Abstract
Proliferation of mesangial cells is a hallmark of glomerular disease, and understanding the regulatory mechanisms is critically important. The purpose of this study was to examine the relationship between mesangial cell proliferation and phosphorylated signal transducer and activator of transcription (STAT) 3 and to determine whether the PDGF receptor tyrosine kinase inhibitor STI 571 inhibited mesangial cell proliferation via modulation of STAT3. In this study, we investigated for the first time, the glomerular expression of phosphorylated STAT3 in paraffin sections from animals with experimental mesangial proliferative glomeronephritis. Phosphorylated STAT3 colocalized with many proliferating mesangial cells. We also demonstrated that treatment with STI 571 reduced mesangial cell proliferation and phosphorylated STAT3 signalling both in vitro and in vivo. In vivo, STI 571 treatment reduced the number of glomerular mesangial cells positive for both phosphorylated STAT3 and proliferating cell nuclear antigen. In summary, phosphorylated STAT3 is strongly expressed during mesangial cell proliferation and STI 571 induced suppression of mesangial cell proliferation involves inhibition of phosphorylated STAT3 signalling.
Keywords: STI 571, STAT3, mesangial proliferation
Introduction
Mesangial cell proliferation plays an important role in the progressive glomerular injury that characterizes many diseases that lead to eventual glomerular sclerosis [1,2]. Platelet-derived growth factor (PDGF) has been widely implicated in the pathogenesis of progressive renal injury in both experimental models and human disease [3]. PDGF-BB has also been reported to be essential for the mesangial cell proliferation that precedes the development of glomerulosclerosis in the remnant kidney model [4]. Specific inhibition of the mitogenic actions of PDGF is therefore a major target for therapy in glomerular disease. In this study, STI 571, a potent and selective inhibitor of PDGF receptor tyrosine kinase [5] was used.
Signal transducer and activator of transcription (STAT) 3 is a member of the STAT protein family. STAT proteins are latent transcription factors that are activated by phosphorylation and STAT3 activation has been implicated in cell proliferation [6–9]. Activated STAT proteins dimerize, translocate to the nucleus and stimulate STAT-specific transcription in cultured mesangial cells [10]. Phosphorylated STAT3 is almost undetectable in the kidneys of normal rats. However, after the induction of anti-Thy1·1 glomerulonephritis (GN), STAT3 phosphorylation increased significantly and in parallel with disease severity with phosphorylated STAT3 being predominantly evident in mesangial cells [9,10]. Anti-Thy1·1 GN in rats is a particularly well-characterized model of mesangial proliferative GN and is induced by a single intravenous injection of a monoclonal anti-rat mesangial cell antibody (OX-7). This results in the acute complement dependent lysis of mesangial cells and is subsequently followed by a phase of intense mesangial cell proliferation and extracellular matrix accumulation (from day 2–3 after injection of monoclonal OX-7) [11–13]. Kalechmam et al. [9] reported that STAT3 was a downstream target of interleukin 10 dependent mesangial cell proliferation in vitro and in vivo. Many studies of experimental mesangial proliferative GN indicate that suppression of PDGF ameliorates mesangial cell proliferation when drug treatment extends from day 0 to the time of sacrifice [14,15]. Here, we report that activated STAT3 was expressed in PDGF-BB induced rat mesangial cell proliferation in vitro. We also report for the first time that activated STAT3 has an important relationship with mesangial cell proliferation in vivo. In the present studies we tested the following hypotheses:
STI 571 will ameliorate mesangial cell proliferation via modulation of STAT3 phosphorylation;
a short duration of STI 571 treatment during the mesangioproliferative phase of anti-Thy1·1 GN will exert a significant anti-proliferative effect.
Material and method
Cell line
A well-characterized, cloned mesangial cell line (1097) isolated from Sprague-Dawley rats [16] was kindly provided by Dr David J. Nikolic-Paterson (Monash Medical Centre, Melbourne, Australia) and used in all in vitro experiments. Cells were used between passage 20–30 and were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin and streptomycin in a humidified 5% CO2 atmosphere at 37 °C.
Reagents
STI 571, generously provided by Novartis Pharma (Basel, Switzerland), was used as previously described in both the in vitro and in vivo studies [14]. The OX-7 hybridoma cell line secreting anti-Thy1·1 monoclonal antibody was purchased from the European Collection of Cell Culture (DERA, Wiltshire, UK). A technique based on the protocol of Morita and colleagues [17] was used for purification of the anti-Thy1·1 monoclonal antibody. Other reagents for these studies were as follows: recombinant human PDGF-BB (Genzyme, Cambridge, MA, USA), FCS and protease inhibitor cocktail for mammalian tissues (Sigma Chemical Co, St Louis, MO, USA) and polyvinylidete difluoride membranes and ECL Western blotting detection system (Amersham Biosciences; Buckinghamshire, UK). Vectorstain ABC Kit, Vector Avidin/biotin Blocking Kit and Vector SG (Vector Laboratory, Burlingame, CA, USA) with 3,3-diaminobenzidine (DAB) (Sigma) were used in immunohistochemical studies.
