Abstract
Previous studies have implicated acetylases and deacetylases in regulating the transcriptional activity of NF-κB. Here, we show that inhibitors of deacetylases such as trichostatin A (TSA) and sodium butyrate (NaBut) potentiated TNF-induced expression of several natural NF-κB-driven promoters. This transcriptional synergism observed between TNF and TSA (or NaBut) required intact κB sites in all promoters tested and was biologically relevant as demonstrated by RNase protection on two instances of endogenous NF-κB-regulated gene transcription. Importantly, TSA prolonged both TNF-induced DNA-binding activity and the presence of NF-κB in the nucleus. We showed that the p65 subunit of NF-κB was acetylated in vivo. However, this acetylation was weak, suggesting that other mechanisms could be implicated in the potentiated binding and transactivation activities of NF-κB after TNF plus TSA versus TNF treatment. Western blot and immunofluorescence confocal microscopy experiments revealed a delay in the cytoplasmic reappearance of the IκBα inhibitor that correlated temporally with the prolonged intranuclear binding and presence of NF-κB. This delay was due neither to a defect in IκBα mRNA production nor to a nuclear retention of IκBα but was rather due to a persistent proteasome-mediated degradation of IκBα. A prolongation of IκB kinase activity could explain, at least partially, the delayed IκBα cytoplasmic reappearance observed in presence of TNF plus TSA.
NF-κB is a ubiquitously expressed family of transcription factors controlling the expression of numerous genes involved in inflammatory and immune responses and cellular proliferation (reviewed in references 4, 5, 19, and 26). There are five known members of the mammalian NF-κB/Rel family: p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), c-Rel, and RelB. The most abundant form of NF-κB is a heterodimer of p50 and p65. In unstimulated cells, NF-κB is sequestered in the cytoplasm in an inactive form through interaction with the IκB inhibitory proteins (including IκBα, IκBβ, and IκBɛ, of which the best studied is IκBα). Upon stimulation of cells by specific inducers, such as the proinflammatory cytokine tumor necrosis factor SF2 (referred to as TNF hereinafter), IκBα is phosphorylated on two specific serine residues by a large cytoplasmic IκB kinase (IKK) complex that consists of the kinase catalytic subunits IKKα and IKKβ and the regulatory subunit NEMO/IKKγ (reviewed in references 23 and 26). This phosphorylation marks IκBα for polyubiquitination by the E3-SCFβ-TrCP ubiquitin ligase complex, a specific ubiquitin ligase belonging to the SCF (i.e., Skp-1/Cul/Fbox) family, and for degradation by the 26S proteasome (reviewed in reference 5). Degradation of IκBα allows a rapid and transient translocation of NF-κB to the nucleus, where it activates transcription from a wide variety of promoters—including that of its own inhibitor, IκBα. The newly synthesized IκBα enters the nucleus and removes NF-κB from its DNA binding sites and transports it back to the cytoplasm, thereby terminating NF-κB-dependent transcription (reviewed in references 19 and 26).
In addition to regulation of NF-κB activity through removal of IκB from NF-κB-IκB complexes, NF-κB activity is also regulated through modulation of its transcriptional function. Changes in NF-κB transcriptional activity have been assigned to inducible phosphorylation of the p65 subunit at Ser276, Ser529, and Ser536 by a large variety of kinases in response to different stimuli (reviewed in references 19 and 48). Additionally, NF-κB-dependent transcription requires multiple coactivators possessing histone acetyltransferase activity: CREB binding protein (CBP) and its homolog p300 (18, 29, 34, 52), p300/CBP-associated factor (P/CAF) (35), SRC-1/NcoA-1, and TIF-2/GRIP-1/NcoA-2 (31, 41, 42). Importantly, recruitment of CBP is enhanced by phosphorylation by the catalytic subunit of PKA (PKAc) of p65 at Ser276 (51, 52). More recently, other findings demonstrated a role for histone deacetylases (HDACs) as well. The first evidence came from the demonstration that inhibition of HDAC activity by trichostatin A (TSA) increases NF-κB-dependent gene expression (17, 24, 46, 49). It was next shown that NF-κB interacts with distinct HDAC isoforms to negatively regulate gene expression, presumably through the deacetylation of histones and/or nonhistone proteins (3, 11, 24, 28, 53). Importantly, the phosphorylation status of p65 determines whether it associates with CBP/p300 or HDAC-1, ensuring that only signal-induced NF-κB entering the nucleus can activate transcription (53).
The studies we describe here demonstrate that potentiation of TNF-induced NF-κB activation by deacetylase inhibitors (such as TSA) is associated with a delay in the cytoplasmic reappearance of IκBα. A prolonged activation of IKK complex promoting persistent IκBα degradation appears to be at least partially responsible for this delay. We thus identified a new regulatory link between deacetylase inhibitors and the NF-κB pathway, which is not at the level of NF-κB/HDAC interactions but rather at the level of IκBα cytoplasmic content.
MATERIALS AND METHODS
Plasmids.
The plasmid pLTR-luc contains the HIV-1LAI 5′ long terminal repeat (LTR; nucleotides 345 to 531) cloned into the reporter vector pGL2-Basic (Promega). To construct pLTRmut-κB-luc, pLTR-luc was used as substrate for mutagenesis of the two κB sites (5′-AACTCACTTTCCGCTGCTCACTTTCCA-3′) by the Quick Change Site-Directed Mutagenesis method (Stratagene).
The pTK-luc reporter plasmid contains the herpes simplex virus (HSV) thymidine kinase (TK) minimal promoter and was described previously (9). The pTK-4xNF-κB-luc was generated by inserting a cassette containing four copies of the interleukin 2 (IL-2) promoter κB site into SmaI-digested pTK-luc.
pIL8-luc and pIL8mut-κB-luc (33), pICAM1-luc (14), and pICAM1mut-κB-luc (1) were previously described.
pIL6-luc and pIL6mut-κB-luc and the p53 expression vector were kindly provided by Guy Haegeman (University of Gent, Gent, Belgium) and Bert Volgestein (The Johns Hopkins Oncology Center, Baltimore, Md.), respectively.
The plasmid pRSV-p65 (15) was obtained from Gary Nabel and Neil Perkins through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health).
Transient transfection and luciferase assays.
HeLa cells were transfected with Fugene6 (Roche) according to the manufacturer's recommended procedures. At 20 h posttransfection, the cells were treated or mock-treated with TSA (450 nM) (Sigma), NaBut (5 mM) (Sigma), TNF (10 ng/ml) (R&D Systems), or combinations of these drugs. At 42 h posttransfection, cells were lysed and assayed for luciferase activity (Promega). Luciferase activities were normalized with respect to protein concentration using the detergent-compatible protein assay (Bio-Rad).
Electrophoretic mobility shift assays.
Nuclear extracts were prepared and electrophoretic mobility shift assays (EMSAs) were performed as previously described with the human immunodeficiency virus type 1 (HIV-1) NF-κB probe (47). As loading controls, the same nuclear extracts were tested for binding of Sp1 to an Sp1 consensus probe, 5′-ATTCGATCGGGGCGGGGCGAGC-3′ (Promega).
In vivo acetylation assay.
