. If hypoxia exists persistently, death programs will be finally activated. Although it has been already speculated that apoptosis or necrosis secondary to hypoxia contributes to islet loss after transplantation, there is lacking clarity about the regulating mechanisms in an islet graft.
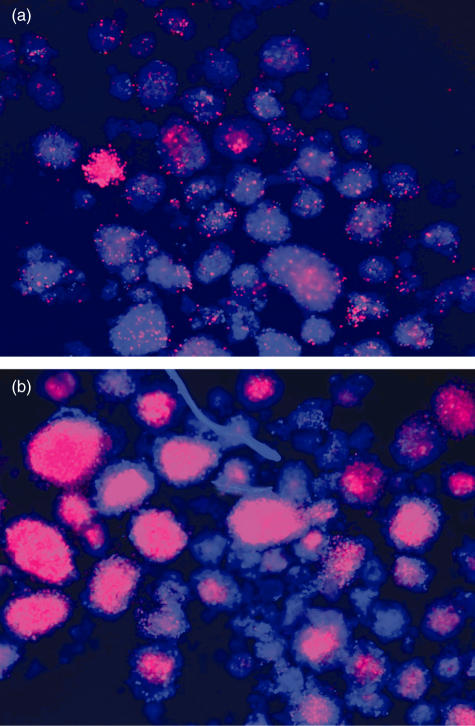
Islets represent approximately 1% of the pancreatic tissue but receive more than 15% of the total pancreatic blood flow [12]. When islets are isolated the capillary network necessary for blood supply is destroyed and cultured islets are supplied with oxygen and nutrients solely by diffusion from the medium. Isolated islets cultured under hypoxic conditions show a zone of central necrosis (Fig. 1). Hypoxic damage in cultured islets depends on different factors, such as depth of the culture vessel, seeding density of the islets, islet size, and synergistic nutrient deprivation [13]. Central cell death in large islets also correlates with the depletion of the intracellular ATP content which significantly deteriorates glucose-stimulated insulin release and morphological viability [14].
Fig. 1.
Dead cells in human islets cultured at normoxia and hypoxia Human islets were cultured under (a) normal air or (b) 2% oxygen for 24 h following overnight culture after isolation. Propidium iodide only penetrates cells with incomplete membranes and stains them red while Hoechst probe enters all cells freely (blue). Photos were taken by Yi Lai.
Prior to necrosis of β-cells hypoxia can result in increased expression of NFκB and genes associated with apoptosis and the stress response (unpublished observations). Experiments in human islets suggest that ischaemia induces general inflammatory responses which include the expression of gene products such as tissue factor and MCP-1 [15]. MCP-1 is known for its potent chemotactic activity for monocytes whereas the expression of tissue factor was identified as the main trigger of an instant blood-mediated inflammatory reaction, a significant mediator of hyperacute islet destruction [16]. Production of these proteins correlates with a negative outcome after islet transplantation.
Of interest, the members of stress and apoptotic-related genes are highly involved in the regulation of survival of islet cells under hypoxia. Stress proteins HSP32 and HSP70 can assist recovery from cellular damage and protect cells from subsequent stress. The HSP70 family is involved in the protection of proteins from denaturation and also helps to refold denatured proteins [17]. Overexpression of HSP70 in rat and human islets provided resistance against injury caused by NO [18]. The expression of HSP 70 in islets of diabetes-prone BB rats was diminished compared to diabetes-resistant rats [19]. HSP32 plays a key role in protection of islets against damages. HSP32 is highly inducible under a variety of conditions associated with oxidative stress [20]. HSP32 mediated protective responses of rat islets against IL-1β protects pancreatic β-cells from apoptosis induced by various stimuli and improved in vivo function after transplantation [21].
Moreover, hypoxia is clearly involved in apoptosis and regulates apoptotic signalling pathways in a complex way [22]. The critical regulators of the intrinsic pathway of apoptosis are the Bcl-2 family members. Bcl-2 and Bcl-xl are antiapoptotic proteins that can keep mitochondrial integrity and avoid the release of cytochrome C that activates the caspase cascade. Bax and Bad are proapoptotic proteins that can induce the release of cytochrome C from the mitochondria. High glucose influences the expression of Bcl-2 family members in human islets [23]. Bax mRNA, but not Bcl-2 mRNA, was expressed at a higher level in islets compared with spleens, which makes islets susceptible to apoptosis.
The supply of oxygen is essential for an adequate cellular energy generation. Maintenance of the aerobic mitochondrial ATP synthesis through continuous oxygen supply during cold storage preserves cellular and metabolic viability during prolonged ischaemia. In particular, the protection of mitochondrial integrity is essential to prevent activation of the cytochrome pathway promoted by increased Bax expression after prolonged ischaemia [24].
