Abstract
There is now considerable clinical evidence that oats do not activate coeliac disease. Nonetheless, a reluctance to include oats in the gluten-free diet remains. Because gluten-induced damage is accompanied by activation of the gastrointestinal immune system, the purpose of this study was to investigate if similar changes were induced by oats ingestion. Small intestinal histological sections from 10 patients who ingested 50 g of oats daily for 3 months were investigated for possible evidence of immune activation. Tissue obtained before and after oats challenge was stained with a series of antibodies directed against the following molecules: human leucocyte antigen D-related (HLA-DR), Ki-67, CD25, CD54 [intercellular adhesion molecule 1 (ICAM-1)] and mast cell tryptase. None of the patients developed clinical or laboratory evidence of adverse effects. The distribution of intestinal HLA-DR expression was not affected by oats ingestion and the crypt epithelium remained unstained. In the pre-oats biopsies, the percentage of Ki-67 positive enterocytes, 29·5 ± 6·9 [95% confidence interval (CI) 13·9–45·0] did not differ significantly from that found in postoats biopsies, 41·2 ± 3·7 (95% CI, 32·8–49·6), P = 0·19, not significant. Furthermore, oats ingestion did not alter the number of CD25 positive and tryptase positive cells. Finally, the distribution and intensity of ICAM-1 staining was unchanged by dietary oats. In summary, detailed immunohistological studies of biopsies from patients ingesting oats for 3 months did not reveal evidence of immune activation. Together with other reported findings, this study strengthens the view that oats can be included safely in the diet of gluten sensitive patients.
Keywords: coeliac disease, immune activation, immunohistochemistry, oats toxicity
Introduction
Coeliac disease is a gluten-sensitive disorder characterized by malabsorption and a typical small intestinal histological lesion [1,2]. Treatment with a strict gluten-free diet results in complete clinical and histological recovery [3]. Recent clinical studies indicate that oats may be included safely in the gluten-free diet [4–7]. Nonetheless, concerns about oats purity and oats immunogenicity have been expressed [8,9], and few countries have endorsed the inclusion of oats in the standard gluten-free diet [10,11].
In our initial study of 10 patients, no clinical, serological or histological evidence of disease activation was observed after 3 months of oats ingestion [5]. However, because of continuing concerns about the safety of including oats in the gluten-free diet, detailed immunohistological evaluations of pre- and post-oats small intestinal tissue sections were performed and are described here. Because it is known that gluten-induced damage is accompanied by activation of the gastrointestinal immune system, the purpose of this study was to investigate if similar changes were induced by oats ingestion. The expression of a range of immunological molecules, considered to reflect immune activation in the coeliac lesion, was examined. These molecules included: the major histocompatibility complex (MHC) class II molecule, human leucocyte antigen D-related (HLA-DR) [12–16]; the alpha chain of the interleukin (IL)-2 receptor, CD25 [17–19]; the intercellular adhesion molecule 1 (ICAM-1) [19–21]; the nuclear proliferating antigen Ki-67 [17,22,23]; and the mast cell enzyme tryptase [24]. Thus, the goal of the study was to determine whether subtle evidence of disease activation developed following oats ingestion and hence provide information on the possibility of oats toxicity.
Materials and methods
Subjects
Details of this study have been reported previously [5]. In brief, 10 adult coeliac patients in disease remission took 50 g of oats daily for a 12-week period while maintaining a strict gluten-free diet. Clinical and immunological markers (including serology) of coeliac disease activation were measured at regular intervals. All patients had small intestinal biopsies taken at the commencement of the study and again at its completion. Informed consent was obtained from each patient and the study was approved by the Hospital Ethics Committee.
Oats challenge
The oats cereal (Peter Kölln, Elmshorn, Germany) used in the study was tested for evidence of gluten contamination using various methodologies and was demonstrated to be entirely gluten-free. The methodologies included reverse-phase high performance liquid chromatography [25], enzyme-linked immunoassay [26] and polymerase chain reaction [27]. Fifty g of oats was taken daily as porridge for a 12-week period. Patient compliance was recorded daily using diaries and all adhered to the study protocol. Patients were monitored closely and assessed clinically at 0, 1, 4 and 12 weeks. Coeliac serological tests (IgG anti-gliadin and IgA anti-endomysial antibodies) were also measured at these time-points. Duodenal biopsies were obtained endoscopically at the beginning of oats challenge and after 12 weeks.