COS-7 cells (3 × 106 cells) were transfected with expression vectors for p65 or p53 (500 ng). At 30 h posttransfection, the cells were resuspended in Dulbecco's modified Eagle's medium containing 1 mCi of sodium [3H]acetate (20 Ci/mmol; Amersham) per ml and 450 nM TSA and incubated for 2 h with or without TNF (10 ng/ml). The cells were washed twice with cold phosphate-buffered saline (PBS), lysed in RIPA buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) supplemented with 10 mM NaBut and protease inhibitors (Roche), and incubated on ice for 20 min. Equal amounts of lysates were used for immunoprecipitation with specific anti-p53 and anti-p65 antibodies (Santa Cruz Biotechnology). The immunoprecipitated proteins were subjected to 10% SDS-polyacrylamide gel electrophoresis (PAGE) and detected by autoradiography.
Western blot analysis.
Nuclear and cytoplasmic extracts were prepared as previously described (references 32 and 39, respectively). Western blots were performed with antibodies against the following proteins: IκBα (Upstate Biotechnology), phospho-IκBα−S32 (New England Biolabs), IκBβ, IκBɛ, p65 (Santa Cruz Biotechnology), phospho-p42/p44 MAPK/ERK, p42/p44 MAPK/ERK, phospho-p38, and p38 (New England Biolabs).
RNase protection analysis.
Total RNA samples were prepared using the commercial RNAqueous Phenol Free Total RNA Isolation Kit (Ambion) from cells treated or mock-treated with TSA or/and TNF.
The HIV-1-specific antisense riboprobe was obtained as previously described (47). An IκBα-specific 32P-labeled antisense riboprobe was synthesized in vitro by transcription of AflIII-restricted pcDNA3-IκBα with SP6 polymerase by standard methods. As control, a glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific antisense probe was synthesized by the same method and used on the same RNA samples. The RNase protection assays were performed with the RPA II Kit (Ambion) or with a custom RPA template system (Pharmingen, San Diego, Calif.) according to the manufacturer's recommendations.
Immunofluorescence confocal microscopy.
HeLa cells were grown on glass coverslips. Following appropriate treatments, cells were washed with PBS and were then fixed and permeabilized by immersion in 100% methanol for 6 min at −20°C. The samples were saturated with PBS containing 0.5% gelatin and 0.25% bovine serum albumin for 1 h and incubated for 1 h at room temperature with an anti-human p65 rabbit polyclonal immunoglobulin G (IgG; C-20; Santa Cruz Biotechnology) and with the monoclonal anti-IκBα antibody 10B (25) (a gift from Ronald Hay) in the same saturation solution. The samples were then washed three times with PBS containing 0.2% gelatin and incubated for 1 h with a 1:300 dilution of the secondary antibodies: Alexa-488-coupled goat anti-mouse IgG and Alexa 546-coupled goat anti-rabbit IgG (Molecular Probe). The samples were then washed three times in PBS containing 0.2% gelatin and mounted for analysis on a Zeiss LSM510 laser-scanning confocal microscope.
Proteasome activity assay.
Proteasome activities in cellular extracts were monitored as described previously (8) by quantifying the chymotrypsin-like and the peptidyl glutamyl peptide hydrolase activities using the fluorogenic substrates N-succinyl-Leu-Leu-Val-Tyr-AMC (LLVY-AMC) and N-CBZ-Leu-Leu-Glu-β-NA (LLE-NA) (Sigma), respectively. Typically, assay mixture contained 50 μg of cellular protein extracts in 25 mM Tris-HCl (pH 7.5) and 25 μM substrate in a final volume of 200 μl. The mixture was incubated at 37°C and analyzed as a time course by spectrofluorometry for release of aminomethylcoumarin (AMC; λex = 350 nm; λem = 440 nm) and naphthylamide (NA; λex = 340 nm; λem = 410 nm). Addition of proteasome inhibitor MG132 (Calbiochem) (200 μM) allowed specific measurement of proteasome peptidase activities.
In vitro IKK assay.
After treatment, HeLa cells (5 × 106 cells) were washed in 1 ml of cold PBS and centrifuged at 15,000 × g for 15 s, resuspended in 300 μl of cold lysis buffer (25 mM HEPES-KOH [pH 7.5], 150 mM NaCl, 0.5% Triton X-100, 10% glycerol, 1 mM dithiothreitol) supplemented with 1 mM Na3VO4, 1 mM NaF, 0.25 mM β-glycerophosphate, and protease inhibitors (Complete; Roche), and then vortex mixed and centrifuged at 15,000 × g for 15 min. Endogenous IKK complexes were immunoprecipitated from cell lysates (500 μg) by using 1.5 μg of anti-IKKγ antibody (FL-419; Santa Cruz Biotechnology) together with 20 μl of protein A-agarose (Santa Cruz Biotechnology) for 4 h at 4°C. Immunoprecipitates were collected by centrifugation and washed three times in the lysis buffer and then twice in kinase buffer (25 mM HEPES-KOH [pH 7.5], 10 mM MgCl2, 1 mM Na3VO4, 1 mM NaF, 0.25 mM β-glycerophosphate, and Complete). Immunoprecipitates were then resuspended in 30 μl of kinase buffer supplemented with ATP (5 mM) in the presence of either wild-type glutathione S-transferase (GST)-IκBα1-54 or mutant GST-IκBS32A/S36A and were incubated at 30°C for 45 min. Reactions were stopped by the addition of SDS loading buffer and were subjected to SDS-PAGE. Proteins were then electroblotted to polyvinylidene difluoride membranes and probed by Western blotting with anti-phospho-IκBα antibody (no. 9246; Cell Signaling), which is specific for IκBα phosphorylated at Ser32 and Ser36. To check loading and confirm the presence of IKKs, the upper part of the SDS-PAGE was analyzed by Western blotting using the anti-IKKβ antibody (T-20; Santa Cruz Biotechnology).
RESULTS
Deacetylase inhibitors potentiate TNF-induced NF-κB-driven gene expression.
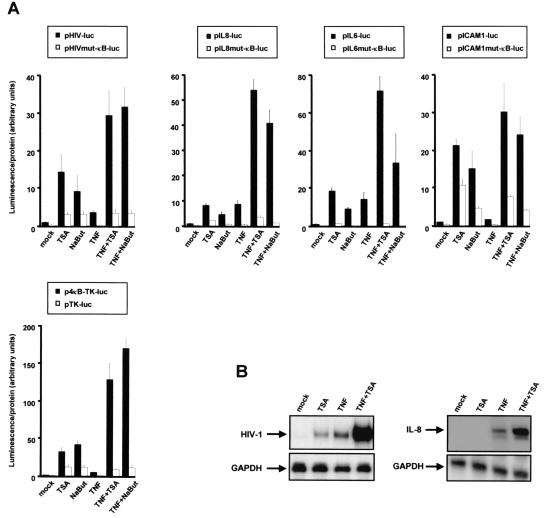
We investigated the functional role of NF-κB binding sites in the inducibility of different NF-κB-regulated promoters by deacetylase inhibitors. To this end, HeLa cells were transfected with luciferase reporter constructs containing the physiological NF-κB-driven promoters, namely from the HIV-1 retrovirus (pHIV-luc), from the IL-8 gene (pIL8-luc), from the IL-6 gene (pIL6-luc), and from the ICAM-1 gene (pICAM1-luc), as well as with a synthetic reporter construct containing multimerized IL-2-κB sites in front of the minimal TK promoter from HSV (p4κB-TK-luc) (Fig. 1A). Transfected cells were assayed for luciferase activity in response to TNF, to an inhibitor of deacetylases (TSA or sodium butyrate [NaBut]), or to a combination of both. For all κB reporters, treatment of cells with TNF alone resulted in increased luciferase gene expression. Moreover, all constructs were also activated by TSA or NaBut alone (Fig. 1A). Remarkably, cotreatment of cells with TNF and TSA (or NaBut) resulted in a strong synergistic upregulation of reporter expression. Mutation or deletion of the κB sites abolished this synergistic activation, demonstrating that the synergism observed between TNF and TSA (or NaBut) required intact κB sites in all promoters tested (Fig. 1A).