At higher glucose concentrations normoxic rodent islets clearly prefer mitochondrial glycolysis whereas low concentrations of glucose stimulates both the aerobic as well as the anaerobic pathway [25]. In an hypoxic environment lactate production is increased in glucose-stimulated islets suggesting that the interrelation of respiratory and anaerobic pathway of glucose breakdown depends on the ambient oxygen concentration [26].
Considering the enormous demand for blood supply and the preference for aerobic glucose metabolization it seems questionable whether islets can adequately adapt to hypoxia at all. Increasing oxygen tension in the culture atmosphere could represent a simple possibility to overcome insufficient oxygen supply to cultured islets. But attempts to decrease the immunogenicity of isolated rat islets by exposure to a high oxygen atmosphere resulted in complete islet disintegration within 4–5 days of culture [27]. Up to now no studies have been performed do define the highest nontoxic oxygen pressure for improving oxygen delivery in cultured islets.
Pancreatic ischaemia will result in the temperature-dependent activation of endogeneous proteases and nonproteolytic enzymes. Normothermic ischaemia will activate these enzymes within a shorter period than cold ischaemia [28]. During enzymatic pancreas digestion oxygen tension will decrease. At the end of the isolation procedure an anoxic level is reached resulting in severe depletion of islet ATP content. This can be efficiently counteracted by the supplementation of preoxygenated perfluorocarbon and the simultaneous administration of ATP precursors during pancreas dissociation [29]. Whether this recently published procedure is feasible for human islet isolation needs further evaluation.
Another aspect in regard to transplantation is function rates of pancreases derived from brain-dead donors are inferior in comparison with living related or unrelated donors. Injury of the central nervous system is a trigger of a cascade of proinflammatory events accompanied by the time-dependent release of cytokines into the serum and activation of T-lymphocyte and macrophage-associated cytokines in the pancreas [30]. After activation of endothelial cells and enhanced expression of molecules for adhesion and costimulation the infiltration of leucocytes is observed [31]. This does not only result in a loss of organ quality for islet isolation but also in enhanced immunogenicity as indicated by increased expression of class I and II major histocompatibility antigens. Further, islets from brain-dead rats were also found to be immunologically highly activated characterized by enhanced expression of cytokines and MCP-1 [32].
Destruction of the natural microenvironment
In their natural environment islets are integrated in heterogeneous tissue mainly composed of acinar, ductal, vascular and nerval cells. Although the metabolic function of the individual components of the pancreas are well defined, the importance of an intact environment for proliferation, differentiation, and regeneration of islets has recently become more evident.
Following islet isolation the enzymatic digestion by proteolytic enzymes, such as collagenase results in the dissociation of islet-attached acinar and ductal tissue. A component of crude collagenase is endotoxin, a proinflammatory substance which is related to poor islet engraftment [33]. It was demonstrated that endotoxin is a significant trigger of inflammatory reactions involving intraislet cytokine production, inhibition of insulin secretion and apoptotic cell death [34,35].
Newly transplanted islets are vulnerable to ischaemia-reperfusion injury, an adverse event that can also cause inflammation and induce cellular apoptosis. Recent data show that tissue factor plays a causative role in triggering a instant blood-mediated inflammatory reaction when islets come into contact with blood [36]. Moreover, it was demonstrated that the expression of a number of cytokines including Il-6, Il-8, and MCP-1 is markedly elevated in freshly isolated human islets, coinciding with partially impaired insulin secretory function in islets before transplantation [37]. Collectively, these factors account for the observation that most functional losses of islet mass ensue during early post-transplant phase [38].
The isolation of islets is believed to be accompanied not only by the reduction of immunogenic connective tissue but also by the depletion of growth factors. This could be of relevance for the absence of a trophic interaction between islets and ductal epithelium which is believed to be essential for regeneration and repair of islet cells [39]. Recently, a positive correlation was observed between metabolic outcome in islet graft recipients and the amount of ductal tissue cotransplanted with allogenic human islets [40].
The loss of peripheral islet cells during poorly controlled pancreas digestion is a consequence of the enzymatic destruction of islet microenvironment which is formed by the extracellular matrix. Matrix is differentiated into two components, the interstitial matrix and the basement membrane (BM). In contrast to motile cells, islet cells require signals from the basal membrane to survive and to maintain their characteristic topographical arrangement essential for secretory function [41]. These Basal membrane–cell interactions seem to be mediated to a large extent by integrins. If detachment of peripheral cells from the basal membrane occurs in a consequence of uncontrolled enzyme activity, the communication between cell surface integrin receptors and matrix molecules is disrupted and apoptosis is induced. Although BM can be re-established during certain culture conditions down-regulation of integrin expression is generally observed in islets isolated from several species [42]. In this context the reduction of the fibronectin receptor integrin α5 is of particular importance since it is linked to the up-regulation of Bcl-2 essential to prevent mitochondrial cytochrome C release as an early event in apoptosis [43].
