Abstract
The aim of this study was to elucidate the time course of the permeability response of endothelial monolayers after exposure to plasma obtained from lipopolysaccharide (LPS)-treated human whole blood; to investigate the role of apoptosis in monolayer permeability, and to inhibit the permeability increase, particularly after addition of the plasma stimulus. Human umbilical vein endothelial cells (HUVEC) were cultured on semiporous membranes and the permeability for albumin was measured after exposure, according to different schedules, to LPS-conditioned plasma. Apoptotic HUVEC were measured by both flow cytometry and ELISA. A variety of agents, including antibodies against cytokines, inhibitors of NF-κB, and a caspase inhibitor, were added to HUVEC, either prior to or after the stimulus. A maximum increase of the permeability was achieved after 4–6 h of exposure to LPS-conditioned plasma. This response was not accompanied by an increase in the number of apoptotic HUVEC. Administration of antibodies against both Tumour Necrosis Factor-α (TNF-α) and Interleukin-1β (IL-1β) to HUVEC within 1 h after stimulation significantly reduced the permeability increase. Similarly, pyrollidine di-thiocarbamate (PDTC), but not N-acetylcysteine, could prevent the permeability response, and was still effective when added within 2 h after LPS-conditioned plasma. The TNF-α/IL-1β signal present in LPS-conditioned plasma appears to increase endothelial permeability through intracellular pathways that very likely involve the activation of NF-κB. Although poststimulatory inhibition of the permeability response proves to be possible with agents such as PDTC, the window of opportunity appears very small if placed in a clinical perspective.
Keywords: endotoxin, HUVEC, cytokines, antioxidants, permeability
Introduction
During severe sepsis and shock, endothelial barrier dysfunction causes massive leakage of fluid and components from the circulation into the surrounding tissue (systemic oedema), severe hypotension, and organ hypoperfusion. These serious conditions may lead to the multiple organ dysfunction syndrome (MODS), which causes substantial mortality among septic patients [1]. The critical role of the endothelial barrier in the pathogenesis of Gram-negative sepsis and associated syndromes thus has made it a valid target for prevention and treatment [2,3].
Bacterial lipopolysaccharide (LPS), and in particular its lipid A component, appears to be a key initiator of endothelial barrier dysfunction during sepsis. After exposure to LPS, endothelial cells undergo several functional and morphological changes, such as cell contraction, disruption of interendothelial junctions, and loss of focal contacts with the underlying extracellular matrix [4]. In addition, it has been demonstrated that LPS may also induce distinct apoptotic signalling pathways in endothelial cells, which would disrupt the integrity of the endothelial barrier [5]. These direct actions require relatively high concentrations of LPS that have been infrequently observed in septic patients. The endothelial response to smaller amounts of LPS presumably occurs through the production of pro-inflammatory cytokines and other mediators produced by blood monocytes and tissue macrophages [6], after activation of their Toll-like receptors [7].
The effect of LPS on endothelial permeability is widely studied in various animal models of sepsis [8,9], but human studies are few and limited to very low LPS doses [10]. However, human whole blood treated ex vivo with LPS constitutes a relevant pool of cytokines and other inflammatory mediators, and remains a valuable and common tool in sepsis-related research [11,12]. Addition of plasma obtained from LPS-treated whole blood (and further referred to as ‘LPS-conditioned plasma’) to monolayers of cultured human umbilical venular endothelial cells (HUVEC) increases their permeability [13] and their expression of cell adhesion molecules (CAMS) [14]. This system can be considered as an in vitro model for the compromised endothelial (barrier) function observed in septic patients. As such, it allows investigations into the efficacy of various agents to prevent or reduce the plasma-induced permeability. In a prior experiment it was established that treatment of LPS-conditioned plasma with excess antibodies against both tumour necrosis factor (TNF)-α and interleukin (IL)-1β, prior to its incubation on HUVEC, can prevent the permeability increase otherwise observed [13]. This raises the question if intervention at a later stage, i.e. after addition of LPS-conditioned plasma to the cell layer, would also be effective. In this respect, not only the possible effect of specific antibodies is of interest but also that of agents that act against the intracellular pathways induced by TNF-α and/or IL-1β. This particularly targets nuclear factor (NF)-κB, a cytokine inducible transcription factor that is involved in the regulation of various pro-inflammatory genes, and that has been recognized as a treatment option for sepsis [15,16]. It was demonstrated recently that pyrollidine di-thiocarbamate (PDTC), an agent that supposedly interferes with the activation of NF-κB, can modulate CAM expression on endothelial cells after induction by LPS-conditioned plasma [14]. A protective effect of PDTC has also been demonstrated in vivo in LPS-treated rats, where it prevents increases in microvascular permeability [17].