Gluten challenge
After the 12-week oats challenge, four of the patients were given a 6-week gluten challenge. In order to establish the sensitivity of patients to low quantities of gluten, two patients agreed to a low-dose gluten challenge and were given 500 mg of gluten (Modern Bakery, Dublin, Ireland) daily. Two further patients were given a standard challenge, consisting of 10 g of gluten daily. All patients were monitored weekly for the development of symptoms and further duodenal biopsies were obtained upon completion of gluten challenge.
Histological evaluation
Standard microscopic examination was performed for evidence of morphological damage. The biopsies were then coded to blind the observers and quantitative histological analysis was carried out. Intraepithelial lymphocyte counts, expressed as a percentage of enterocyte numbers, were performed by two independent observers. Enterocyte height (in µm) from the middle one-third of five villi was measured by computerized image analysis (Quantimet 570 analyser, Leica Cambridge Instruments, Milton Keynes, UK).
Immunohistochemistry studies
Tissue sections were stained with a series of immunohistochemical reagents (Table 1), which included antibodies to HLA-DR, ICAM-1 (CD54), Ki-67, CD25 and mast cell tryptase. In all cases, slides were coded and examined by two independent observers. Details of the techniques employed are given in Table 1. Bound antibody was detected with biotinylated rabbit anti-mouse antibody. As detailed below, various methods were used to quantify the level of staining, according to the antibody employed.
Table 1.
Antibodies used in immunohistology studies.
| Antibody | Molecule | Source | Dilution | Incubation | Tissue | Antigen retrieval |
|---|---|---|---|---|---|---|
| CD25 | CD25 | Dako | 1 : 50 | 1 h | Frozen | Nil |
| ICAM-1 | CD54 | Becton Dickinson | 1 : 90 | 1 h | Frozen | Nil |
| HLA-DR | MHC II | Dako | 1 : 30 | Overnight, 4°C | Paraffin | Microwave |
| Anti-tryptase | Mast cell tryptase | Dako | 1 : 50 | Overnight, 4°C | Paraffin | Pronase digestion |
| Ki-67 | Ki-67 nuclear antigen | Dako | 1 : 80 | Overnight, 4°C | Paraffin | Microwave |
Previous studies have shown that in normal duodenal tissue, HLA-DR staining is observed on the upper half of villi, whereas in active coeliac disease staining of the entire villus and crypt enterocytes is found [11–13]. A three-point semiquantitative scale of staining was established: 1+ to indicate weak staining of tips of villi; 2+ to indicate moderate staining of the upper half of villi; and 3+ to indicate staining of entire villi and also the crypt enterocytes.
In the pre- and post-oats challenge biopsies, the absolute number of CD25 positive cells per mm2 was evaluated in eight of the 10 subjects. Employing a graticule, the entire tissue section was examined. The majority of stained cells had a lymphocyte morphology, but in cell counts no attempt was made to differentiate between lymphocytes and macrophages. Samples from two patients given a gluten challenge were also examined.
Histological sections from eight of the 10 subjects were examined for ICAM-1 staining. The intensity and distribution of tissue staining was evaluated on a composite basis, with scoring of blood vessel, individual cell and lamina propria connective tissue reactivity. Scores ranging from 0 to 3 were given to each item: 0 indicating zero, 1 mild, 2 moderate and 3 marked staining.
Ki-67 staining of crypt enterocytes was evaluated in the 10 patients and the percentage of positive cells was estimated following counting of 500 enterocytes per biopsy sample. Mast cell tryptase staining of a limited number of oats challenged patients was also performed − in five pre-oats and in the matching post-oats samples. The total number of positively stained cells located in the lamina propria and the crypts in five high-power fields were counted.
Statistical analysis
For the mean difference in antibody staining pre- and post-challenge, 95% confidence intervals (CI) were constructed. Analysis of results for paired non-parametric data was performed using Wilcoxon's signed-rank test. A P-value > 0·05 was considered to be not significant (n.s.). Calculation of statistical values was performed using Instat software.