Islet microvasculature and blood flow
Pancreatic islets comprise four functionally distinct cell types, namely glucagon-secreting α-cells, insulin secreting β-cells, somatostatin-secreting δ-cells and pancreatic polypeptide-secreting cells [44]. These cells are highly organized with β-cells clustered in the central core and other nonβ-cell types located on the periphery of islets. Such a structural organization of islets is physiologically important in orchestrating intra-islet cellular communication between different cell types for modulating the release of islet hormones in response to changes in metabolic states.
In keeping with this spatial arrangement of cells within islets, islet microvasculature is also organized in a nonrandom manner for its functional adaptation to metabolic regulation. Using rodent islets as a model system it was demonstrated that islet microcirculation is supplied by a few different arterioles that pass through the nonβ-rim into the central core where they ramify into fenestrated capillaries, and drain coalescing into an efferent plexus exiting the islet through several venules. Such a core-to-mantle islet angioarchitecture is thought to maintain blood flow from central β-cells to peripheral α cells providing an anatomic priority for β-cells to detect blood glucose fluctuations and cross-talk to downstream nonβ-cells via rapid release of insulin in response to a rise in blood glucose levels [45].
The pancreatic islet angioarchitecture entails a blood perfusion of the pancreatic islets that is 10 times higher than that in the exocrine pancreas and similar to that seen in the renal cortex (5–7 ml/min/g). However, during the process of isolation and in vitro culture of pancreatic islets preceding transplantation, the islet vasculature is believed to dedifferentiate or degenerate. Therefore, immediately after transplantation, the pancreatic islets are supplied with oxygen and nutrients solely by diffusion from the surrounding tissues. The revascularization process is initiated within a few days, and the islets are generally thought to be fully revascularized by 1 month post-transplantation. However, oxygen tension and blood flow was reported to be reduced in islet grafts independent from the site of transplantation [46].
Recent clinical data indicate that even when fasting glycaemia is corrected in diabetic recipients, the functional performance of engrafted islets with respect to the first-phase insulin secretion in response to intravenous glucose load is about 20% of normal [47]. Furthermore, it was observed that diabetic rats suffer from hypoglycaemia in response to prolonged exercise due to impaired glucagon secretion in transplanted islets [48]. Whether or not these abnormalities in insulin and glucagon release in transplant subjects are related to the lack of adequate islet revascularization remain to be determined but there is evidence that a lack of adequate islet revascularization can reduce microvascular perfusion to islet grafts and compromise the function of transplanted islets, contributing to suboptimal performance of functional islets post transplantation [49]. Furthermore, a significant gain in islet revascularization, caused by VEGF-mediated augmentation of angiogenesis in islet grafts, is associated with improved glucose-stimulated insulin release and enhanced glucose tolerance in diabetic recipient mice [50]. While inadequate islet mass has been considered a culprit for prolonged postprandial blood glucose excursions as well as for impaired ability to tolerate intravenously injected glucose, these data implicate inadequate islet revascularization as another underlying cause for the observed metabolic anomaly in transplant subjects.
Islet angiogenesis in vitro and in vivo
Intra-islet endothelial cells were described before in rodent and also human pancreas [51]. We demonstrated spontaneous sprouting activity of islet endothelia embedding islets into fibrin gels soaked with VEGF and bFGF [52]. With this approach it is possible to study mechanisms of the angiogenic process under standardized conditions. β-cells and endothelial cells are focally delivered into a three-dimensional fibrin matrix, allowing sprouting to occur by invasion into the extracellular matrix. Furthermore, utilizing a replication-deficient retrovirus transducing the PymT protein we generated islet endothelioma cells and confirmed that endothelial cells were present in the islets either proliferating or in a quiescent state.
The initiation of neovascularization is triggered by local hypoxia and requires angiogenic factors released from the transplanted islets. The change of the expression of angiogenic factors directs the revascularization process. Hypoxia has multiple effects on the metabolism and function of cells. Islets respond to a decrease in local oxygen supply by a number of strategies to increase oxygen delivery including the elevation of VEGF-A165 expression [53]. Rat islets cultures at 1% oxygen demonstrated an up-regulation of VEGF; a similar increase of VEGF expression was found in rat islet grafts subsequent to transplantation [54]. It is not well defined in what way hypoxia modulates angiogenic properties of islets in the earliest phase after transplantation. We have also demonstrated that human and porcine islets respond with specific expression patterns of VEGF-A165 and bFGF; however, this was not sufficient to prevent a decay of islet endothelial cells in culture [55].