In the present study, we have investigated the time course of the permeability response of HUVEC monolayers to LPS-conditioned plasma and the possibility that apoptosis is a mechanism which contributes to this phenomenon. Subsequently, we have examined the potential of both antibodies against TNF-α and IL-1β, and of PDTC to modulate the permeability response after addition of LPS-conditioned plasma to the endothelial monolayer.
Materials and methods
Materials
Culture medium M199 (containing 25 mM HEPES, Earl's salts, and L-Glutamate) and heat-inactivated newborn calf serum, penicillin-streptomycin, and trypsin/EDTA were obtained from Life Technologies (Paisley, UK). Heat-inactivated normal human serum was purchased from ICN (Costa Mesa, CA, USA). A crude fraction of endothelial cell growth factors (ECGF) was extracted from calf brain, and kindly provided by the Department of Paediatrics, University Medical Centre Nijmegen, the Netherlands. Culture flasks, dishes, and multiwell tissue culture inserts containing collagen precoated PTFE-membranes (Transwell-COL, 0·4 µM pore diameter, ± 1 cm2 growth area) were obtained via Corning B.V. Life Sciences, Schiphol-Rijk, the Netherlands. Neutralizing antibodies to human TNF-α and IL-1β (clone 1825 and 8516, respectively) were obtained from R & D Systems, Abingdon, UK. According to the manufacturer, 100 ng/ml of anti-IL-1β and anti-TNF-α completely neutralized the bioactivity of 50 and 250 pg/ml of recombinant human IL-1β and TNF-α, respectively.
Preparation of microporous membranes and cell seeding
Endothelial cells were isolated from umbilical cords as described previously [18]. Approximately 105 HUVEC/cm2 in 0·5 ml of serum-completed medium were seeded at the upper (or luminal) side of the tissue culture inserts, while 1·5 ml of medium was added to the lower (or abluminal) compartment of the 12-well culture dishes. Both compartments were frequently replenished with fresh culture medium. Cultures were grown for five days, when confluence was confirmed through phase contrast microscopy.
Incubation of monolayers with LPS conditioned plasma
LPS-conditioned plasma was prepared as described previously [13]. Briefly, E. coli LPS (055:B5) was added to heparinized human whole blood in a final concentration of 1 ng/ml and incubated for 18 h at 37 °C. Plasma was diluted to 20% v/v in culture medium without serum components, and incubated at 37 °C on the luminal side of the tissue culture insert. In experiments where the time course of the permeability response was investigated, incubation with LPS-conditioned plasma was preceded or followed by incubation with untreated control plasma of the same donor, at different time intervals. Plasma of the same donors was used throughout the experiments. Our previous study demonstrated that the response of HUVEC to plasma obtained from these donors was essentially similar [14].
Intervention studies
Neutralizing antibodies to human TNF-α and IL-1β were diluted in M199 and added directly to the cultures at various time points after the addition of LPS-conditioned plasma, at a final concentration of 1 mg/ml. In addition, LPS-conditioned plasma was pre-incubated with these neutralizing antibodies for 1 h at 37 °C, prior to the incubation of the mixture on the cultures, and served as a control.
The following chemical compounds were tested individually on the capacity to inhibit plasma-induced endothelial permeability: Pyrollidine dithiocarbamate (PDTC), N-acetylcysteine (NAC), both obtained from Sigma, and benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD-fmk, R & D systems, Abingdon, UK). These compounds were diluted in complete medium and either pre-incubated on the cultures for 1 h prior to the addition of LPS-conditioned plasma, or added together with LPS-conditioned plasma. In case of significant inhibitory effects of pre- or co-incubation of these agents, intervention studies were performed where the compound was added to the cultures at various time points after the stimulation with LPS-conditioned plasma.