FIG. 1.
Deacetylase inhibitors potentiate TNF-induced NF-κB-driven gene expression. (A) HeLa cells were transiently transfected with 500 ng of the indicated luciferase reporter constructs. Cells were treated with TSA, NaBut, TNF, TNF plus TSA, or TNF plus NaBut (or were untreated), and cellular lysates were tested for luciferase activity. The mock-treated value of each wild-type reporter construct was arbitrarily set to a value of 1. Values represent the means of triplicate samples. A representative experiment of three independent transfections is shown. (B) RPA after a 6-h treatment of the latently HIV-1-infected cell line U1 with TNF and/or TSA. To detect HIV-1 RNA, total RNA samples were incubated with an antisense riboprobe corresponding to the HIV-1 LTR (left panel). RPA after a 2-h treatment of HeLa cells with TNF and/or TSA. Total cellular RNA was harvested after the indicated treatments and used in an RPA with a human IL-8 gene-specific riboprobe (right panel). GAPDH is shown as loading controls.
The biological relevance of the TNF-TSA synergistic effect was then addressed by RNase protection assays on endogenous κB-dependent gene expression. We analyzed HIV-1 transcription in the infected U1 cell line containing integrated proviruses; we also analyzed endogenous IL-8 gene transcription in HeLa cells. As shown on Fig. 1B, TSA treatment resulted in an increase of the steady-state HIV-1 mRNA level to a degree similar to that observed in response to TNF alone. Importantly, a synergistic induction of the steady-state HIV mRNA level was observed after treatment with TNF plus TSA, whereas the mRNA level of the housekeeping gene GAPDH was unaffected. Transcript levels of endogenous IL-8 behaved in a similar manner, with a synergistic increase after the combined treatment (i.e., TNF plus TSA) (Fig. 1B).
Taken together, these results demonstrate that deacetylase inhibitors functionally synergized with TNF to activate NF-κB-dependent gene expression. The TNF-TSA synergism was strictly dependent on the presence of intact κB sites.
TSA prolongs TNF-induced NF-κB DNA-binding activity.
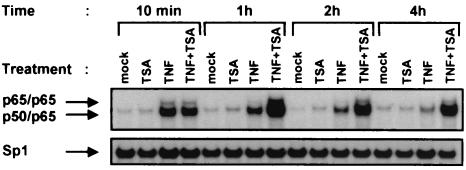
To address the influence of TSA on DNA-binding activity of NF-κB, nuclear extracts were prepared from HeLa cells that were either mock-treated or treated with TNF and/or TSA for different periods of time (10 min, 1, 2, and 4 h). These samples were subjected to EMSA using a DNA probe containing the HIV-1-κB sites (Fig. 2, upper panel). As expected, NF-κB appeared rapidly after a 10-min treatment with TNF and faded away after a 2-h treatment (Fig. 2). Supershift assays identified the major retarded complex as p50/p65 heterodimers and the fainter complex as p65/p65 homodimers (data not shown). By itself, TSA caused no induction of NF-κB binding activity even after a 4-h treatment (Fig. 2). When TSA was combined with TNF, an induction of NF-κB binding activity similar to that obtained with TNF alone was observed after a 10-min treatment. Remarkably, at later times (1, 2, and 4 h), TSA prolonged TNF-induced NF-κB binding activity for at least up to 4 h and also slightly increased this binding activity. Additionally, supershift analysis of the NF-κB complexes at the different activation times and conditions demonstrated no change in the dimer composition of the two retarded NF-κB complexes (data not shown). TSA did not modify the binding of the constitutively expressed Sp1 transcription factor in either the presence or absence of TNF (Fig. 2, lower panel).
FIG. 2.
EMSA analysis of NF-κB binding activity after TNF plus TSA versus TNF treatment. Nuclear extracts were prepared from HeLa cells treated with TNF and/or TSA (or untreated) for various times. An oligonucleotide corresponding to the HIV-1-κB sites was used as the probe. As control for equal loading, the lower panel shows comparability of the various nuclear extracts assessed by EMSA with an Sp1 consensus probe.
These results demonstrate that, while TSA alone did not stimulate NF-κB DNA-binding activity, it prolonged and increased TNF-induced NF-κB binding without alteration in NF-κB subunit composition.
The p65 subunit of NF-κB is not significantly acetylated in vivo.
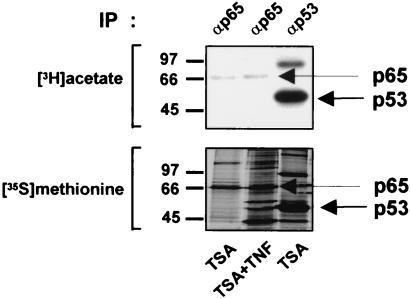
It has become clear that the acetylases and deacetylases not only modify histones but also a variety of nonhistone proteins, including general and specific transcription factors (reviewed in reference 10). For some of these transcription factors—such as p53 (21) and GATA-1 (7)—acetylation has been shown to lead to increased DNA binding and transactivation capacities. Since our results described above showed that TSA enhanced TNF-induced NF-κB binding and transcriptional activities, we considered that these effects could be mediated by direct acetylation of the p65 NF-κB subunit. To test p65 acetylation in vivo, COS-7 cells were transfected with a p65 expression vector or, as a positive control, a p53 expression vector and labeled with [3H]sodium acetate or [35S]methionine. Whole-cell extracts were immunoprecipitated using a p65- or p53-specific antiserum. We found that intracellularly expressed p65 (Fig. 3) (as well as endogenous p65 protein in untransfected HeLa and COS-7 cells; data not shown) were acetylated in vivo. However, the level of acetylation was very weak despite a good p65 protein expression as revealed by the [35S]methionine labeling. This was in striking contrast with p53, whose acetylation was easily detectable. Stimulation with TNF had no enhancing effect on this weak p65 acetylation (Fig. 3). Moreover, despite multiple attempts, we could not demonstrate the acetylation of p65 in vitro by using a series of purified known acetyltransferases (i.e., CBP, p300, p/CAF, and GCN5) (data not shown), a finding supported by others (21, 30, 41, 52, 53).
FIG. 3.
p65 is not significantly acetylated in vivo. COS-7 cells were transfected with a p65- or p53-expression vector and labeled either with [3H]sodium acetate or [35S]methionine. Whole-cell extracts from transfected cells were immunoprecipitated with an anti-p65 or anti-p53 antibody. The immunoprecipitated proteins were analyzed by SDS-PAGE followed by autoradiography.
The level of acetylated p65 we detected in vivo appears very low, thus suggesting that this level represents only a very restricted fraction of the total cellular pool of p65. These results led us to surmise that other mechanisms might be implicated in the enhanced binding and transcriptional activities of NF-κB observed after TNF plus TSA versus TNF treatment.