VEGF plays a role in pancreatic organogenesis and normal physiological function [56]. It is a major stimulant of neovascularization by inducing proliferation and migration of endothelial cells and tube formation. VEGF regulates down-stream events including activation of MAPK/Erk and inhibition of SAPK/JK signalling transduction [57].
High expression levels of vascular endothelial growth factors observed in endocrine cells have been recently hypothesized to be responsible for the pronounced vascularization of native islets.
Effect of endothelial cells on islet survival and function
Islet endothelium is involved in development, physiology and the pathogenesis of diseases of the endocrine pancreas. Insulin gene expression in the fetal pancreas is induced by the mutual interaction of endothelium and endoderm [56]. The dorsal pancreatic bud arises during embryogenesis from the portion of the foregut endoderm that contacts the endothelium of the dorsal aorta and two vitelline veins. Recombination of dorsal endoderm with aortic endothelium can induce pancreatic endocrine differentiation, whereas recombination with tissues such as the adjacent notochord or neural tube cannot [58]. Removal of endothelial cell precursors in embryos resulted in failure of endocrine gene expression patterns in pancreatic endoderm. These results suggest that EC contact and signalling is necessary and sufficient for endocrine pancreas development.
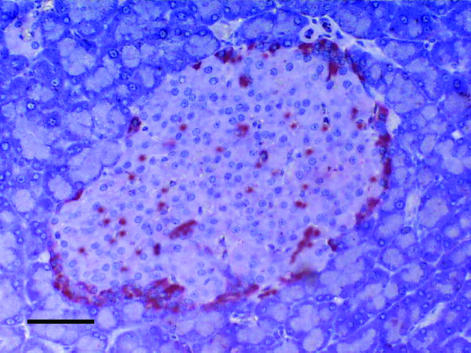
Adult islets are highly and specifically vascularized compared to the surrounding exocrine pancreatic tissue (Fig. 2). The islet microcirculation is characterized by a network of sinusoidal capillaries with a distinctive fenestrated endothelium branching from arterioles entering the islet [59]. Each endocrine cell is in close proximity to an endothelial cell. This facilitates rapid exchange of signals and substances. It is an open issue if the islet endothelium is involved in the regulation of insulin release. Insulin could enter the vascular lumen through fenestrated capillaries or by transcytosis through the endothelial cell [60]. In VEGF-A165 deficient islets 10 times less endothelial fenestrae were observed and blood glucose levels were elevated [61] implying that fenestrated capillary network is required for the full secretory function of islets. Another study suggests that insulin is secreted directly to the interstitial space between the β cells without detour through endothelial cells [62].
Fig. 2.
Pancreatic rat islet stained with CD31 (PECAM) antibody for endothelial cells demonstrating high vascular density compared to surrounding exocrine pancreas tissue (black bar 100 µm). Photo taken by Thomas Linn.
During an immune attack on pancreatic islets, endothelial cells adopt an activated phenotype and are likely to be involved in regulating mononuclear cell accumulation in the islets. Adhesion molecules activate costimulatory pathways in endothelial cells allowing mononuclear cells such as lymphocytes and monocytes to transmigrate and home to pancreatic islets [63]. Therefore studying the characteristics of islet endothelial cells is of major interest in terms of potential anti-inflammatory therapy against autoimmune or allogenic processes within the endocrine pancreas.
Signals from endothelium may also have a role in postnatal islet cell proliferation and neogenesis. We and others have found that intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets [52,64,65]. Induction of endothelial cell proliferation in transplanted islets by VEGF resulted in increased functional β-cell mass in murine and human islets [55,66].
Using a chemical diabetes model experimental evidence was found that bone-marrow derived cells were recruited to the pancreas in response to islet injury. A major proportion of the donor-derived bone marrow cells developed the characteristics of endothelial cells so that the authors concluded they had observed endothelial progenitor cells from the bone marrow differentiate into endothelium [67]. Furthermore, human islets could be protected from instant blood-mediated inflammatory reaction when they were cocultured with primary human aortic endothelial cells. This inflammatory reaction is elicited when islets come into contact with blood after intraportal transplantation. Islets coated with endothelial cells were transplanted and showed less infiltration with CD11b positive immune cells [68].