Permeability measurement
For detection of macromolecular passage across the Transwell culture inserts, a tracer solution containing FITC-BSA (250 µg/ml) was prepared in complete medium. At the upper side of the monolayer, culture medium was replaced by 0·5 ml of the tracer solution. After 1 and 2 h of incubation, duplicate 100 µl samples were drawn from the lower compartment and transferred to 96-well microtiter plates. For the detection of FITC-BSA, microtiter plates were read on a Wallac Victor II plate reader (Perkin Elmer, Boston, MA, USA) at emission and excitation wavelengths of 490 and 520 nm, respectively. In general, samples drawn 2 h after the start of the assay showed less variation in the concentration of FITC-BSA than samples drawn after 1 h. In order to allow quantitative comparison, the 2 h data are presented, and expressed as relative permeability (with respect to a control stimulus run in each individual experiment).
Apoptosis assay
For the detection of endothelial cell death HUVEC cultured on gelatinized 6-well dishes were analysed for Annexin-V-FITC binding using a previously described method [19]. Briefly, after incubation with stimulus, a pool of cells that contained both detached and (trysinized) adherent cells was incubated in the dark with 1% v/v FITC-conjugated Annexin-V (2 µg/ml, Bender Medsystems, Vienna, Austria) and 10% v/v propidium iodide (PI, 100 µg/ml, Calbiochem) for 10 min on ice. Cells were analysed on a Coulter® Epics® XL-MCL cell sorter. Necrotic cells were defined as cells demonstrating positive staining for both Annexin-V-FITC and PI, while apoptotic cells were Annexin-V-FITC-positive and PI-negative. Viable cells were defined as double negative for both stains. Fluorescence of 5000 events per sample was expressed on a double parameter histogram using log scales.
Cell death detection assay
A cell death detection ELISA (Roche Diagnostics GmbH, Mannheim, Germany) that determines cytoplasmic histone-associated DNA fragments was performed on plasma-stimulated and control HUVEC cultured on gelatinized 96-well microtiter plates according to the manufacturers manual, and absorbance of the samples was read at 405 nm on a Bio-Rad microtiter plate reader.
Statistical analysis
Comparison of different treatments and differences between stimulated and control cultures were analysed by one-way anova followed by Tukey and Dunnett Multiple Comparisons Test, respectively, using GraphPad Prism version 4·00 for Windows, GraphPad™ Software, San Diego, CA, USA.
Results
Effect of LPS-conditioned plasma on endothelial permeability
Under nonstimulated conditions (i.e. incubation with untreated control plasma), over a 2-h period the HUVEC monolayers allowed the passage of FITC-BSA from the upper compartment (250 µg/ml) into the lower compartment to a concentration of 4·1 ± 0·4 µg/ml (range 1·6–7·7 µg/ml), or 6·6% of the calculated equilibrium concentration. Incubation with plasma obtained from whole blood that was treated with 1 ng/ml of LPS resulted in the generation of plasma that always induced a significant increase (on the average 80%) of the FITC-BSA concentration in the lower compartment. However, between the experiments considerable variations existed in the amount of FITC-BSA that passed the monolayer both under basal and stimulated conditions. In order to allow comparison between multiple experiments, in each experiment the response to unstimulated control plasma and LPS-conditioned plasma was set at 0 and 100%, respectively.
Time course of the permeability response to LPS-conditioned plasma
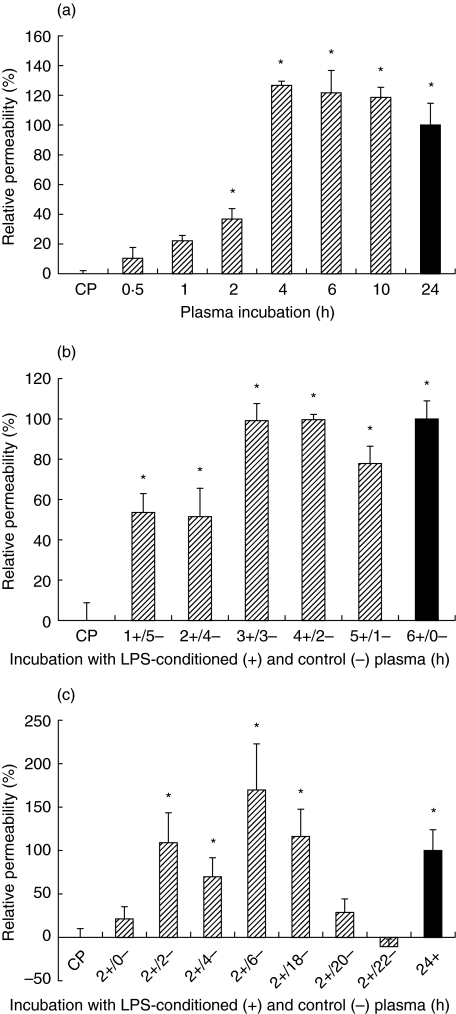
LPS-conditioned plasma was added to the HUVEC monolayer for periods of 0·5–24 h, immediately prior to the permeability analysis (Fig. 1a). Incubations for 2 h induced a significant permeability increase, while more than 4 h was required to reach maximum permeability.