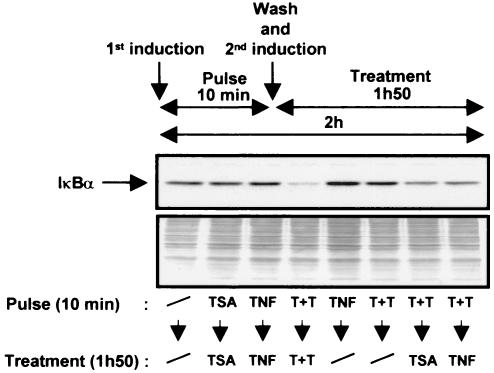
TSA delays the cytoplasmic reappearance of IκBα in cells treated with TNF plus TSA versus TNF alone.
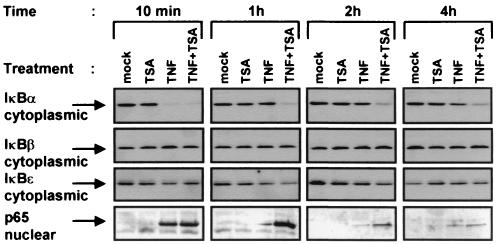
A critical level of regulation of NF-κB activity is through its retention in the cytoplasm via interactions with the inhibitors IκBs. We therefore probed the effects of TSA on the protein levels of IκBα, IκBβ, and IκBɛ in the cytoplasm of HeLa cells treated with TNF and/or TSA for periods ranging from 10 min to 4 h. Western blotting analysis showed that TSA alone did not induce IκBα degradation at any time point (Fig. 4). As expected, TNF induced a rapid degradation of the IκBα protein, thereby leading to its complete disappearance by 10 min. IκBα degradation was followed by its reappearance, with the amount of IκBα returning to a level similar to that seen in unstimulated cells by 1 h (Fig. 4). After a 10-min treatment with TNF plus TSA, IκBα was degraded as observed with TNF alone. In contrast, complete reappearance of IκBα was not detected even at the 4-h time point of TNF plus TSA stimulation (Fig. 4), indicating a delay in the IκBα cytoplasmic reappearance in presence of TNF plus TSA versus TNF alone. Pulse treatments of cells with TSA and/or TNF showed that the continuous presence of both TNF and TSA was required to observe the delay in the cytoplasmic reappearance of IκBα (Fig. 5). Although IκBβ and IκBɛ are two other major IκB isoforms responsible for inhibition of NF-κB nuclear translocation, no change in their respective cytoplasmic concentration was observed after any treatment (Fig. 4). Importantly, Western blot analysis of the corresponding nuclear extracts with a specific antibody against p65 revealed prolonged nuclear presence of p65 after TNF plus TSA versus TNF treatment, which correlated temporally with the delayed cytoplasmic reappearance of IκBα (Fig. 4).
FIG. 4.
Delay in the cytoplasmic IκBα reappearance in response to TNF plus TSA versus TNF treatment. Cytoplasmic and nuclear extracts were prepared from HeLa cells treated with TNF and/or TSA (or untreated) for various times. Equal amounts of proteins were analyzed by Western blotting with antibodies against IκBα, IκBβ, IκBɛ, and p65.
FIG. 5.
The continuous presence of both TNF and TSA is required for the delayed cytoplasmic reappearance of IκBα. HeLa cells were pulsed for 10 min with TSA, TNF, or a combination of both. Medium was then removed after this initial 10-min treatment (i.e., after IκBα disappearance), and the cells were washed twice with PBS and then replenished for the remainder of the 2-h period (i.e., for 1 h 50 min) either with fresh medium or with fresh medium supplemented with TSA, TNF, or both. Cytoplasmic extracts were then prepared and equal amounts of proteins were resolved on 10% SDS-PAGE and immunoblotted with an anti-IκBα antibody. Coomassie blue-stained membrane after immunoblotting (bottom panel) is shown as a loading control.
Our results therefore demonstrate that the sustained NF-κB DNA-binding activity we observed above in gel shifts upon TNF plus TSA versus TNF stimulation appears to result from a prolonged intranuclear presence of NF-κB due to a profound reduction of the IκBα cytoplasmic content.
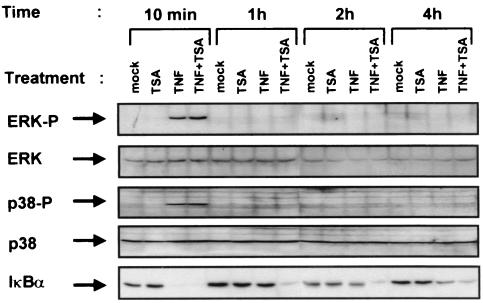
TSA does not affect the activation by TNF of p38 or ERK mitogen-activated protein (MAP) kinases.
Since it was of interest to determine whether other TNF-elicited signaling pathways were altered in TNF plus TSA- versus TNF-treated cells, we next examined the effect of TSA on ERK and p38 MAP kinase activation. To that end, we treated HeLa cells with TNF and/or TSA for different periods of time, and we examined cell lysates for the presence of phosphorylated (i.e., activated) forms of the ERK and p38 MAP kinases by immunoblot analysis (Fig. 6). Both TNF and TNF plus TSA induced a marked increase in the phosphorylation of the ERK and the p38 MAP kinases at 10 min that was no longer observed after 1 h.
FIG. 6.
TSA does not affect the activation by TNF of the p38 and ERK MAP kinases. Whole-cell extracts were prepared from HeLa cells treated with TNF and/or TSA (or untreated) for various times. Equal amounts of proteins were analyzed by Western blotting with the anti-phospho-p38 or anti-phospho-ERK antibody. Blots were reprobed with anti-p38 or anti-ERK to verify equal loading.
Thus, TSA did not prolong or increase the activation by TNF of the p38 or ERK MAP kinases, indicating that not all TNF-induced signaling pathways were persistently activated. This result indicates that TSA did not induce a generalized and prolonged activation of all TNF-responsive signaling pathways, pointing out a specificity of the TSA effect on the TNF-induced NF-κB activation cascade.
The delayed cytoplasmic reappearance of IκBα appears to result from a persistent proteasome-mediated degradation of the IκBα protein.
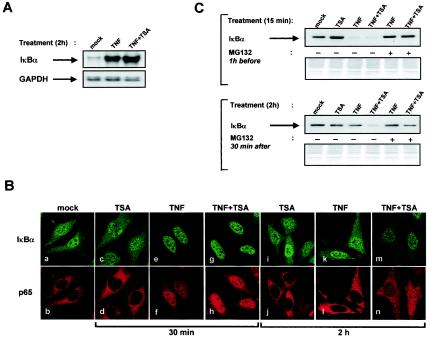
At least three mechanisms, not mutually exclusive, should be considered for the delayed IκBα cytoplasmic reappearance: (i) a defect in IκBα mRNA production, (ii) a retention of IκBα in the nucleus, and/or (iii) an increased proteolysis of IκBα.
To address the first possible mechanism, we analyzed the steady-state level of IκBα mRNA by RNase protection assay in HeLa cells treated or not treated for 2 h with TNF or TNF plus TSA (Fig. 7A). A very low level of IκBα mRNA expression was detectable in samples from unstimulated cells. In contrast, we observed similar strong activations of IκBα transcription following both TNF and TNF plus TSA treatments, thereby indicating that TSA did not impair the TNF-dependent transcriptional activation of the IκBα promoter. Control RNase protection analysis of the same mRNA samples using a GAPDH riboprobe showed no difference in the mRNA levels (Fig. 7A, lower panel). These results indicate that the delayed IκBα cytoplasmic reappearance was not due to a defect in the steady-state level of IκBα mRNA.