Therapeutic implications of reducing environmental stress using angiogenesis
Gene therapy with VEGF was demonstrated to increase the success of islet revascularization subsequent to transplantation [50]. This may be due to the distribution of the VEGF receptors within the pancreatic islet and a natural high expression of VEGF of the β-cells. VEGFR2 is highly expressed by the vascular endothelial cells in the islet while the other VEGF-A receptor, VEGFR1, is mainly expressed by the cells of the islet periphery [61]. VEGFR2 is the main signalling receptor for VEGF-A, while VEGFR1 is thought to be an antagonist of VEGF-A signalling. According to the distribution of the VEGF-A receptors, Konstantinova and Lammert [69] proposed that high VEGF-A levels would be locally active within the islet core and attract vessels to the islet centre where they branch into a fine capillary network while the VEGF-R1 expressing islet cell mantle reduces the spreading of these factors into the exocrine tissue.
Therapeutic angiogenesis is an innovative application to induce collateral vessel formation and restore blood flow to tissue with chronic ischaemia via local delivery of pro-angiogenic growth factors in the form of proteins or genes. This approach as opposed to systemic administration of angiogenic factors, is designed to achieve therapeutic effect locally within target tissue without significant elevation of plasma levels of angiogenic factors. Therapeutic angiogenesis via local delivery of plasmids or adenoviral vectors encoding VEGF gene to enhance new vessel formation has been used for treating myocardial or lower limb ischaemia in a number of clinical trials [70,71]. Significant improvements in clinical symptoms were achieved and no severe adverse side-effects associated with local production of VEGF in tissue were reported [55,72–74].
There is evidence that islets preconditioned ex vivo with augmented angiogenic function with either VEGF gene or protein induce revascularization more efficiently following transplantation in diabetic mice. Also there is a principal effect, still there are critical issues, for instance the introduction of angiogenic proteins or genes into islets must not alter their structural and functional integrity; and the expression profile or kinetic release of angiogenic proteins within islet grafts should match the transient nature of islet revascularization to allow rapid onset of angiogenesis and formation of functional microvasculature in islets. It would be ideal to construct a system for controlled release to achieve short-term release of angiogenic molecules locally in transplanted islets.
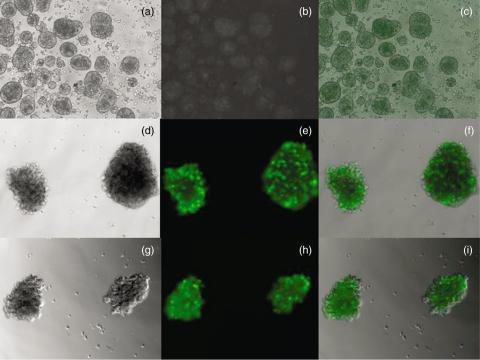
Adenovirus is the most commonly used vector system in preclinical studies due to its high transduction efficiency for both proliferating and quiescent cell types. Figure 3 demonstrates efficient transduction of the VEGF gene by adenovirus in human pancreatic islets. This virus is capable of accommodating large DNA inserts and can be produced in a large quantity and a relatively high titre. The recombinant adenoviral vectors are replication-defective. Adenoviral transduction of islets is carried out ex vivo in culture media and free un-transduced adenovirus will be washed off before islet transplantation. This may hopefully prevent systemic exposure to free adenovirus after islet transplantation and the expression of angiogenic factors will be limited locally within islet grafts. Adenovirus-mediated transgene expression is short-lived which matches the transient nature of islet revascularization. A drawback for adenovirus-mediated gene delivery is its immunogenicity associated with leaky expression of residual adenoviral proteins [75].
Fig. 3.
Microscopic images of human pancreatic islets 3 days after infection with Adenovec-hVEGF-A165 and Adenovec-0 (no VEGF) both expressing green fluorescent protein. (a–c) show controls without Adenovec infection, (d–f) represent islets infected with Adenovec-0 and (g–i) show islets infected with Adenovec-hVEGF-A165. Magnification is ×10 (a–c) and ×20 (d–i), respectively. Infection experiments and photos from Ingrid Hauck-Schmalenberger.
Conclusions and future research
Over recent years significant knowledge has been accumulated on the detrimental effects of ischaemia to pancreatic islets. Optimal blood flow protects pancreatic islets from both inflammatory and nutrient stress. Vascular growth factors were demonstrated to successfully induce a functional vascular network in islet grafts. However, in spite of the development of attenuated viruses the delivery of genes by viral carriers still is an ethical issue for human islet transplantation. Future research may focus on endothelial cells attached to islets in vitro prior to transplantation. Endothelial cells may also be beneficial when provided to islets under inflammatory attack in the native pancreatic environment.