Fig. 1.
Stimulation of endothelial permeability by LPS-conditioned plasma. HUVEC monolayers are incubated with LPS-conditioned plasma and albumin permeability is expressed relative to that induced under reference conditions (▪). Data represent mean ± sem (n = 4) (a) Effect of increasing incubation times. (b) Effect of replacing LPS-conditioned plasma with control plasma after various time periods. (c) Effect of increasing incubation time with control plasma after a 2 h incubation with LPS-conditioned plasma. *P < 0·05 versus incubation with control plasma (CP) alone.
In a second experiment, LPS-conditioned plasma was added and subsequently replaced by control plasma at different time intervals over a 6-h period, prior to the permeability assay (Fig. 1b). If compared to continuous exposure for 6 h, replacement of LPS-conditioned plasma after 1 h or 2 h with control plasma resulted in a half-maximal, though significant, rise in the permeability. Replacement of LPS-conditioned at or later than 3 h after the start of the incubation did not affect the permeability response.
Finally, endothelial cultures were exposed to a submaximal 2-h stimulus with LPS-conditioned plasma followed by incubation with control plasma for extended periods, to a maximum of 22 h, prior to the permeability assay. As shown in Fig. 1c, the permeability increase to a 2 h stimulus with LPS-conditioned plasma reached maximum levels approximately 6 h later. Interestingly, in the later stages the permeability response gradually decreased over time toward control levels.
Role of apoptosis
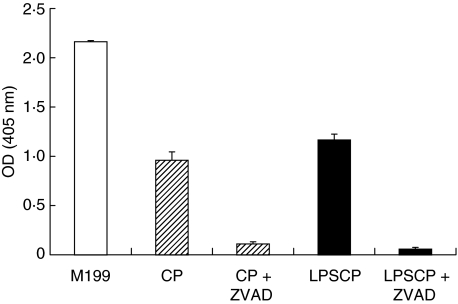
While serum starvation induced a marked increase of double-positive (dead) and Annexin-V only positive (apoptotic) cells, endothelial cultures exposed to LPS-conditioned plasma did not reveal increased numbers of Annexin-V- and propidium iodide-positive cells if compared to cultures exposed to untreated control plasma (Table 1). Cell death was also analysed by an ELISA which measures the amount of cytoplasmic histone-bound DNA fragments (Fig. 2). Again, cell cultures exposed to serum-free medium showed a high level of cell death. Lower, but still highly significant, levels of histone-bound DNA fragments were generated by incubation with either control plasma (OD 0·96 ± 0·08) or LPS-conditioned plasma (OD 1·17 ± 0·06). The generation of cytoplasmic DNA in both control and stimulated cultures was prevented almost completely by the presence of the apoptosis inhibitor Z-VAD-fmk.
Table 1.
Effect of LPS-conditioned plasma on cell death of HUVEC.
| Dead (%) | Apoptotic (%) | |||
|---|---|---|---|---|
| Exp 1 | Exp 2 | Exp 1 | Exp 2 | |
| Serum free medium | 29 | 40 | 21 | 38 |
| Culture medium | 12 | 12 | 13 | 13 |
| Control plasma | 5 | 6 | 16 | 19 |
| LPS-conditioned plasma | 4 | 4 | 13 | 14 |
Cells were incubated for 6 h with various media prior to analysis with flow cytometry. Data are derived from two independent experiments and represent the relative amount of positive cells (5000 events).
Fig. 2.