Results
Oats challenge: clinical and histological events
The clinical and histological events following oats challenge have been reported previously [5]. Briefly, none of the 10 coeliac patients challenged with a 50 g daily oats supplement for 12 weeks experienced any adverse symptoms. No change in IgG anti-gliadin antibody status occurred, the antibodies being undetectable in nine of the 10 patients. IgA anti-endomysial antibodies were consistently negative in all patients during the study. Standard histological evaluation of post-oats challenge duodenal biopsies showed no evidence of morphological damage. No significant change in intraepithelial lymphocyte counts or enterocyte height was observed [5].
Gluten challenge: clinical and histological events
Four patients were given a 6-week gluten challenge after completing the oats study. All developed histological manifestations of active coeliac disease − two with villous atrophy (including one patient given 500 mg of gluten daily) and in the remaining patients an increase in intraepithelial lymphocytes was observed. Three patients became anti-endomysial antibody positive: the fourth patient became ill with vomiting, diarrhoea and fatigue on 500 mg of daily gluten, and the challenge was stopped after 4 weeks.
Immunohistochemistry studies
HLA-DR
Using the semiquantitative scale for estimating HLA-DR staining, no increase in staining was observed after oats ingestion for 3 months: a median score of 1·6 ± 0·1 (95% CI 1·3–1·9) was observed pre-oats challenge and 1·8 ± 0·1 post-challenge (95% CI 1·6–2·0), P = 0·296, n.s. In no post-challenge patient was crypt enterocyte staining observed. In contrast, HLA-DR staining of villi and crypts was evident in all four patients after gluten challenge (each with a score of 3) and this concurred with other evidence of disease activation in these patients (Fig. 1).
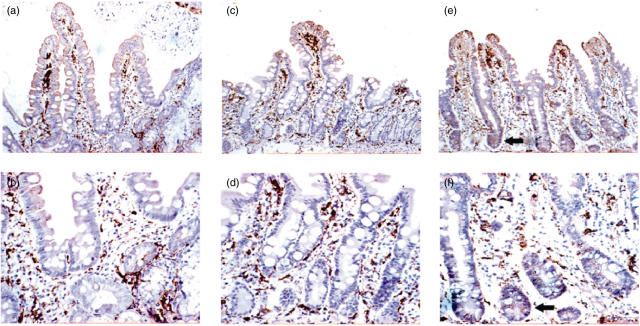
Fig. 1.
Human leucocyte antigen D-related (HLA-DR) staining of duodenal histological sections before oats challenge (a, b), after oats challenge (c, d) and after gluten challenge (e, f). The magnification of (a), (c) and (e) is ×20, with the corresponding sections shown at ×40 in (b), (d) and (f). In the pre- and post-oats sections, staining of lamina propria cells and villous enterocytes is observed. In the post-gluten sections, in addition to the above structures, staining of crypt enterocytes is observed (arrows).
CD25
The number of CD25 positive cells per mm2 was evaluated in eight of the 10 subjects, pre- and post-oats challenge. The entire tissue section was examined and only a small number of positively stained cells were observed. No attempt was made to differentiate between lymphocyte and macrophage-stained cells. Oats challenge was not associated with an increase in CD25 positive cells: a mean of 6·9 ± 1·9 cells (95% CI 2·5–11·3) per mm2 pre-oats were enumerated, compared with 4·5 ± 0·8 cells (95% CI 2·6–6·4) per mm2 post-oats, P = 0·25, n.s. Of the four subjects who were subsequently given a gluten challenge, biopsy samples were suitable for evaluation in two patients and in both an increase in CD25 positive cells was found, with 16 and 17 positive cells per mm2 observed.
ICAM-1
Histological sections from eight of the 10 subjects were examined. A mean score of 5·6 ± 0·8 (95% CI 3·7–7·6) was accorded to the pre-oats tissue sections and this did not differ from that observed in the post-oats samples with a mean of 4·8 ± 0·8 (95% CI 2·9–6·6), P = 0·062, n.s.