FIG. 7.
The delayed cytoplasmic reappearance of IκBα appears to result from a persistent proteasome-mediated degradation of IκBα. (A) The delayed cytoplasmic reappearance of IκBα is not due to a defect in IκBα mRNA production. Total RNA was isolated from HeLa cells treated with TNF or TNF plus TSA for 2 h (or untreated), and IκBα mRNA was analyzed by RPA with a human IκBα gene-specific riboprobe. GAPDH is shown as loading controls. (B) The delayed cytoplasmic reappearance of IκBα is not due to a nuclear retention of IκBα. HeLa cells were treated with TNF and/or TSA (or untreated) and analyzed by confocal microscopy at the indicated time points. Endogenous IκBα and p65 were localized by indirect immunofluorescence. (C) The delayed cytoplasmic reappearance of IκBα appears to result from a persistent proteasome-mediated degradation of IκBα. Cytoplasmic extracts were prepared from HeLa cells treated for 15 min with TNF and/or TSA as indicated after a 1-h pretreatment with MG132 (25 μM) and then analyzed for the presence of IκBα by Western blotting (top). Cytoplasmic extracts were prepared from HeLa cells treated for 2 h with TNF and/or TSA as indicated. After 30 min of this initial treatment, MG132 (25 μM) was added for the rest of the 2-h period. Extracts were immunoblotted with anti-IκBα (bottom). The Coomassie blue-stained membrane after immunoblotting is shown as a loading control.
To determine whether the delayed IκBα cytoplasmic recovery we observed after treatment with TNF plus TSA versus TNF treatment was associated with a retention of IκBα in the nucleus, we monitored by confocal fluorescence microscopy the subcellular localization of endogenous IκBα and p65 during stimulation with TNF and/or TSA (Fig. 7B). In unstimulated HeLa cells, we detected IκBα in both the cytoplasm and the nucleus (as previously demonstrated [35, 45]), whereas p65 was localized predominantly in the cytoplasmic compartment (Fig. 7B, panels a and b, respectively). Treatment with TSA alone for 30 min or 2 h did not alter this subcellular distribution (Fig. 7B, panels c and d or i and j). Treatment with TNF led after 30 min to a substantial reduction of the cytoplasmic amount of IκBα accompanied by the translocation of p65 to the nucleus (Fig. 7B, panels e and f). This was transient since, following 2 h of TNF treatment, we observed replenishment of the cytoplasmic pool of IκBα by newly synthesized protein and the concomitant return of the nuclear p65 to the cytoplasm (Fig. 7B, panels k and l, respectively). The patterns of IκBα and p65 distribution in response to a 30-min treatment with TNF plus TSA were identical to those observed with TNF alone (Fig. 7B, panels g and h). In contrast, after a 2-h treatment with TNF plus TSA, p65 was still abundantly present in the nucleus (Fig. 7B, compare panels n and l), while the neosynthesized IκBα was barely detectable in the cytoplasm (Fig. 7B, panel m), confirming our Western blot results (see Fig. 4 above). Interestingly, IκBα appeared to be present to a lesser degree in the nucleus by 2 h of TNF plus TSA treatment compared to all the other conditions (Fig. 7B, compare panel m with panels a, c, e, g, i, and k). This indicates that TSA did not delay the cytoplasmic reappearance of IκBα by preventing its retrograde transport from the nucleus to the cytoplasm, in which case we would have observed a nuclear accumulation of IκBα.
A persistent proteasome-mediated degradation of IκBα could explain the delayed IκBα cytoplasmic reappearance in cells treated with TNF plus TSA, despite the presence of high levels of IκBα mRNA in such cells. To investigate the potential involvement of the proteasome pathway, we tested the effect of the proteasome inhibitor MG132 on IκBα cytoplasmic levels during treatment with TNF and/or TSA. Pretreatment of cells for 1 h with MG132 completely abolished IκBα degradation that had been induced by a 15-min treatment with either TNF or TNF plus TSA (Fig. 7C, top panel), thereby demonstrating the functional activity of MG132. Importantly, in cells treated for 2 h with TNF plus TSA, addition of MG132 (30 min after the TNF or TNF plus TSA initial treatment, i.e., after the disappearance of IκBα) for the rest of the 2-h period led to a partial abolition of the delayed IκBα cytoplasmic reappearance (Fig. 7C, bottom panel), thus suggesting that the delay resulted from a persistent proteasome-mediated degradation of the IκBα protein.
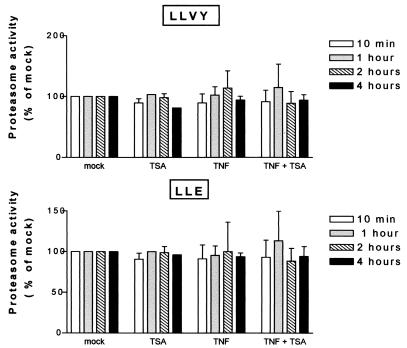
TSA does not affect proteasome activity in presence or absence of TNF.
Given that several studies have reported a modulatory effect of deacetylase inhibitors (such as TSA and NaBut) on cellular proteasome activity (20, 50), we next investigated whether, in our experimental setting, differences existed in intrinsic proteolytic activities of cells treated with TNF plus TSA versus TNF alone. To this end, cytosolic extracts were prepared from HeLa cells treated with TNF and/or TSA for various times, and two proteasome peptidase activities (chymotrypsin-like and peptidyl-glutamyl-peptide hydrolase activities) were evaluated in the extracts by using appropriate fluorogenic peptide substrates LLVY-AMC and LLE-NA, respectively. The time course presented in Fig. 8 showed that neither of these two peptidase activities was significantly altered as a result of treatment with any drug alone or in combination.
FIG. 8.
TSA does not affect proteasome activity in presence or absence of TNF. Cell lysates were prepared from HeLa cells treated with TNF and/or TSA (or untreated) for various times and analyzed for proteasome activity. Chymotrypsin-like and peptidyl glutamyl peptide hydrolase proteasome activities were measured in lysates by using the fluorogenic peptides LLVY-AMC and LLE-NA, respectively. The proteasome inhibitor MG132 (200 μM) was used to ensure that measured activities were due to the proteasome. The mock-treated value at each time point was arbitrarily set to a value of 100%. Values represent the means of triplicate samples.
TSA prolongs TNF-induced IKK kinase activity.
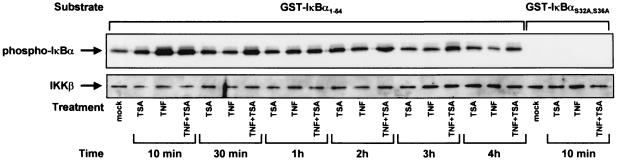
Since phosphorylation of IκBα at S32,36 by the IKK complex is a prerequisite to its proteasome-mediated degradation, we examined whether TSA could prolong TNF-induced IKK activity and could therefore account for the delayed IκBα cytoplasmic reappearance. Endogenous IKK activity was measured in HeLa cells treated with TSA, TNF, or both for different periods of time. IKKs were immunoprecipitated with an anti-IKKγ antibody, and associated kinase activity was assayed by using GST-IκBα fusion protein as a substrate. As expected, TNF treatment caused a transient induction of IKK activity as measured by the enhanced phosphorylation of GST-IκBα. The activation of IKK peaked 10 min post-TNF treatment and decreased at later times (from 30 min to 4 h) (Fig. 9, top panel). When both TNF and TSA were used in combination, an induction of IKK activity identical to that obtained with TNF alone was observed after a 10-min treatment (Fig. 9, top panel). At later time points, while IKK activity slightly decreased, this activity was always higher than that observed following TNF treatment (Fig. 9, top panel). All these reactions were specific because no phosphorylation of the mutated GST-IκBα S32,36A was detected under these conditions (Fig. 9, top panel).