Endothelial cell death after exposure to LPS-conditioned plasma. Data represent the absorbance (OD) value (n = 2) as a measure of cytoplasmic histone-bound DNA fragments in HUVEC cultures incubated for 6 h with serum-free medium (M199), with 20% control plasma (CP), and with 20% LPS-conditioned plasma (LPSCP). Cells were also pre-incubated with the apoptosis-inhibitor Z-VAD-fmk.
Intervention studies with antibodies against IL-1β and TNF-α
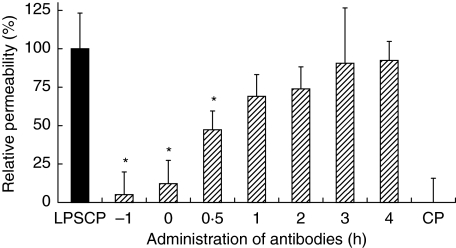
The permeability increase, induced by incubation with LPS-conditioned plasma, could almost be completely prevented if the plasma was pre-incubated with antibodies against both TNF-α and IL-1β (Fig. 3). If the antibodies were added to the culture medium 30 min after LPS-conditioned plasma, the increase in permeability could still be significantly reduced, to 47% (P< 0·05) of the maximal response. If the antibodies were added at a later time point, average permeability was still somewhat reduced, although not significantly so.
Fig. 3.
Stimulation of endothelial permeability by LPS-conditioned plasma: prevention by antibodies against TNF-α and IL-1β. HUVEC monolayers are incubated with LPS-conditioned plasma (LPSCP) for 6 h and at various time points both antibodies are added. Data (mean ± sem, n = 8) depict the permeability relative to that induced by LPSCP (▪) alone (= 100%) and to that induced by control plasma (CP, 0%). *P < 0·05 versus LPSCP.
Effects of inhibitors of apoptosis and intracellular signalling pathways
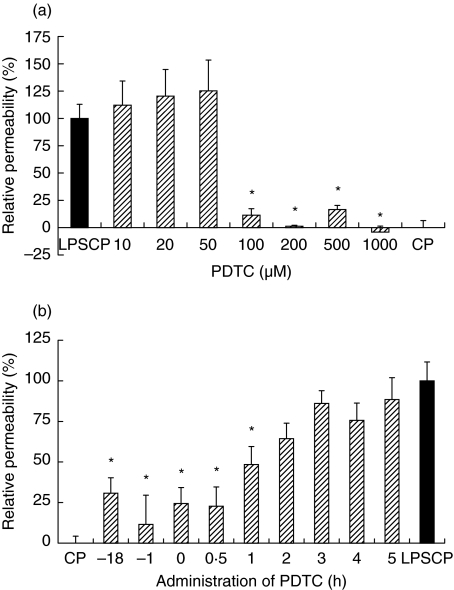
Pre- and co-incubation with the apoptosis inhibitor Z-VAD-fmk (100 µM) or the antioxidant NAC (100 µM) did not affect the permeability response induced by LPS-conditioned plasma (data not shown). On the other hand, the addition of PDTC at a final concentration of 100 µM or more to endothelial monolayers prior to stimulation by LPS-conditioned plasma almost completely prevented the permeability response (Fig. 4a). Addition of PDTC (200 µM) to cultures up to 1 h after the stimulation with LPS-conditioned plasma significantly reduced the permeability response to approximately 48%, while later additions of PDTC resulted in nonsignificant reductions of the permeability response, ranging from 64 to 88% when compared to the effect induced by LPS-conditioned plasma alone (Fig. 4b).
Fig. 4.
Effect of PDTC on endothelial permeability induced by LPS-conditioned plasma. Data (mean ± sem, n = 8) depict the permeability relative to that induced by LPS-conditioned plasma (LPSCP, black bars) alone (100% reflects increase versus incubation with control plasma, CP). (a) PDTC (10–103 µM) is added to the to the cultures 1 h prior to LPSCP. (b) Effect of administration of 200 µM PDTC at various time points in relation to the addition of LPSCP (at 0 h). *P < 0·05 versus LPSCP.