Ki-67
The percentage of Ki-67 positive cells was evaluated in the 10 subjects, pre- and post-oats challenge. The percentage of positive cells, 29·5 ± 6·9 (95% CI 13·9–45·0) in the pre-oats sections did not differ significantly from that found in post-oats sections, 41·2 ± 3·7 (95% CI 32·8–49·6), P = 0·19, n.s. In the four gluten-challenged subjects, an increased percentage of Ki-67 positive cells was found with mean percentage values of 54, 53, 51 and 73 observed (Fig. 2).
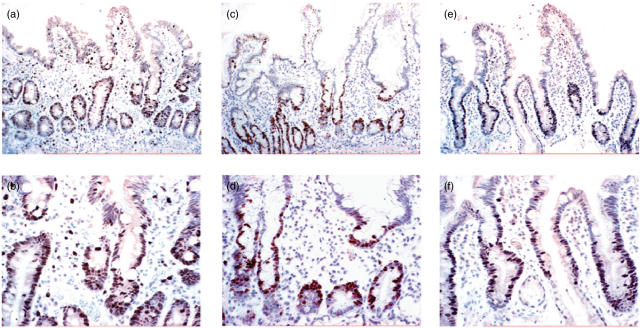
Fig. 2.
Ki-67 staining of duodenal histological sections before oats challenge (a,b), after oats challenge (c,d) and after gluten challenge (e,f). The magnification of (a), (c) and (e) is ×20 with the corresponding sections shown at ×40 in (b), (d) and (f). Positive Ki-67 staining of crypt enterocytes is shown in all sections.
Tryptase
Tryptase staining was evaluated in five of the 10 subjects, in whom paired pre- and post-oats challenge samples were available. No change resulted following oats challenge with a mean score of 31 ± 5·7 pre-oats (95% CI 15·2–46·8) and 30·2 ± 34·8 (95% CI 16·9–43·5) post-oats observed, P = 1·0, n.s. This contrasted with a mean score of 47 ± 6·6 in the four gluten-challenged individuals.
Discussion
Oats cereal, a nutritious food source, is traditionally excluded from the diet of patients with coeliac disease [3]. In recent years, several clinical studies have investigated the inclusion of oats in the diet of coeliac patients and, with the exception of one recent report [9] (discussed below) no evidence of toxicity has been adduced [4–7]. The studies included patients with adult [4,5,7] and paediatric coeliac disease [6] and with dermatitis herpetiformis [28,29]. Despite these findings, there has been a reluctance to recommend the inclusion of oats in the standard gluten-free diet [10,11]. To examine further these issues in this study, using a range of immunohistochemical markers we investigated the duodenal biopsy tissue of coeliac patients after oats ingestion, looking for possible evidence of immune activation. Oats ingestion in these patients was not associated with increased expression in any of these activation molecules.
In our original study [5] we investigated the effect of 50 g of oats taken daily for 12 weeks and found no clinical or laboratory evidence of disease activation. This study included careful microscopic evaluation of duodenal tissue before and after the challenge period. No increase in intraepithelial lymphocyte numbers was found and, employing computer-aided image analysis, no decrease in enterocyte height was observed. Furthermore, no change in gliadin or endomysial antibody status was observed, in agreement with two further studies that investigated antibody status [29,30]. An increase in antibody levels would be expected if patients were exposed to disease activating cereal prolamin [31].
Increased MHC class II molecule expression by small intestinal enterocytes has been reported repeatedly in untreated or active coeliac disease [12–14]. Indeed, up-regulation of this molecule has been employed in both in vivo [15] and in vitro [16] studies to determine evidence of disease activation. In one in vitro study, this event was reported to develop within 2 h of gliadin exposure [19]. It is likely that the increased production of interferon gamma, which is associated with active coeliac disease, is responsible for this effect, and other studies have reported the rapid appearance of interferon gamma mRNA in in vitro experiments [32,33]. In coeliac disease, the increased intensity of MHC class II staining and the presence of this molecule on crypt enterocytes is very characteristic of active disease [12–14]. These features were absent in all the biopsies examined here, both before and after oats ingestion. This finding is in keeping with a study of patients with dermatitis herpetiformis, in whom oats ingestion similarly failed to cause up-regulation of HLA-DR [29]. In contrast, in the current study, when four of the patients were later given a gluten challenge, increased MHC class II staining developed.