FIG. 9.
TSA prolongs TNF-induced IKK kinase activity. Endogenous IKK activity was measured in HeLa cells treated with TNF and/or TSA for different periods of time. IKKs were immunoprecipitated with an anti-IKKγ antibody, and associated kinase activity was assayed by using purified GST-IκBα1-54 fusion protein as a substrate (top). The presence of equal amounts of the IKK catalytic subunit, IKKβ, was confirmed in each sample by Western blotting (bottom). Equivalent GST-IκBα loading was verified by staining the membrane with Coomassie brilliant blue after immunoblotting (data not shown).
In conclusion, our results indicate that TSA appears to prolong the TNF-induced IKK activity by up to more than 4 h. The phosphorylation of IκBα by IKK is critical to its proteasome-mediated degradation. Therefore, the prolongation of IKK activity could explain the persistent degradation of neosynthesized IκBα observed in presence of TNF plus TSA and the resulting prolonged presence of NF-κB in the nucleus.
DISCUSSION
In this report, we define a new regulatory link between κB-mediated gene expression and deacetylase inhibitors, which is distinct from the NF-κB-HDAC interactions but involves the inhibitory protein IκBα.
IκBα plays a pivotal role in the NF-κB signaling pathway. The primary level of regulation of NF-κB activity is through its retention in the cytoplasm via interactions with IκBα in preinduction states (reviewed in reference 26). Moreover, following stimulation with proinflammatory cytokines such as TNF, the resynthesis of de novo IκBα leads to the postinduction nuclear accumulation of IκBα, thereby inducing nuclear export of NF-κB. This latter event is part of a negative feedback system that ensures a transient NF-κB transcriptional response and the restoration of the preinduction state NF-κB-IκB complexes (2). Here, we provide evidence that deacetylase inhibitors affect this negative feedback regulation known as postinduction repression of NF-κB function. Indeed, we showed by immunoblotting and confocal immunofluorescence microscopy that the replenishment of the cytoplasmic pool of IκBα was delayed in cells treated with TNF plus TSA versus TNF alone. This delay was due neither to a defect in IκBα mRNA production nor to a nuclear retention of IκBα but rather appeared to result from a persistent proteasome-mediated degradation of IκBα. Consequently, IκBα can no longer accumulate in the nucleus to remove NF-κB from target gene promoters and transport it back to the cytoplasm, thereby terminating the NF-κB response. Consistent with this scenario, our results revealed that the intranuclear DNA binding and presence of p65 were prolonged in presence of TNF plus TSA versus TNF alone. This could account, at least in part, for the transcriptional synergism we observed between TNF and TSA or NaBut on several NF-κB-regulated promoters.
Our results are in contrast with those of other publications that indicate that NaBut and sodium valproate (a newly identified deacetylase inhibitor) inhibit NF-κB activation (22, 50). A critical difference between these previous reports and our results may be that these studies entailed long-term incubations with deacetylase inhibitors. As such, their data likely involve broader changes in cellular physiology. Cell-type-specific variables may also explain the different responses.
It is important to note that the molecular mechanisms mediating the TNF-TSA synergism are likely to be highly complex and to implicate phenomena other than the delayed IκBα cytoplasmic reappearance and persistent NF-κB nuclear levels reported here. It has now become clear that histone acetylation and chromatin remodeling are key regulatory processes for NF-κB-dependent transcription. Recently, it has been demonstrated that two classes of NF-κB-activable genes exist: those containing constitutively and immediately accessible NF-κB binding sites in their promoter (thus recruiting NF-κB dimers immediately after their nuclear entry) and those that have to be hyperacetylated to become accessible to NF-κB before it is taken back to the cytoplasm (36). For a subset of stimulus-induced cytokine and chemokine genes, it would be the p38 pathway that, at least in part through induction of histone H3 phosphorylation and phosphoacetylation, would orchestrate the modifications that lead to enhanced accessibility of NF-κB sites in response to inflammatory stimuli (37). On the other hand, previous studies have also reported the critical role of competing acetylases and deacetylases in regulating the transcriptional activity of NF-κB. In unstimulated cells, p50/p50 homodimers are constitutively nuclear and serve as transcriptional repressors associated with HDAC-1 (53). Moreover, p65 can interact with distinct HDAC isoforms, HDAC-1 (3, 24, 28, 53) or HDAC-3 (11, 27). Thus, these HDACs associated with p65 and p50 could repress expression of NF-κB-regulated genes by maintaining histones and/or other proteins in a deacetylated state. In several of these studies, treatment of cells with TSA blocks the HDAC activity, thereby resulting in histone hyperacetylation and subsequently a higher level of gene expression (3, 36, 53). TSA or NaBut would thus increase NF-κB-dependent transcription by alleviating the chromatin- and/or factor-mediated block to transcriptional activation. Therefore, the TNF-TSA synergism would result from a bimodal phenomenon consisting of both the inhibition by TSA of the HDAC corepressor complexes interacting with NF-κB as well as the delayed IκBα cytoplasmic reappearance in the presence of TNF plus TSA versus TNF alone.
We cannot exclude that direct acetylation of Rel family members could also intervene in the molecular mechanisms of synergistic activation by TNF and TSA. However, despite multiple attempts, we detected only a very weak acetylation of p65 in vivo and we were unable to detect it in vitro. This is consistent with several previous studies (21, 30, 41, 52, 53). The reasons why p65 acetylation under our conditions was so weak are unclear, despite the recent observations made by two groups that five lysines in p65 are acetylated in vivo (12, 27). Surprisingly, these two groups did not identify the same lysine residues as mediating the in vivo acetylation of p65. Moreover, the function attributed to p65 acetylation contrasts sharply between these two studies. One group proposed that p65 acetylation (on residues K218, K221, and K310) enhances its DNA binding and impairs assembly with IκBα and therefore impedes IκBα-dependent nuclear export of the NF-κB complex, allowing the duration of the NF-κB response (11, 12). In contrast, the other group proposed that acetylation of p65 contributes to the mechanism of postinduction turnoff of NF-κB-mediated transcription (27). They demonstrate that a dual acetylation (on residues K122 and K132) reduces binding of p65 to κB-containing DNA, thereby facilitating its removal by IκBα and subsequent export to the cytoplasm (27). The reasons for these discrepancies are unclear at this time. Overall, our data indicate the existence of an additional TSA-sensitive mechanism regulating the NF-κB pathway consisting in delaying IκBα reappearance.