Antibodies
Anti-phosphorylated (Tyr705)-STAT3 (p-STAT3) antibody and phospho-STAT3 (Tyr705) blocking peptide were purchased from Cell Signaling Technology (Beverly, MA, USA) and anti-non-phosphospecific STAT3 (total-STAT3) antibody was from Upstate Biotechnology, Inc (Lake Placid, NY, USA). Anti-human PDGF antibody was from R & D systems (Minneapolis, MN, USA). Horse radish peroxidase (HRP)-conjugated donkey anti-rabbit IgG was from Amersham Pharmacia Biotech (Little Chalfont, UK). Monoclonal mouse anti-proliferating cell nuclear antigen (PCNA), goat anti-mouse IgG conjugated with HRP and peroxidase anti-peroxidase mouse monoclonal antibody were from Dakocytomation (Denmark). Biotin conjugated goat anti-rabbit IgG was from Zymed (San Francisco, CA, USA) whilst mouse anti-rat CD68 (ED1) was from Serotec (Oxford, UK).
Western blotting studies of STAT3 proteins
For detection of p-STAT3 signalling, rat mesangial cells were cultured in 6-well flat-bottomed plates in DMEM/10% FCS and incubated for 24 h. Subconfluent cells were then starved for 2 days in DMEM/0·1% FCS and pre-incubated with either PDGF neutralizing antibody for 1 h or STI 571 for 30 min before being stimulated with PDGF-BB for 15 min. Cells were then washed three times with cold phosphate-buffered saline (PBS) and lysed by thawing in 100 µl of lysis buffer (20 mM Tris-HCl, pH 7·4, 100 mM NaCl, 1 mM ethylene glycol tetraacetate, 5 mM NaF, 1 mM NaVO4, 1% Triton X-100, 10% glycerol, 1% deoxycholate, 100 mM phenylmethylsulphonyl fluoride, and 10% protease inhibitor cocktail for mammalian tissues). The cell lysates were stirred on ice for 1 h and then scraped into 1·5 ml Eppendorf tubes followed by centrifugation at 18 400 g for 20 min at 4°C. The protein content of cell lysates was separated on 7·5% polyacrylamide gels using SDS-PAGE and transferred to polyvinylidene difluoride membranes. The blots were blocked with 20 mM Tris-HCl pH 7·4 and 140 mM NaCl with 0·05% Tween 20 (TBST buffer) containing 5% nonfat dry milk at room temperature for 1 h, washed three times in TBST buffer and incubated with each primary antibody at 4 °C overnight (p-STAT3 at 1 : 1000 dilution or total-STAT3 at 1 : 500 dilution). The membranes were then incubated with the secondary antibody (HRP-conjugated donkey anti-rabbit IgG) at 1 : 5000 dilution at room temperature for 1 h with the reaction products being detected with the ECL Western blotting detection system.
Proliferation assay
Mesangial cells were plated at 5 × 103 cells per well in 96-well flat-bottomed microtitre plates in DMEM/10% FCS and allowed to adhere for 24 h. Subconfluent cells were then starved for 2 days in DMEM/0·1% FCS. PDGF-BB (in the presence or absence of STI 571) was added at a final concentration of 20 ng/ml and cell proliferation determined 24 h later by the addition of 0·5 µCi [3H]-thymidine (Amersham Pharmacia Biotech, Little Chalfont, UK) to each well during the last 6 h of culture. After washing three times in PBS, cells were solubilized in 1 M NaOH. The lysate was then neutralized with 1 M HCl and then Clear-sol II scintillation fluid (Nacalai Tesque, Kyoto, Japan) was added and radioactive emissions determined with a liquid scintillation counter (LSC5100; Aloka Tokyo, Japan). Replicates of six wells were used in each experiment and all experiments were performed four times.
Animals
Male Wistar rats (6 weeks old, 180–200 g) were purchased from SLC (Kyoto, Japan). Animals were kept in standard conditions with free access to water and standardized food. This study was carried out in accordance with the Guidelines for Animal Experiments in Hiroshima University and the Committee of Research Facilities for Laboratory Animal Science, Natural Science Center for Basic Research and Development, Hiroshima University.