Increased numbers of CD25 positive T cells are also a known feature of active coeliac mucosa [17] and develop after 24 h of gluten exposure in organ culture experiments [18,19]. Indeed, advantage of this effect of gluten has been employed in the production of gluten-reactive T cell lines and clones [34]. However, 3 months of oats ingestion had no effect on the numbers of CD25 positive cells in the present study and only small numbers of cells were found to express this activation marker, in keeping with the patients having well-treated coeliac disease [17]. In two of the four patients subsequently given a gluten challenge, frozen tissue sections were available for staining and increased numbers of CD25 positive cells were found.
A further molecule employed frequently to investigate immune activation in coeliac tissue is ICAM-1 [20,21]. Expression of this molecule may be observed on endothelial cells and also cells of the immune system, including T cells and macrophages [21] but with absent staining of the epithelial layer, as was confirmed in this study. The level of expression is diffusely increased in active coeliac tissue [20,21] and this has been reported to develop within 2 h of gliadin exposure in organ culture experiments [19]. In this study, a composite scoring system (based on blood vessel, individual cell and connective tissue staining) was employed and, although some interindividual variation in the level of expression was noted, 3 months of oats ingestion had no effect on the extent of expression.
Two additional markers of disease activation were investigated: staining for Ki-67 and tryptase. Ki-67 is a nuclear proliferation antigen and increased numbers of positively stained enterocytes have been described after in vitro gluten challenge, in keeping with increased crypt cell division observed in the coeliac lesion [22]. In a further study, the number of Ki-67 positive cells was found to correlate with the number of apoptotic enterocytes [23]. In addition, increased numbers of Ki-67-stained intraepithelial lymphocytes have been reported in untreated coeliac disease [17]. In this study, although a small increase in Ki-67 cells was noted after oats challenge (median cell number pre-oats 29·5 ± 6·9 versus 41·2 ± 3·7 post-oats), the difference was not significant (P= 0·19). Mast cell numbers identified by tryptase staining were also investigated, as in one study the number of tryptase-stained cells was deemed to be the best indicator of disease activity [24]. Again, oats challenge did not affect the number of tryptase-stained cells in the tissues investigated.
In studies reported elsewhere, histological sections from these patients were investigated for lactase expression [35] and for changes in blood vessel morphology: the findings in both studies favoured the safety of oats in the diet. Lactase expression, detected by an anti-lactase monoclonal antibody, was unaffected by oats ingestion [35]. Similarly, blood vessel size was unaltered by the oats challenge (Srinivasan et al. submitted).
Taken together, the investigations performed on the tissue of these patients after oats challenge support the viewpoint that oats has neither immunogenic nor toxic effects on coeliac mucosa. This finding is in keeping with the many clinical reports that oats is well tolerated by coeliac patients. Nonetheless, it should be noted that one recent study reported evidence of oats activation of coeliac disease in three of nine patients [9,37]. Moreover, avenin stimulation of gliadin-reactive T cell lines, generated from coeliac mucosa, has also been reported [36–38]. Hence, the safety of oats ingestion by coeliac subjects should continue to be reviewed, as occasional patients may react to this dietary cereal.
Acknowledgments
Thanks to J. Kelly, U. O’Shea, S. Wilson and J. Jackson for advice and technical assistance with the manuscript.