Discussion
Model investigations into mediator-induced endothelial permeability may help understand the pathways and indicate means for prevention of systemic oedema and shock in septic patients. Such investigations require the exposure of endothelial cells to a relevant pool of inflammatory mediators, rather than to single molecules. So far, in most in vitro studies where endothelial permeability has been examined, the permeability response was measured after stimulation with single inflammatory mediators, including LPS [4] and TNF-α [20], but seldom a relevant combination of these mediators, such as present in LPS-stimulated whole blood. Furthermore, while examination of the time course of the permeability response to individual mediators, such as LPS or cytokines has been quite superficial, it has not previously been studied in the case of LPS-stimulated whole blood. The latter system has been used widely as a model to investigate the production of inflammatory mediators during sepsis [21].
At least 2 h of continuous exposure to LPS-conditioned plasma is required to induce a significant increase of endothelial monolayer permeability, and it reaches maximum levels 4–6 h after the start of the incubation. This result corresponds quite well to observations in studies where LPS was added alone to cultured endothelial cells [22,23] and to studies where LPS was infused in vivo in animals [24]. However, a significant permeability increase has not been reported in studies where healthy volunteers were exposed to single infusions of LPS [25]. This apparent discrepancy may be explained by the fact that human studies only allowed the administration of low (and often single) doses of LPS, in contrast to the lethal doses often used in animals. It is conceivable that infusion with (single) low doses of LPS results in a short and submaximal raise in the plasma levels of various cytokines and other inflammatory host mediators, but insufficient to alter endothelial barrier function. In our model, short exposures (< 2 h) to LPS-conditioned plasma (followed by incubation with control plasma) did not significantly increase the permeability, which agrees with the in vivo observations in humans. It has been shown that direct stimulation of endothelial permeability requires up to several ng of LPS [18,26], depending on the species and origin of the endothelial cells. In our experiments, a direct effect of LPS on the permeability seems unlikely, since the estimated amount of LPS in the plasma dilutions is approximately 0·2 ng, and maybe even lower, since LPS was pre-incubated for 18 h in whole blood, prior to the addition of plasma to the endothelial cultures.
The data also indicate that cell death or apoptosis does not to play an important role in the plasma-induced permeability increase. In fact, a significant and similar apoptotic signal was present in both control and stimulated HUVEC, and pre-incubation with the caspase-inhibitor Z-VAD-fmk blocked apoptosis in both cultures to an equal degree. Unsurprisingly, apoptosis, which is a common feature of primary cell cultures such as HUVEC, is found to occur here. However, the observation that stimulated cultures do not show an increased number of apoptotic cells is somewhat surprising, since the major mediators of increased permeability (e.g. IL-1β, TNF-α and LPS) have also proven to be at least partially involved in the induction of apoptosis signalling pathways [27]. We have indeed observed an increased number of detached cells in HUVEC cultures that were stimulated with plasma obtained from LPS-treated (10 µg/ml) whole blood (unpublished observations). This suggests that LPS-conditioned plasma at least can influence the growth and turnover of HUVEC cultures to a certain extent. However, in the experiments presented here a 104-fold lower amount of LPS was used.
In a prior experiment we have shown that LPS needs to be incubated in whole blood for at least 2 h in order to generate plasma that enhances endothelial permeability [13]. Here, it is demonstrated that at least another 2 h of incubation of endothelial cells with such plasma is required to significantly enhance the permeability, and that it takes an additional 4–6 h to achieve a maximum permeability response. In theory, this would leave, at the very most, approximately 8–10 h after the first encounter with LPS to successfully interfere with its actions.
Previously, we have reported that inhibition of the plasma-induced permeability increase required the pre-incubation of neutralizing antibodies against both IL-β and TNF-α in LPS-conditioned plasma, prior to its addition to the endothelial monolayer [13]. The present data show that a partial inhibition of the permeability response remains possible when these antibodies are added to HUVEC within 1 h after addition of LPS-conditioned plasma. Apparently, during this relatively short period, the antibodies can still prevent to some extent the binding of the inflammatory cytokines to their receptors on the endothelial cells.
Several other agents that are supposed to interfere with the intracellular signals generated by LPS, IL-1β or TNF-α, were tested on the capacity to inhibit the plasma-induced permeability increase. The fact that pre- or co-incubation of HUVEC with ZVAD-fmk did not prevent the permeability response to LPS-conditioned plasma supports the conclusion that apoptosis of HUVEC is not the mechanism responsible.