Acknowledgments
Supported by Juvenile Diabetes Research Foundation, technical work of Doris Erb, Gundula Hertl is appreciated.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki E, Abiru N, Eguchi K. Prevention of type 1 diabetes: from the viewpoint of beta cell damage. Diabetes Res Clin Prac. 2004;66(Suppl. 1):S27–32. doi: 10.1016/j.diabres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Eizirik DL, Sandler S, Welsh N, et al. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994;93:1968–74. doi: 10.1172/JCI117188. and May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo C. Roles of poly (ADP-ribose) polymerase activation in the pathogenesis of diabetes mellitus and its complications. Pharmacol Res. 2005;52:60–71. doi: 10.1016/j.phrs.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson PO, Sandler S, Jansson L. Pancreatic islet blood perfusion in the nonobese diabetic mouse: diabetes-prone female mice exhibit a higher blood flow compared with male mice in the prediabetic phase. Endocrinology. 1998;139:3534–41. doi: 10.1210/endo.139.8.6153. [DOI] [PubMed] [Google Scholar]
- 6.Kilcullen JK, Ly QP, Chang TH, et al. Nonviable Staphylococcus aureus and its peptidoglycan stimulate macrophage recruitment, angiogenesis, fibroplasia, and collagen accumulation in wounded rats. Wound Repair Regen. 1998;6:149–56. doi: 10.1046/j.1524-475x.1998.60209.x. [DOI] [PubMed] [Google Scholar]
- 7.Esposito I, Menicagli M, Funel N, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630–6. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Donnelly E, Kobayashi H, et al. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 2005;52:1585–94. doi: 10.1002/art.21016. [DOI] [PubMed] [Google Scholar]
- 9.Menger MD, Yamauchi JI, Vollmar B. Revascularization and microcirculation of freshly grafted islets of Langerhans. World J Surg. 2001;25:509–15. doi: 10.1007/s002680020345. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson PO, Palm F, Andersson A, Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation. 2000;69:761–6. doi: 10.1097/00007890-200003150-00015. [DOI] [PubMed] [Google Scholar]
- 11.Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA. 1996;93:9493–8. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson L. Dissociation between pancreatic islet blood flow and insulin release in the rat. Acta Physiol Scand. 1985;124:223–8. doi: 10.1111/j.1748-1716.1985.tb07655.x. [DOI] [PubMed] [Google Scholar]
- 13.Jahr H, Hussmann B, Eckhardt T, Bretzel RG. Successful single donor islet allotransplantation in the streptozotocin diabetes rat model. Cell Transplant. 2002;11:513–8. [PubMed] [Google Scholar]
- 14.Giuliani M, Moritz W, Bodmer E, et al. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14:67–76. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 15.Johansson U, Olsson A, Gabrielsson S, et al. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun. 2003;308:474–9. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 16.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–62. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 17.Sonna LA, Gaffin SL, Pratt RE, et al. Effect of acute heat shock on gene expression by human peripheral blood mononuclear cells. J Appl Physiol. 2002;92:2208–20. doi: 10.1152/japplphysiol.01002.2001. [DOI] [PubMed] [Google Scholar]
- 18.Scarim AL, Heitmeier MR, Corbett JA. Heat shock inhibits cytokine-induced nitric oxide synthase expression by rat and human islets. Endocrinology. 1998;139:5050–7. doi: 10.1210/endo.139.12.6366. [DOI] [PubMed] [Google Scholar]
- 19.Wachlin G, Heine L, Kloting I, et al. Stress response of pancreatic islets from diabetes prone BB rats of different age. Autoimmunity. 2002;35:389–95. doi: 10.1080/0891693021000014989. [DOI] [PubMed] [Google Scholar]
- 20.Burkart V, Liu H, Bellmann K, Wissing D, et al. Natural resistance of human b cells toward nitric oxide Is mediated by heat shock protein 70. J Biol Chem. 2000;275:19521–8. doi: 10.1074/jbc.M002265200. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro MM, Klein D, Pileggi A, et al. Heme oxygenase-1 fused to a TAT peptide transduces and protects pancreatic b-cells. Biochem Biophys Res Commun. 2003;305:876–81. doi: 10.1016/s0006-291x(03)00856-8. [DOI] [PubMed] [Google Scholar]
- 22.Brunelle JK, Chandel NS. Oxygen deprivation induced cell death: an update. Apoptosis. 2002;7:475–82. doi: 10.1023/a:1020668923852. [DOI] [PubMed] [Google Scholar]
- 23.Federici M, Hribal M, Perego L, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki TY, Tanioka Y, et al. Pancreas preservation by the 2-layer cold storage method before islet isolation protects isolated islets against apoptosis through the mitochondrial pathway. Surgery. 2003;134:437–45. doi: 10.1067/s0039-6060(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 25.Tamarit-Rodriguez J, Idahl LA, Gine E, Alcazar O, Sehlin J. Lactate production in pancreatic islets. Diabetes. 1998;47:1219–23. doi: 10.2337/diab.47.8.1219. [DOI] [PubMed] [Google Scholar]