References
- 1.Maki M, Collin P. Celiac disease. Lancet. 1997;349:1755–9. doi: 10.1016/S0140-6736(96)70237-4. [DOI] [PubMed] [Google Scholar]
- 2.Feighery C. Celiac disease fortnightly review. BMJ. 1999;319:236–9. doi: 10.1136/bmj.319.7204.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke WT, Holmes GKT. Celiac disease. Edinburgh: Churchill Livingstone; 1984. [Google Scholar]
- 4.Janatuinen EK, Pikkarainen PH, Kemppainen TA, et al. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med. 1995;333:1033–7. doi: 10.1056/NEJM199510193331602. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan U, Leonard N, Jones E, et al. Absence of oats toxicity in adult celiac disease. BMJ. 1996;313:1300–1. doi: 10.1136/bmj.313.7068.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffenberg EJ, Haas J, Drescher A, et al. A trial of oats in children with newly diagnosed celiac disease. J Pediatr. 2000;137:361–3. doi: 10.1067/mpd.2000.109003. [DOI] [PubMed] [Google Scholar]
- 7.Janatuinen EK, Kemppainen TA, Julkunen RJ, et al. No harm from five year ingestion of oats in celiac disease. Gut. 2002;50:332–5. doi: 10.1136/gut.50.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troncone R, Mazzarella G, Leone A, et al. Oats prolamines are immunogenic for the celiac intestinal mucosa. In: Stern M, editor. Proceedings of the 12th Meeting Group on Prolamin Analysis and Toxicity. Tubingen. 1998. pp. 69–74. [Google Scholar]
- 9.Lundin KEA, Nilsen EM, Scott HG, et al. Oats induced villous atrophy in celiac disease. Gut. 2003;52:1649–52. doi: 10.1136/gut.52.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovik A, Fluge G, Dybdahl JH, et al. Dietary treatment of celiac disease. Tidsskr Nor Laegeforen. 1999;119:1888–91. [PubMed] [Google Scholar]
- 11.Thompson T. Oats and the gluten-free diet. J Am Diet Assoc. 2003;103:376–9. doi: 10.1053/jada.2003.50044. [DOI] [PubMed] [Google Scholar]
- 12.Arnaud-Battandier F, Cerf-Bensussan N, Amsellem R, Schmitz J. Increased HLA-DR expression by enterocytes in children with celiac disease. Gastroenterology. 1986;91:1206–12. doi: 10.1016/s0016-5085(86)80018-x. [DOI] [PubMed] [Google Scholar]
- 13.Scott H, Sollid LM, Fausa O, Brandtzaeg P, Thorsby E. Expression of MHC class II subregion products by jejunal epithelium in patients with celiac disease. Scand J Immunol. 1987;26:563–71. doi: 10.1111/j.1365-3083.1987.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 14.Kelly J, O'Farrelly C, O'Mahony C, Weir DG, Feighery C. Immunoperoxidase demonstration of the cellular composition of the normal and celiac small bowel. Clin Exp Immunol. 1987;68:177–88. [PMC free article] [PubMed] [Google Scholar]
- 15.Marley NJE, Macartney JC, Ciclitira PJ. HLA-DR, DP and DQ expression in the small intestine of patients with celiac disease. Clin Exp Immunol. 1987;70:386–93. [PMC free article] [PubMed] [Google Scholar]
- 16.Fais S, Maiuri L, Pallone F, et al. Gliadin-induced changes in the expression of MHC class II antigens by human small intestinal epithelium. Organ culture studies with celiac disease mucosa. Gut. 1992;33:472–5. doi: 10.1136/gut.33.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstensen TS, Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki-67) of alpha/beta and gamma/delta cells in the epithelium. Eur J Immunol. 1993;23:505–10. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- 18.Halstensen TS, Scott H, Fausa O, Brandtzaeg P. Gluten stimulation of celiac mucosa in vitro induces activation (CD25) of lamina propria CD4+ T cells and macrophages but no crypt-cell hyperplasia. Scand J Immunol. 1993;38:581–90. doi: 10.1111/j.1365-3083.1993.tb03245.x. [DOI] [PubMed] [Google Scholar]
- 19.Maiuri L, Picarelli A, Boirivant M, et al. Definition of the initial immunologic modifications upon in vitro gliadin challenge in the small intestine of celiac patients. Gastroenterology. 1996;110:1368–78. doi: 10.1053/gast.1996.v110.pm8613040. [DOI] [PubMed] [Google Scholar]