Rat anti-Thy1·1 GN
Acute mesangial proliferative GN was induced in male Wistar rats with a single intravenous injection of a monoclonal antibody to the Thy1·1 antigen (OX-7, 1 mg/kg) at day 0. Control rats received the same volume of intravenous saline (Control group, n = 7). The STI 571 treated anti-Thy1·1 GN group (STI treated GN, n = 7) received a daily intraperitoneal injection of STI 571 (50 mg/kg) from day 4 to day 6 (3 h prior to sacrifice). Vehicle treated GN (control GN, n = 7) received a daily intraperitoneal injection of vehicle (10% dimethyl sulphoxide in saline) at the same times. All animals were sacrificed at day 6.
Immunohistochemistry
Kidney sections were fixed in 4% formalin and embedded in paraffin. 4 µm thick sections were used for immunohistochemistry. Tissue sections were placed in 0·01 M citrate buffer, pH 6·0 and heated for 10 min in a microwave oven [18] prior to immunostaining for p-STAT3, PCNA and macrophages/monocytes (ED-1). Sections were sequentially blocked in 10% FCS, 10% normal goat serum and 5% bovine serum albumin in PBS (1 h each) and then incubated overnight at 4 °C with the primary antibody diluted in PBS containing 10% normal goat serum and 5% normal rat serum. After washing, endogenous peroxidase was inhibited by incubation in 0·6% H2O2 in methanol for 20 min. Next, sections were incubated sequentially with secondary antibodies. The ABC method was used for the detection of p-STAT3 whereas the indirect immunoperoxidase technique was performed as described previously for the detection of PCNA and ED-1 [18]. Two colour immunostaining was used to detect colocalization of p-STAT3/PCNA and p-STAT3/ED-1. After staining for p-STAT3 using the ABC method followed by developing with 3,3-DAB to give a brown colour, tissue sections underwent a second round of microwave treatment to block antibody cross-reactivity followed by inactivation of endogenous peroxidase and enhanced detection of the PCNA or ED-1 antigen. Sections were then blocked as above, washed and incubated overnight at 4°C with the PCNA or ED-1 monoclonal antibody. Sections were then washed and incubated sequentially with HRP conjugated goat anti-mouse IgG and then developed with Vector SG to give a blue/grey colour [18,19].
Quantification of immunohistochemistry
Fifty full size and nonmesangiolytic hilar glomeruli were assessed for each animal under high power (×400) and the average cell number or staining score per glomerular cross-section (gcs) determined.
Sections double stained with p-STAT3/PCNA antibodies were used to quantify the p-STAT3 staining score and determine the number of p-STAT3/PCNA double positive cells. All scoring was performed with the observer masked to the study group. Semiquantification of glomerular p-STAT3 staining was performed using the following glomerular staining score: 0, very weak or absent staining; 1+, weak staining with < 25% of the glomerular tuft exhibiting focally increased staining; 2+, 25–49% of the glomerular tuft exhibits focally increased staining; 3+, 50–75% of the glomerular tuft exhibits increased staining; 4+, >75% of the glomerular tuft stains strongly [20]. The scoring data were expressed as the mean ± SD per gcs.
Statistics
All data are shown as mean ± SD unless otherwise specified. Data were analysed by analysis of variance (anova) using the Stat View IV program (Brainpower, Calabasas, CA, USA) on a Windows 2000 platform. Data derived from in vivo experiments (7 animals per group) are expressed as the mean ± SD. Comparisons between experimental groups were compared by anova using the Tukey's post hoc test. A P-value <0·05 was considered statistically significant.