From a mechanistic point of view, we showed that TSA induced a prolongation of the TNF-induced activation of the IKK complex likely being responsible for the persistent phosphorylation- and proteasome-mediated IκBα degradation observed in cells cotreated with TNF and TSA. It is worth noting that the TSA effect on IKK activity could also have an impact on the transactivation capacity of p65, since IKKs have been demonstrated to phosphorylate p65 on Ser536 (38). This effect on IKK activity does not exclude that TSA could in addition modulate the downstream pathway leading to IκBα degradation. We demonstrated that TSA did not alter the intrinsic cellular proteasome activity of cells treated with TNF. Nevertheless, TSA action could target the ubiquitination step. In agreement with this hypothesis, β-TrCP—which is the specific IκBα ubiquitin-ligase—could be indirectly upregulated following TSA treatment. Indeed, TSA and NaBut have been reported to upregulate Tcf activity (6). Interestingly, the expression of β-TrCP mRNA and protein can be increased in some cells by the activation of the Wnt-β-catenin-Tcf signaling pathway, resulting in the acceleration of β-catenin (another target of β-TrCP) degradation (43). This upregulation of β-TrCP levels also results in the activation of the NF-κB pathway in response to the stimuli that induce IκB phosphorylation (43, 44). A Tcf-mediated upregulation of β-TrCP by TSA could also contribute, at least in part, to the prolongation of IκBα degradation observed in cells treated with TNF plus TSA.
The mechanism by which TSA produces a persistent activation of IKK remains to be determined. IKK activity could be modulated by direct acetylation of at least one of the IKK subunits (α or β). The acetylation status of IKKα and/or -β would control its activity or regulate its association with factors modulating its activity such as IKKγ. In addition, TSA could very well influence other components of the signaling pathway situated upstream of the IKKs, such as yet unidentified upstream kinases or proteins associated with the TNF receptor. Interestingly, a TSA-mediated regulation has been previously reported for several kinases, notably for protein kinase C and calmodulin kinase II (16). These hypotheses are currently under investigation.
In conclusion, we have identified a novel mechanism involved in the regulation of NF-κB signaling by deacetylase inhibitors. Our results could provide additional targets for the discovery of inhibitors of NF-κB, which is involved in a number of different inflammatory diseases.
Acknowledgments
E.A. and V.Q. contributed equally to this work.
We thank Guy Haegeman (University of Gent, Gent, Belgium), Ronald Hay (University of St. Andrews, United Kingdom), and Bert Vogelstein (The Johns Hopkins Oncology Center, Baltimore, Md.) for reagents used in this study. We are grateful to Claire Josse (Université de Liège) for her assistance with the RNase protection assays.
This work was supported by grants to C.V.L. from the Fonds National de la Recherche Scientifique (FNRS; Belgium), the Télévie-Program, the Université Libre de Bruxelles (ULB; ARC program no. 98/03-224), the Internationale Brachet Stiftung, the CGRI-INSERM cooperation, the Région Wallonne-Commission Européenne FEDER, the Agence Nationale de Recherches sur le SIDA (ANRS; France), and the Theyskens-Mineur Foundation. V.Q. is a designated Aspirant of the FNRS. A.C. is Chercheur Qualifié of the FNRS. C.V.L. is Maître de Recherches of the FNRS. J.P. and V.B. are Directeurs de Recherches of the FNRS. E.A. is supported by a postdoctoral fellowship from the ULB (ARC program no. 98/03-224). V.G. and T.L.-A. N. are fellows of the Belgian Fonds pour la Recherche dans l'Industrie et l'Agriculture (FRIA).
REFERENCES
- 1.Aoudjit, F., N. Brochu, B. Belanger, C. Stratowa, J. Hiscott, and M. Audette. 1997. Heterodimeric retinoic acid receptor-β and retinoid X receptor-α complexes stimulate expression of the intercellular adhesion molecule-1 gene. Cell Growth Differ. 8:335-342. [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos, F., P. Turpin, M. Rodriguez, D. Thomas, R. T. Hay, J. L. Virelizier, and C. Dargemont. 1997. Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. J. Cell Sci. 110:369-378. [DOI] [PubMed] [Google Scholar]
- 3.Ashburner, B. P., S. D. Westerheide, and A. S. Baldwin, Jr. 2001. The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 21:7065-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin, A. S., Jr. 2001. Series introduction: the transcription factor NF-κB and human disease. J. Clin. Investig. 107:3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Neriah, Y. 2002. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 3:20-26. [DOI] [PubMed] [Google Scholar]
- 6.Bordonaro, M., J. M. Mariadason, F. Aslam, B. G. Heerdt, and L. H. Augenlicht. 1999. Butyrate-induced apoptotic cascade in colonic carcinoma cells: modulation of the β-catenin-Tcf pathway and concordance with effects of sulindac and trichostatin A but not curcumin. Cell Growth Differ. 10:713-720. [PubMed] [Google Scholar]
- 7.Boyes, J., P. Byfield, Y. Nakatani, and V. Ogryzko. 1998. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature 396:594-598. [DOI] [PubMed] [Google Scholar]
- 8.Bulteau, A., I. Petropoulos, and B. Friguet. 2000. Age-related alterations of proteasome structure and function in aging epidermis. Exp. Gerontol. 35:767-777. [DOI] [PubMed] [Google Scholar]
- 9.Calomme, C., T.-L. Nguyen, Y. de Launoit, V. Kiermer, L. Droogmans, A. Burny, and C. Van Lint. 2002. Upstream stimulatory factors binding to an E box motif in the R region of the bovine leukemia virus long terminal repeat stimulates viral gene expression. J. Biol. Chem. 277:8775-8789. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H., M. Tini, and R. M. Evans. 2001. HATs on and beyond chromatin. Curr. Opin. Cell Biol. 13:218-224. [DOI] [PubMed] [Google Scholar]
- 11.Chen, L., W. Fischle, E. Verdin, and W. C. Greene. 2001. Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653-1657. [DOI] [PubMed] [Google Scholar]
- 12.Chen, L., Y. Mu, and W. C. Greene. 2002. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 21:6539-6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai, R.-M., E. Chen, D. L. Longo, C. M. Gorbea, and C.-C. H. Li. 1998. Involvement of valosin-containing protein, an ATPase co-purified with IκBα and 26 S proteasome, in ubiquitin-proteasome-mediated degradation of IκBα. J. Biol. Chem. 273:3562-3573. [DOI] [PubMed] [Google Scholar]
- 14.de Launoit, Y., M. Audette, H. Pelczar, S. Plaza, S., and J. L. Baert. 1998. The transcription of the intercellular adhesion molecule-1 is regulated by Ets transcription factors. Oncogene 16:2065-2073. [DOI] [PubMed] [Google Scholar]
- 15.Duckett, C. S., N. D. Perkins, T. F. Kowalik, R. M. Schmid, E. S. Huang, A. S. Baldwin, Jr., and G. J. Nabel. 1993. Dimerization of NF-κB2 with RelA(p65) regulates DNA binding, transcriptional activation, and inhibition by an IκBα (MAD-3). Mol. Cell. Biol. 13:1315-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eickhoff, B., L. Germeroth, C. Stahl, G. Köhler, S. Rüller, M. Schlaak, and J. van der Bosch. 2000. Trichostatin A-mediated regulation of gene expression and protein kinase activities: reprogramming tumor cells for ribotoxic stress-induced apoptosis. Biol. Chem. 381:1127-1132. [DOI] [PubMed] [Google Scholar]