- 20.Sturgess RP, Mccartney JC, Makgoba MW, Hung CH, Haskard DO, Ciclitira PJ. Differential upregulation of intercellular adhesion molecule-1 in celiac disease. Clin Exp Immunol. 1990;82:489–92. doi: 10.1111/j.1365-2249.1990.tb05477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smart CJ, Calabrese A, Oakes DJ, Howdle PD, Trejdosiewicz LK. Expression of the LFA-1 beta 2 integrin (CD11a/CD18) and ICAM-1 (CD54) in normal and celiac small bowel mucosa. Scand J Immunol. 1991;34:299–305. doi: 10.1111/j.1365-3083.1991.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 22.Maiuri L, Ciacci C, Raia V, et al. FAS engagement drives apoptosis of enterocytes of celiac patients. Gut. 2001;48:418–24. doi: 10.1136/gut.48.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss SF, Attia L, Scholes JV, Walters JR, Holt PR. Increased small intestinal apoptosis in celiac disease. Gut. 1996;39:811–7. doi: 10.1136/gut.39.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotalova R, Lojda Z, Smidova J, Jodl J. Histochemical and immunohistochemical study of jejunal mucosa cells in children with celiac sprue. Cesk Pediatr. 1993;48:398–403. [PubMed] [Google Scholar]
- 25.Bietz JA. HPLC of cereal proteins. Adv Cereal Sci Technol. 1986;8:105–70. [Google Scholar]
- 26.Skerritt JH, Hill AS. Enzyme immunoassay for determination of gluten in foods: a collaborative study. J Assoc Off Anal Chem. 1991;74:257–64. [PubMed] [Google Scholar]
- 27.Allmann M, Candrian U, Hofelein C, Luthy J. Polymerase chain reaction (PCR): a possible alternative to immunochemical methods assuring safety and quality of food. Detection of wheat contamination in non-wheat food products. Zeit Lebensm Unters Forsch. 1993;196:248–51. doi: 10.1007/BF01202741. [DOI] [PubMed] [Google Scholar]
- 28.Hardman CM, Garioch JJ, Leonard JN, et al. Absence of toxicity of oats in patients with dermatitis herpetiformis. N Engl J Med. 1997;337:1884–7. doi: 10.1056/NEJM199712253372604. [DOI] [PubMed] [Google Scholar]
- 29.Reunala T, Collin P, Holm K, et al. Tolerance to oats in dermatitis herpetiformis. Gut. 1998;43:490–3. doi: 10.1136/gut.43.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janatuinen EK, Kemppainen TA, Pikkarainen PH, et al. Lack of cellular and humoral immunological responses to oats in adults with celiac disease. Gut. 2000;46:327–31. doi: 10.1136/gut.46.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catassi C, Rossini M, Ratsch I-M, et al. Dose dependent effects of protracted ingestion of small amounts of gliadin in celiac disease children: a clinical and jejunal morphometric study. Gut. 1993;34:1515–9. doi: 10.1136/gut.34.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsen EM, Jahnsen FL, Lundin KE, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 33.Kilmartin C, Lynch S, Abuzakouk M, Wieser H, Feighery C. Avenin fails to induce a Th 1 response in celiac tissue following in vitro culture. Gut. 2003;52:47–52. doi: 10.1136/gut.52.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundin KE, Scott H, Hansen T, et al. Gliadin-specific, HLA-DQ (alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993;178:187–96. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan U, Jones E, Weir DG, Feighery C. Lactase enzyme, detected immunohistochemically, is lost in active celiac disease, but unaffected by oats challenge. Am J Gastroenterol. 1999;94:2936–41. doi: 10.1111/j.1572-0241.1999.01441.x. [DOI] [PubMed] [Google Scholar]
- 36.Vader LW, Stepniak DT, Bunnik EM, et al. Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterology. 2003;125:1105–13. doi: 10.1016/s0016-5085(03)01204-6. [DOI] [PubMed] [Google Scholar]
- 37.Arentz-Hansen H, Fleckenstin B, Mulberg O, et al. The molecular basis for oats intolerance in celiac disease patients. PloS Med. 2004. Epub 19 October. [DOI] [PMC free article] [PubMed]
- 38.Kilmartin C, Wieser H, Abuzakouk M, et al. Intestinal T cell responses to cereal proteins in celiac disease. Dig Dis Sci. 2006;51:202–9. doi: 10.1007/s10620-006-3108-0. [DOI] [PubMed] [Google Scholar]