Results
p-STAT3 expression in rat mesangial cells
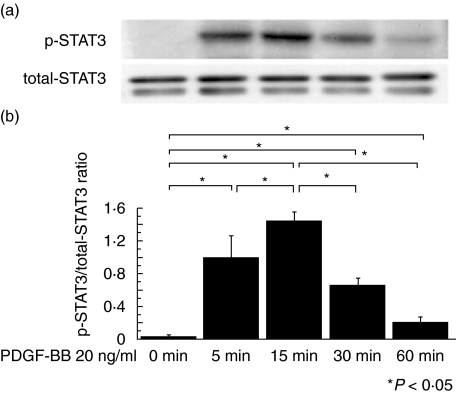
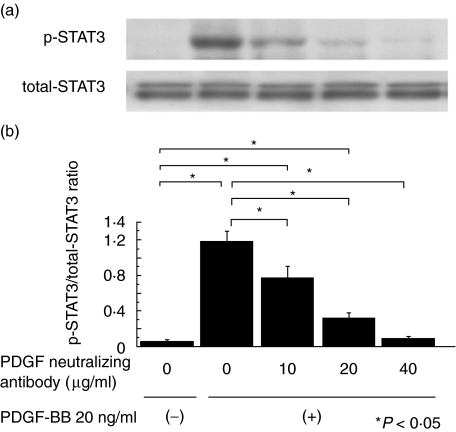
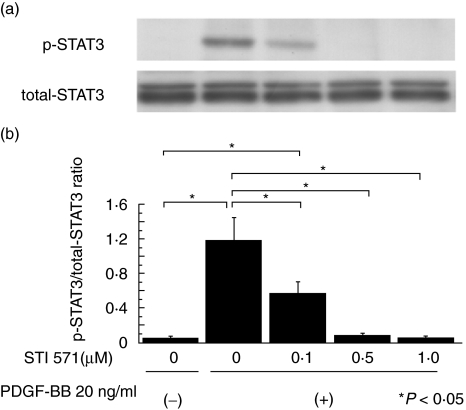
Twenty ng/ml of PDGF-BB was added to quiescent mesangial cells for 0, 5, 15, 30 or 60 min and the time course of p-STAT3 expression examined (Fig. 1). p-STAT3 expression was most strongly expressed at 15 min and we therefore used this dose and incubation time in further experiments. We then examined the suppressive effect of a neutralizing anti-PDGF antibody in order to confirm that stimulation of PDGF-BB specifically induced STAT3 activation. Quiescent mesangial cells were preincubated with anti-PDGF neutralizing antibody for 1 h before the cells were stimulated with PDGF-BB 20 ng/ml for 15 min. p-STAT3 expression was suppressed in a dose-dependent manner thereby demonstrating that PDGF-BB specifically activates STAT3 in vitro (Fig. 2). Next, we examined the suppressive effect of STI 571. Quiescent mesangial cells were preincubated with STI 571 for 30 min before the cells were stimulated with PDGF-BB 20 ng/ml for 15 min. STAT3 activation was remarkably suppressed in a dose-dependent manner thereby indicating that STI 571 specifically inhibits the effects of PDGF-BB upon STAT3 activation (Fig. 3).
Fig. 1.
Time course of phosphorylated STAT3 expression. (a) The well characterized cloned mesangial cell line (1097) was used in all experiments. Quiescent mesangial cells were stimulated with PDGF-BB (20 ng/ml) for the indicated times. Cells were lysed and immunoblotted with phosphorylated (p-) and nonphosphospecific (total-) STAT3 antibodies. (b) Graph shows the ratio of p-STAT3 to total-STAT3. p-STAT3 expression is maximal at 15 min. The data represent the mean ± SD of four individual experiments. *P < 0·05 by anova with Tukey's post hoc test.
Fig. 2.
Effect of a neutralizing PDGF antibody on phosphorylated STAT3 expression in mesangial cells stimulated by PDGF-BB. (a) Quiescent mesangial cells were preincubated for 1 h with a neutralizing anti-PDGF antibody that effectively inhibits the action of PDGF. Cells were then stimulated with PDGF-BB (20 ng/ml) for 15 min. Cells were lysed and immunoblotted with phosphorylated (p-) and non phosphospecific (total-) STAT3 antibodies. (b) Graph shows the ratio of p-STAT3 to total-STAT3. PDGF neutralizing antibody effectively suppressed p-STAT3 expression in a dose-dependent manner. The data represent the mean ± SD of four individual experiments. *P< 0·05 by anova with Tukey's post hoc test.
Fig. 3.
Effect of STI 571 on phosphorylated STAT3 expression in the mesangial cells stimulated by PDGF-BB. (a) Quiescent mesangial cells were preincubated with STI 571 for 30 min and then stimulated with PDGF-BB (20 ng/ml) for 15 min. Cells were lysed and immunoblotted with phosphorylated (p-) and non phosphospecific (total-) STAT3 antibodies. (b) Graph shows the ratio of p-STAT3 to total-STAT3. STI 571 effectively suppressed p-STAT3 expression in a dose-dependent manner. The data represent the mean ± SD of four individual experiments. *P< 0·05 by anova with Tukey's post hoc test.
Effect of STI 571 on PDGF-BB induced mesangial cell proliferation in vitro
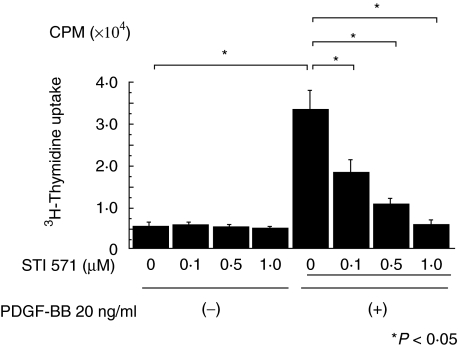
The ability of PDGF-BB to stimulate proliferation in serum-starved mesangial cells was inhibited by pretreatment of cells with STI 571 in a dose-dependent fashion (Fig. 4). Mesangial cells remained viable as evidenced by the trypan blue exclusion test and the presence of normal nuclear morphology.