- 26.Malaisse WJ, Rasschaert J, Zahner D, Sener A. Hexose metabolism in pancreatic islets: the Pasteur effect. Diabetes Res. 1988;7:53–8. [PubMed] [Google Scholar]
- 27.Lacy PE, Davie JM, Finke EH. Prolongation of islet allograft survival following in vitro culture (24 degrees C) and a single injection of ALS. Science. 1979;204:312–3. doi: 10.1126/science.107588. [DOI] [PubMed] [Google Scholar]
- 28.Tanioka Y, Hering BJ, Sutherland BE, et al. Effect of pancreatic warm ischemia on islet yield and viability in dogs. Transplantation. 1997;64:1637–4. doi: 10.1097/00007890-199712270-00001. [DOI] [PubMed] [Google Scholar]
- 29.Tanioka Y, Tanaka T, Gotoh T, et al. Augmentation of tissue ATP level during digestion using preoxygenated perfluorocarbon results in high islet yield-new strategy for islet isolation. Transplant Proc. 2005;37:220–2. doi: 10.1016/j.transproceed.2004.12.232. [DOI] [PubMed] [Google Scholar]
- 30.Contreras JL, Eckstein C, Smyth CA, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52:2935–42. doi: 10.2337/diabetes.52.12.2935. [DOI] [PubMed] [Google Scholar]
- 31.Obermaier R, von Dobschuetz E, Keck T, et al. Brain death impairs pancreatic microcirculation. Am J Transplant. 2004;4:210–5. doi: 10.1046/j.1600-6143.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 32.Toyama H, Takada M, Suzuki Y, Kuroda Y. Activation of macrophage-associated molecules after brain death in islets. Cell Transplant. 2003;12:27–32. doi: 10.3727/000000003783985205. [DOI] [PubMed] [Google Scholar]
- 33.Jahr H, Pfeiffer G, Hering BJ, Federlin K, Bretzel RG. Endotoxin-mediated activation of cytokine production in human PBMCs by collagenase and Ficoll. J Mol Med. 1999;77:118–20. doi: 10.1007/s001090050316. [DOI] [PubMed] [Google Scholar]
- 34.Vargas F, Vives-Pi M, Somoza N, et al. Endotoxin contamination may be responsible for the unexplained failure of humanpancreatic islet transplantation. Transplantation. 1998;65:722–7. doi: 10.1097/00007890-199803150-00020. [DOI] [PubMed] [Google Scholar]
- 35.Eizirik DL, Welsh M, Strandell E, Welsh N, Sandler S. Interleukin-1 beta depletes insulin messenger ribonucleic acid and increases the heat shock protein hsp70 in mouse pancreatic islets without impairing the glucose metabolism. Endocrinology. 1990;127:2290–7. doi: 10.1210/endo-127-5-2290. [DOI] [PubMed] [Google Scholar]
- 36.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islets as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–45. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 37.Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–68. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 38.Davalli AM, Scaglia L, Zangen DH, et al. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–7. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 39.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic b-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 40.Street CN, Lakey JR, Shapiro AM, et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107–14. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 41.Lucas-Clerc C, Massart C, Campion JP, et al. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9–20. doi: 10.1016/0303-7207(93)90046-m. [DOI] [PubMed] [Google Scholar]
- 42.Ris F, Hammar E, Bosco D, et al. Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human b-cells in vitro. Diabetologia. 2002;45:841–50. doi: 10.1007/s00125-002-0840-7. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 44.Orci L, Unger RH. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet. 1975;2:1243–4. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- 45.Samols E, Stagner JI, Ewart RB, Marks V. The order of islet microvascular cellular perfusion is B-A-D in the perfused rat pancreas. J Clin Invest. 1998;82:350–3. doi: 10.1172/JCI113593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic isletsirrespective of the implantation site. Diabetes. 2001;50:489–95. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 47.Ryan EA, Lakey JR, Paty B, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 48.Omer A, Duvivier-Kali VF, Aschenbach W, et al. Exercise induces hypoglycemia in rats with islet transplantation. Diabetes. 2004;53:360–5. doi: 10.2337/diabetes.53.2.360. [DOI] [PubMed] [Google Scholar]
- 49.Mattsson G, Jansson L, Nordin A, et al. Evidence of functional impairment of syngenically transplanted nouse pancreatic islets retrieved from the liver. Diabetes. 2004;53:948–54. doi: 10.2337/diabetes.53.4.948. [DOI] [PubMed] [Google Scholar]