- 17.El Kharroubi, A., G. Piras, R. Zensen, and M. A. Martin. 1998. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol. Cell. Biol. 18:2535-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerritsen, M. E., A. J. Williams, A. S. Neish, S. Moore, Y. Shi, and T. Collins. 1997. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. USA 94:2927-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109:S82-S96. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano, M., M. Lauricella, G. Calvaruso, M. Carabillo, S. Emanuele, R. Vento, and G. Tesoriere. 1999. The apoptotic effects and synergistic interaction of sodium butyrate and MG132 in human retinoblastoma Y79 cells. Cancer Res. 59:5586-5595. [PubMed] [Google Scholar]
- 21.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 22.Ichiyama, T., K. Okada, J. M. Lipton, T. Matsubara, T. Hayashi, and S. Furukawa. 2000. Sodium valproate inhibits production of TNF-α and IL-6 and activation of NF-κB. Brain Res. 857:246-251. [DOI] [PubMed] [Google Scholar]
- 23.Israel, A. 2000. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 10:129-133. [DOI] [PubMed] [Google Scholar]
- 24.Ito, K., P. J. Barnes, and I. M. Adcock. 2000. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin 1β-induced histone H4 acetylation on lysines 8 and 12. Mol. Cell. Biol. 20:6891-6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffray, E., K. M. Wood, and R. Hay. 1995. Domain organization of IκBα and sites of interaction with NF-κB p65. Mol. Cell. Biol. 15:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karin, M., and Y. Ben Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 27.Kiernan, R., V. Brès, R. W. M. Ng, M.-P. Coudart, S. El Messaoudi, C. Sardet, D.-Y. Jin, S. Emiliani, and M. Benkirane. 2003. Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 278:2758-2766. [DOI] [PubMed] [Google Scholar]
- 28.Lee, S. K., J. H. Kim, Y. C. Lee, J. Cheong, and J. W. Lee. 2000. Silencing mediator of retinoic acid and thyroid hormone receptors as a novel transcriptional corepressor molecule of activating protein 1, nuclear factor-κB, and serum response factor. J. Biol. Chem. 275:12470-12474. [DOI] [PubMed] [Google Scholar]
- 29.Merika, M., A. J. Williams, G. Chen, T. Collins, and D. Thanos. 1998. Recruitment of CBP/p300 by the IFN-β enhanceosome is required for synergistic activation of transcription. Mol. Cell 1:277-287. [DOI] [PubMed] [Google Scholar]
- 30.Munshi, N., M. Merika, J. Yie, K. Senger, G. Chen, and D. Thanos. 1998. Acetylation of HMG I(Y) by CBP turns off IFN-β expression by disrupting the enhanceosome. Mol. Cell 2:457-4467. [DOI] [PubMed] [Google Scholar]
- 31.Na, S. Y., S. K. Lee, S. J. Han, H. S. Choi, S. Y. Im, and J. W. Lee. 1998. Steroid receptor coactivator-1 interacts with the p50 subunit and coactivates nuclear factor κB-mediated transactivations. J. Biol. Chem. 273:10831-10834. [DOI] [PubMed] [Google Scholar]
- 32.Osborn, L., S. Kunkel, and G. J. Nabel. 1989. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κ B. Proc. Natl. Acad. Sci. USA 86:2336-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott, M., J. L. Lovett, L. Mueller, and E. Verdin. 1998. Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-κB factors. J. Immunol. 160:2872-2880. [PubMed] [Google Scholar]
- 34.Perkins, N. D., L. K. Felzien, J. C. Betts, K. Leung, D. H. Beach, and G. J. Nabel. 1997. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science 275:523-527. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez, M. S., J. Thompson, R. T. Hay, and C. Dargemont. 1999. Nuclear retention of IκBα protects it from signal-induced degradation and inhibits nuclear factor κB transcriptional activation. J. Biol. Chem. 274:9108-9115. [DOI] [PubMed] [Google Scholar]
- 36.Saccani, S., S. Pantano, and G. Natoli. 2001. Two waves of nuclear factor κB recruitment to target promoters. J. Exp. Med. 193:1351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saccani, S., S. Pantano, and G. Natoli. 2002. p38-dependent marking of inflammatory genes for increased NF-κ B recruitment. Nat. Immunol. 3:69-75. [DOI] [PubMed] [Google Scholar]
- 38.Sakurai, H., H. Chiba, H. Miyoshi, T. Sugita, and W. Toriumi. 1999. IκB kinase phosphorylates NF-κB p65 subunit on serine 536 in the transactivation domain J. Biol. Chem. 274:30353-30356. [DOI] [PubMed] [Google Scholar]
- 39.Schoonbroodt, S., V. Ferreira, M. Best-Belpomme, J. R. Boelaert, S. Legrand-Poels, M. Korner, and J. Piette. 2000. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of IκB alpha in NF-κB activation by an oxidative stress. J. Immunol. 164:4292-4300. [DOI] [PubMed] [Google Scholar]
- 40.Seigneurin-Berny, D., A. Verdel, S. Curtet, C. Lemercier, J. Garin, S. Rousseaux, and S. Khochbin. 2001. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol. Cell. Biol. 21:8035-8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheppard, K. A., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 19:6367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spencer, T. E., G. Jenster, M. M. Burcin, C. D. Allis, J. Zhou, C. A. Mizzen, N. J. McKenna, S. A. Onate, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1997. Steroid receptor coactivator 1 is a histone acetyltransferase. Nature 389:194-198. [DOI] [PubMed] [Google Scholar]
- 43.Spiegelman, V. S., T. J. Slaga, M. Pagano, T. Minamoto, Z. Ronai, and S. Y. Fuchs. 2000. Wnt/β-catenin signaling induces the expression and activity of βTrCP ubiquitin ligase receptor. Mol. Cell 5:877-882. [DOI] [PubMed] [Google Scholar]
- 44.Spiegelman, V. S., P. Stavropoulos, E. Latres, M. Pagano, Z. Ronai, T. J. Slaga, and S. Y. Fuchs. 2001. Induction of β-transducin repeat-containing protein by JNK signaling and its role in the activation of NF-κB. J. Biol. Chem. 276:27152-27158. [DOI] [PubMed] [Google Scholar]
- 45.Turpin, P., R. H. Hay, and C. Dargemont. 1999. Characterization of IκBα nuclear import pathway. J. Biol. Chem. 274:6804-6812. [DOI] [PubMed] [Google Scholar]
- 46.Vanden Berghe, W., K. De Bosscher, E. Boone, S. Plaisance, and G. Haegeman. 1999. The nuclear factor-κB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin 6 gene promoter. J. Biol. Chem. 274:32091-32098. [DOI] [PubMed] [Google Scholar]
- 47.Van Lint, C., S. Emiliani, M. Ott, and E. Verdin. 1996. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 15:1112-1120. [PMC free article] [PubMed] [Google Scholar]
- 48.Vermeulen, L., G. De Wilde, S. Notebaert, W. Vanden Berghe, and G. Haegeman. 2002. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem. Pharmacol. 64:963-970. [DOI] [PubMed] [Google Scholar]
- 49.West, M. J., A. D. Lowe, and J. Karn. 2001. Activation of human immunodeficiency virus transcription in T cells revisited: NF-κB p65 stimulates transcriptional elongation. J. Virol. 75:8524-8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin, L., G. Laevsky, and C. Giardina. 2001. Butyrate suppression of colonocyte NF-κ B activation and cellular proteasome activity. J. Biol. Chem. 276:44641-44646. [DOI] [PubMed] [Google Scholar]
- 51.Zhong, H., H. SuYang, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]
- 52.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]
- 53.Zhong, H., M. J. May, E. Jimi, and S. Ghosh. 2002. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol. Cell 9:625-636. [DOI] [PubMed] [Google Scholar]