Fig. 4.
Effect of STI 571 on PDGF-BB stimulated DNA synthesis. Quiescent mesangial cells were preincubated with and without STI 571 for 30 min and then stimulated with PDGF-BB (20 ng/ml) for 24 h. A [3H]-thymidine uptake test was performed during the last 6 h of culture using 0·5 µCi/well. STI 571 suppressed PDGF-BB induced mesangial cell proliferation in a dose-dependent fashion.
STI 571 inhibits proliferating glomerular cells in anti-Thy1·1 GN
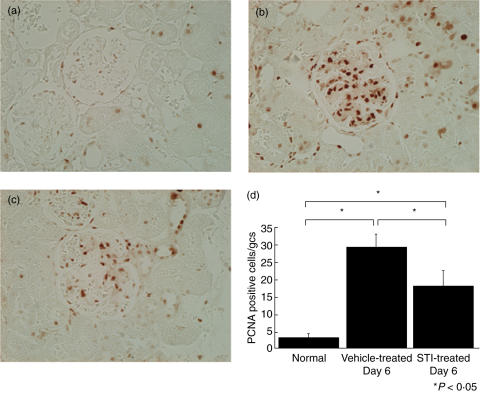
The microscopic lesions present in rats with anti-Thy1·1 GN took the form of the diffuse mesangial proliferation and glomerular hypertrophy. STI 571 treated rats with anti-Thy1·1 GN exhibited comparable morphological changes as control rats with anti-Thy1·1 GN. We then examined whether STI 571 treatment modulated mesangial cell proliferation by comparing the numbers of PCNA positive glomerular cells to control rats with anti-Thy1·1 GN. Immunostaining for PCNA was performed using 4 µm thick sections and developed with 3,3-DAB to produce a brown colour (Fig. 5a–c). ST1571 treatment significantly reduced the number of PCNA positive glomerular cells compared to disease controls (Fig. 5d).
Fig. 5.
Immunostaining of renal tissue from normal rats and rats with anti-Thy1·1 GN. Kidney sections (4 µm) were immunostained for proliferating cell nuclear antigen (PCNA) and developed with 3,3-diaminobenzidine to produce a brown colour. (a) Normal, (b) anti-Thy1·1 glomerulonephritis treated with 10% dimethyl sulphoxide in saline at day 6 (Vehicle-treated GN), (c) anti-Thy1·1 glomerulonephritis treated with STI 571 at day 6. (STI-treated GN). (d) Quantification of Proliferating Cell Nuclear Antigen positive glomerular cells. Fifty hilar glomeruli were scored under high power (×400) and the number of proliferating cell nuclear antigen (PCNA) positive glomerular cells was counted. Treatment with STI 571 significantly reduced the number of PCNA positive cells/glomerular cross-section (gcs). The data represent the mean ± SD for groups of 7 animals. *P< 0·05 by anova with Tukey's post hoc test.
Double immunostaining of p-STAT3/PCNA and p-STAT3/ED-1
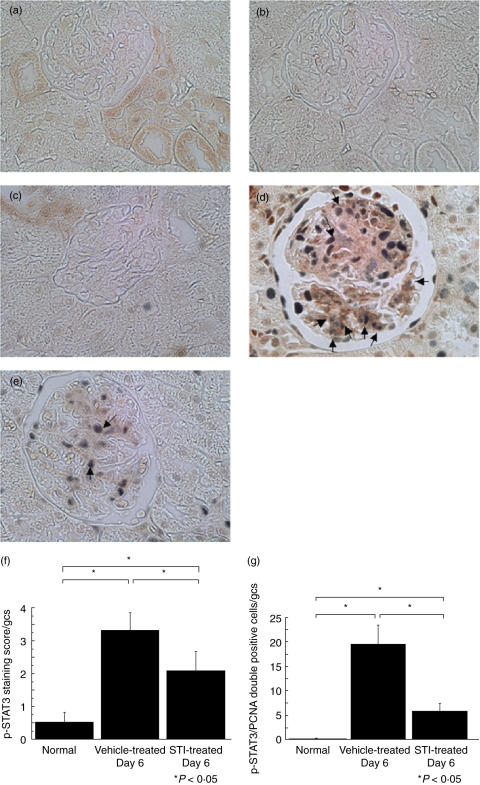
We were able to demonstrate positive glomerular immunostaining of p-STAT3 using paraffin embedded tissue sections and used the staining score indicated previously to quantify p-STAT3 expression. Very limited glomerular staining was evident in normal control animals. In contrast, strong glomerular expression of p-STAT3 was present in rats with anti-Thy1·1 GN. The specificity of the immunohistochemical staining was demonstrated in two ways. First, omission of the primary antibody gave no staining (data not shown). Second, incubation of antibodies with the immunizing peptide (phospho-STAT3 Tyr705) blocking peptide) completely blocked the respective immunostaining signal (Fig. 6a–e). The p-STAT3 staining score was significantly reduced at day 6 in STI 571 treated rats with anti-Thy1·1 GN compared with untreated disease controls (Fig. 6f).