- 50.Zhang N, Richter A, Suriawinata J, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963–70. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 51.Suschek C, Fehsel K, Kroncke KD, et al. Primary cultures of rat islet capillary endothelial cells. Constitutive and cytokine-inducible macrophagelike nitric oxide synthases are expressed and activities regulated by glucose concentration. Am J Pathol. 1994;145:685–95. [PMC free article] [PubMed] [Google Scholar]
- 52.Linn T, Schneider K, Hammes HP, et al. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003;17:881–3. doi: 10.1096/fj.02-0615fje. [DOI] [PubMed] [Google Scholar]
- 53.Vasir B, Aiello LP, Yoon KH, et al. Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes. 1998;47:1894–903. doi: 10.2337/diabetes.47.12.1894. [DOI] [PubMed] [Google Scholar]
- 54.Vasir B, Jonas C, Steil GM, et al. Gene expression of VEGF and its receptors Flk-1/KDR and Flt-1 in cultured and transplanted rat islets. Transplantation. 2001;71:924–35. doi: 10.1097/00007890-200104150-00018. [DOI] [PubMed] [Google Scholar]
- 55.Lai Y, Schneider D, Kidszun A, et al. Vascular endothelial growth factor increases functional b-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation. 2005;79:1530–6. doi: 10.1097/01.tp.0000163506.40189.65. [DOI] [PubMed] [Google Scholar]
- 56.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–7. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 57.Gupta K, Kshirsagar S, Li W, et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signalling. Exp Cell Res. 1999;247:495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 58.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–17. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 59.Stagner JI, Samols E. The induction of capillary bed development by endothelial growth factor before islet transplantation may prevent islet ischemia. Transplant Proc. 1990;22:824–8. [PubMed] [Google Scholar]
- 60.Predescu D, Predescu S, McQuistan T, Palade GE. Transcytosis of a-1-acidic glycoprotein in the continuous microvascular endothelium. Proc Natl Acad Sci USA. 1998;95:6175–80. doi: 10.1073/pnas.95.11.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lammert E, Gu G, McLaughlin M, et al. Role of VEGF-A in vascularization of pancreatic islets. Current Biol. 2003;13:1070–4. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi N, Kishimoto T, Nemoto T, et al. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–52. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- 63.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med. 2003;197:643–56. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brissova M, Fowler M, Wiebe P, et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318–25. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 65.Nyqvist D, Köhler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287–93. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
- 66.Zhang N, Richter A, Suriawinata J, et al. Elevated vascular endothelial growth factor production in islet graft revascularization. Diabetes. 2004;53:963–70. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 67.Mathews V, Hanson PT, Ford E, et al. Recruitment of bone marrow-derived endothelial cells to sites of pancreatic b-cell injury. Diabetes. 2004;53:91–8. doi: 10.2337/diabetes.53.1.91. [DOI] [PubMed] [Google Scholar]
- 68.Johansson U, Elgue G, Nilsson B, Korsgren O. Composite islet-endothelial cell grafts: a novel approach to counteract innate immunity in islet transplantation. Am J Transplant. 2005;5:2632–9. doi: 10.1111/j.1600-6143.2005.01076.x. [DOI] [PubMed] [Google Scholar]
- 69.Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. Bioessays. 2004;26:1069–75. doi: 10.1002/bies.20105. [DOI] [PubMed] [Google Scholar]
- 70.Isner JM. Myocardial gene therapy. Nature. 2002;415:234–9. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 71.Khan TA, Sellke FW, Laham RJ. Gene therapy progress and prospects. therapeutic angiogenesis for limb and myocardial ischemia. Gene Ther. 2003;10:285–91. doi: 10.1038/sj.gt.3301969. [DOI] [PubMed] [Google Scholar]
- 72.Cheng K, Fraga D, Zhang C, et al. Adenovirus based vascular endothelial growth factor gene delivery to human pancreatic islets. Gene Ther. 2004;11:1105–16. doi: 10.1038/sj.gt.3302267. [DOI] [PubMed] [Google Scholar]
- 73.Narang AS, Cheng K, Henry J, et al. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21:15–25. doi: 10.1023/b:pham.0000012147.52900.b8. [DOI] [PubMed] [Google Scholar]
- 74.Linn T, Erb D, Schneider D, et al. Polymers for induction of revascularization in the rat fascial flap: application of vascular endothelial growth factor and pancreatic islet cells. Cell Transplant. 2003;12:769–78. doi: 10.3727/000000003108747244. [DOI] [PubMed] [Google Scholar]
- 75.Zhang N, Schroppel B, Chen D, et al. Adenovirus transduction induces expression of multiple chemokines and chemokine receptors in murine b cells and pancreatic islets. Am J Transplant. 2003;3:1230–41. doi: 10.1046/j.1600-6143.2003.00215.x. [DOI] [PubMed] [Google Scholar]