Fig. 6.
Two colour immunohistochemistry of phosphorylated STAT3 and proliferating cell nuclear antigen. Kidney sections (4 µm) were stained using two colour immunohistochemistry with phosphorylated STAT3 (p-STAT3) being stained brown and proliferating cell nuclear antigen stained blue/grey. (a) Normal rat single stained with p-STAT3, (b) Normal rat in which staining is abolished by incubation of p-STAT3 with the p-STAT3 blocking peptide (a and b are serial). (c–e) Two colour immunohistochemistry. (c) Normal, (d) anti-Thy1·1 glomerulonephritis treated with 10% dimethyl sulphoxide in saline at day 6 (Vehicle-treated GN), (e) anti-Thy1·1 glomerulonephritis treated with STI 571 at day 6 (STI-treated GN). Magnification × 400. (f) Staining score of phosphorylated STAT3 (0–4). Phosphorylated STAT3 (p-STAT3) staining score was quantified using staining score (0–4). Levels of p-STAT3 staining per glomerular cross-section (gcs) were significantly reduced by STI 571 treatment. The data represent the mean ± SD for groups of 7 animals. *P< 0·05 by anova with Tukey's post hoc test. (g) Quantification of phosphorylated STAT3/proliferating cell nuclear antigen double positive cell numbers. Phosphorylated STAT3 (p-STAT3)/proliferating cell nuclear antigen (PCNA) double positive cells per glomerular cross-section (gcs) were counted in 50 hilar glomeruli. In anti-Thy1·1 glomerulonephritis treated with STI 571 (STI-treated GN), colocalization of p-STAT3 and PCNA positive cells was significantly reduced in comparison with anti-Thy1·1 glomerulonephritis treated with 10% dimethyl sulphoxide in saline (Vehicle-treated GN). The data represent the mean ± SD for groups of 7 animals. *P< 0·05 by anova with Tukey's post hoc test.
We also identified and quantified the number of p-STAT3 and PCNA double positive glomerular cells in each experimental group. Many PCNA positive glomerular cells were double stained with p-STAT3 in control rats with anti-Thy1·1 GN whereas such double positive cells were very few in normal rats. The number of double positive cells was significantly lower in STI 571 treated rats with anti-Thy1·1 GN compared with control rats with anti-Thy1·1 GN (Fig. 6c).
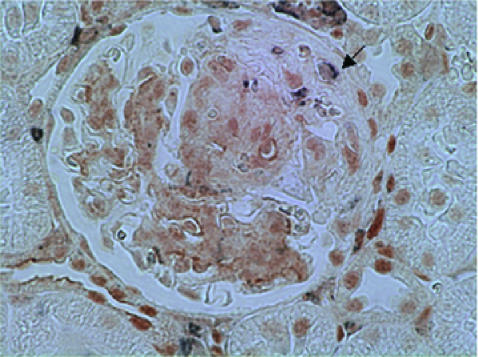
We also performed double staining of p-STAT3 and ED-1 in control rats with anti-Thy1·1 GN in order to ascertain the expression of p-STAT3 by macrophages and monocytes (Fig. 7). We detected very few ED-1 positive macrophages/monocytes exhibiting double staining for p-STAT3. These results demonstrate that the p-STAT3 and PCNA double positive glomerular cells are likely to represent resident glomerular cells, especially mesangial cells. The number of ED-1 positive cells in STI 571 treated rats with anti-Thy1·1 GN was comparable with control rats with anti-Thy1·1 GN (data not shown).
Fig. 7.
Two colour immunohistochemistry of phosphorylated STAT3 and the macrophages/monocyte marker ED-1 in anti-Thy1·1 glomerulonephritis treated with 10% dimethyl sulphoxide in saline at day 6. Kidney sections (4 µm) were stained using two colour immunohistochemistry with phosphorylated STAT3 being stained brown and the macrophages/monocyte marker ED-1 stained blue/grey. Very few ED-1 positive macrophages were double stained by p-STAT3. Magnification ×400.
Discussion
Recent studies have demonstrated that inhibitors of intracellular signalling pathways may well represent novel therapeutic agents in the treatment of mesangial proliferative GN [21,22] and in this study we have focused upon the role of STAT3 in mesangial cell proliferation. We examined PDGF-BB induced STAT3 activation in rat mesangial cells and hypothesized that STAT3 activation plays a key role in mesangial cell proliferation.
STI 571 is a pharmacological drug which is clinically used as a treatment of chronic myelocytic leukaemia by acting as Bcr-Abl kinase inhibitor. STI 571 reduced PDGF-BB induced expression of p-STAT3 thereby suggesting a role for STAT3 phosphorylation in mesangial cell proliferation and that STI 571 exerts its anti-proliferative effect by indirectly inhibiting STAT3 phosphorylation. STI 571 is a selective and potent inhibitor of PDGF receptor tyrosine kinase [14]. Our data indicate that STI 571 suppressed PDGF-BB induced rat mesangial cell proliferation in vitro and reduced the proliferation of mesangial cells in a well described model of mesangial proliferative GN and this reinforces the pivotal role of the growth factor PDGF in anti-Thy1·1 GN [4,23]. In addition, the results of our study suggest that STI 571 should be considered as a new potential therapeutic agent for patients with mesangial proliferative GN. The anticipated plasma levels of STI 571 in the animals in this study may be estimated from human data [24] and would be approximately 10 µM. This concentration is 10 times the level used in the in vitro study and 6 times the blood concentration found in patients with human chronic myelocytic leukaemia (CML).
Anti-Thy1·1 GN is a well established model in rats and is characterized by an acute phase of complement-dependent mesangiolysis followed by mesangial cell proliferation and expansion of the mesangial matrix such that it resembles the morphological features of human mesangial proliferative GN. Previous work has demonstrated that treatment with either STI 571 or a neutralizing PDGFβ receptor antibody from day 0 ameliorates mesangial proliferation in anti-Thy1·1 GN [14,15]. However, the anti-proliferative effect of drugs administered for the short proliferative phase (day 4–6) of anti-Thy1·1 GN has not been examined previously. In our study, treatment with STI 571 for 3 days (day 4–6) effectively reduced the number of PCNA positive glomerular cells and the level of glomerular p-STAT3 expression. In addition, treatment with STI 571 significantly reduced the colocalization of p-STAT3 and PCNA in proliferating mesangial cells. Zang et al. [25] reported that almost all PCNA positive glomerular cells exhibit double staining with α smooth muscle actin during the proliferative phase of anti-Thy1·1 GN such that PCNA positive glomerular cells during the proliferative phase of anti-Thy1·1 GN could be considered to represent proliferating mesangial cells. Furthermore, our in vivo data suggest that infiltrating ED-1 positive macrophages are not a significant source of p-STAT3 positive cells and we therefore consider p-STAT3/PCNA double positive cells to represent p-STAT3 positive proliferating mesangial cells. We demonstrate for the first time the localization and relationship of p-STAT3 and proliferating mesangial cells in a well established model of experimental mesangial proliferative GN. Furthermore, we show that treatment with STI 571 limited to the proliferative phase of Th1·1 GN significantly reduced the level of mesangial cell proliferation. These findings indicate a strong relationship between mesangial cell proliferation and STAT3 activation in experimental mesangial proliferative GN.
In vivo, prolonged activation of STAT3 was seen in proliferative mesangial areas and some normal tubules and STI 571 did not completely suppress STAT3 activation. Other mediators such as interleukin 10 and Gas6 that may activate STAT3 [9,10] may play a role in STAT3 activation and osmotic stress may activate STAT3 in tubules [18]. In addition, as STI 571 is a specific inhibitor of PDGF receptor it is likely to suppress mesangial cell proliferation induced by other isoforms of PDGF such as PDGF-D.
In this study, we have demonstrated that STI 571 treatment suppresses mesangial cell proliferation in association with inhibition of STAT3 activation suggesting that STAT3 may be a key downstream signalling molecule during PDGF-BB induced mesangial cell proliferation. In conclusion, STI 571 is an effective and practical drug that is capable of suppressing mesangial cell proliferation associated with STAT3 phosphorylation both in vitro and in vivo.
References
- 1.Fogo A, Ichikawa I. Evidence for the central role of glomerular growth promoters in the development of sclerosis. Semin Nephrol. 1989;9:329–42. [PubMed] [Google Scholar]
- 2.Striker LJ, Doi T, Elliot S, et al. The contribution of glomerular mesangial cells to progressive glomerulosclerosis. Semin Nephrol. 1989;9:318–28. [PubMed] [Google Scholar]
- 3.Border WA, Yamamoto T, Noble NA. Transforming growth factor beta in diabetic nephropathy. Diabetes Metab Rev. 1996;12:309–39. doi: 10.1002/(SICI)1099-0895(199612)12:4<309::AID-DMR171>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Ostendorf T, Kunter U, Grone HJ, et al. Specific antagonism of PDGF prevents renal scarring in experimental glomerulonephritis. J Am Soc Nephrol. 2001;12:909–18. doi: 10.1681/ASN.V125909. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Tamura S, Buchdunger E, et al. Effect of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–6. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 6.Fukada T, Hibi M, Yamanaka Y, et al. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–60. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg JF, Horvath CM, Besser D, et al. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–8. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharti AC, Domato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 9.Kalechman Y, Gafter U, Weinstein T, et al. Inhibition of interleukin-10 by the immunomodulator AS101 reduces mesangial cell proliferation in experimental mesangioproliferative glomerulonephritis: association with dephosphorylation of STAT3. J Biol Chem. 2004;279:24724–32. doi: 10.1074/jbc.M312006200. [DOI] [PubMed] [Google Scholar]
- 10.Yanagita M, Arai H, Nakano T, et al. Gas6 induces mesangial proliferation via latent transcription factor STAT3. J Biol Chem. 2001;45:42364–9. doi: 10.1074/jbc.M107488200. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Wilson CB. Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int. 1987;32:514–25. doi: 10.1038/ki.1987.240. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Iida H, Alpers CE, et al. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–58. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson RJ, Garcia RL, Pritzl P, et al. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990;136:369–74. [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert RE, Kelly DJ, McKay T, et al. PDGF signal transduction inhibition ameliorates experimental mesangial proliferative glomerulonephritis. Kidney Int. 2001;59:1324–32. doi: 10.1046/j.1523-1755.2001.0590041324.x. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Abe H, Arai H, et al. Activation of STAT3/Smad1 is a key signaling pathway for progression to glomerulosclerosis in experimental glomerulonephritis. J Biol Chem. 2005;G280:7100–6. doi: 10.1074/jbc.M411064200. [DOI] [PubMed] [Google Scholar]
- 16.Kakizaki Y, Kraft N, Atkins RC. Differential control of mesangial cell proliferation by interferon-gamma. Clin Exp Immunol. 1991;85:157–63. doi: 10.1111/j.1365-2249.1991.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita H, Maeda K, Obayashi M, et al. Induction of irreversible glomerulosclerosis in the rat by injection of a monoclonal anti-Thy-1. 1 Antibody Nephron. 1992;60:92–9. doi: 10.1159/000186711. [DOI] [PubMed] [Google Scholar]
- 18.Masaki T, Foti R, Hill PA, et al. Activation of the ERK pathway precedes tubular proliferation in the obstructed rat kidney. Kidney Int. 2003;63:1256–64. doi: 10.1046/j.1523-1755.2003.00874.x. [DOI] [PubMed] [Google Scholar]
- 19.Ikezumi Y, Hurst LA, Masaki T, et al. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 20.Daniel C, Wiede J, Krutzsch HC, et al. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–68. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 21.Bokemeyer D, Panek D, Kramer HJ, et al. In vivo identification of the mitogen-activated protein kinase cascade as a central pathogenic pathway in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 2002;13:1473–80. doi: 10.1097/01.asn.0000017576.50319.ac. [DOI] [PubMed] [Google Scholar]
- 22.Clarke HC, Kocher HM, Khwaja A, et al. Ras antagonist farnesylthiosalicylic acid (FTS) reduces glomerular cellular proliferation and macrophage number in rat Thy-1 nephritis. J Am Soc Nephrol. 2003;14:848–54. doi: 10.1097/01.asn.0000057543.55318.8b. [DOI] [PubMed] [Google Scholar]
- 23.Floege J, Eng E, Young BA, et al. Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int Suppl. 1993;39:S47–54. [PubMed] [Google Scholar]
- 24.Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22:935–42. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Nakazawa K, Shigematsu H. Participation of endothelial cells and transformed mesangial cells in remodeling of glomerular capillary loops in Thy-1 nephritis. Pathol Int. 2001;51:227–39. doi: 10.1046/j.1440-1827.2001.01196.x. [DOI] [PubMed] [Google Scholar]