The decisive role of TNF-α and IL-1β suggest that activation of NF-kB may be involved in the mediation of the permeability response. At higher concentrations, the antioxidant PDTC, which supposedly inhibits the activation of NF-κB, appears to be capable of reducing the plasma-induced permeability response, and was effective even if added to the endothelial cells after LPS-conditioned plasma. PDTC has been reported to reduce the LPS-induced and NF-κB-mediated expression of endothelial tissue factor [28]in vitro and to reduce mortality in a rat model for endotoxin-induced shock [29]. We have previously demonstrated that PDTC also reduces the expression of E-Selectin on endothelial cells that were stimulated with LPS-conditioned plasma [14]. Together these findings support the idea that NF-κB-activation constitutes a key mechanism in the mediation of endothelial inflammation and permeability.
We could not demonstrate a protective effect of the antioxidant NAC on endothelial permeability, either added prior to or after LPS-conditioned plasma. Like PDTC, NAC inhibits the formation of intracellular reactive oxygen species, and may subsequently prevent the activation of NF-κB in endothelial cells [30]. However, it has also been shown that at higher concentrations of LPS, NAC does not prevent endothelial cell activation [31]. It has even been suggested that the inhibitory effect of PDTC on NF-κB activation in endothelial cells may be reversed by NAC [32].
In conclusion, it has been shown that there exist several possibilities for poststimulatory intervention in the development of the LPS-induced, plasma-mediated, increase in endothelial permeability. Further exploration of the IL-1β and TNF-α-dependent intracellular pathways may be helpful to diminish or prevent the consequences of generalized capillary leakage observed in sepsis. However, the data indicate the presence of only a relatively small window of opportunity where effective intervention is possible. The latter finding, together with the fact that in patients the actual onset moment of sepsis remains mostly unknown, raises serious questions as to the clinical feasibility of such a strategy.
Acknowledgments
This work was financially supported by a PhD grant from the Radboud University Nijmegen, the Netherlands.
References
- 1.Brun BC. The epidemiology of the systemic inflammatory response. Intens Care Med. 2000;26:S64–S74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volk T, Kox WJ. Endothelium function in sepsis. Inflamm Res. 2000;49:185–98. doi: 10.1007/s000110050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–77. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman DD, Goldblum SE. Direct effects of endotoxin on the endothelium. barrier function and injury. Laboratory Invest. 1999;79:1181–99. [PubMed] [Google Scholar]
- 5.Hull C, McLean G, Wong F, Duriez PJ, Karsan A. Lipopolysaccharide signals an endothelial apoptosis pathway through TNF receptor-associated factor 6-mediated activation of c-Jun NH2-terminal kinase. J Immunol. 2002;169:2611–8. doi: 10.4049/jimmunol.169.5.2611. [DOI] [PubMed] [Google Scholar]
- 6.Pugin J, Ulevitch RJ, Tobias PS. Activation of endothelial cells by endotoxin. direct versus indirect pathways and the role of CD14. Prog Clin Biol Res. 1995;392:369–73. [PubMed] [Google Scholar]
- 7.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 8.Gao X, Xu N, Sekosan M, et al. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J Immunol. 2001;167:2895–901. doi: 10.4049/jimmunol.167.5.2895. [DOI] [PubMed] [Google Scholar]
- 9.Holbeck S, Grande PO. Endotoxin increases both protein and fluid microvascular permeability in cat skeletal muscle. Crit Care Med. 2003;31:560–5. doi: 10.1097/01.CCM.0000048620.88344.70. [DOI] [PubMed] [Google Scholar]
- 10.Renckens R, Weijer S, de Vos AF, et al. Inhibition of plasmin activity by tranexamic acid does not influence inflammatory pathways during human endotoxemia. Arterioscler Thromb Vasc Biol. 2004;24:483–8. doi: 10.1161/01.ATV.0000118280.95422.48. [DOI] [PubMed] [Google Scholar]
- 11.Mathiak G, Kabir K, Grass G, et al. Lipopolysaccharides from different bacterial sources elicit disparate cytokine responses in whole blood assays. Int J Mol Med. 2003;11:41–4. [PubMed] [Google Scholar]
- 12.Wurfel MM, Park WY, Radella F, et al. Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J Immunol. 2005;175:2570–8. doi: 10.4049/jimmunol.175.4.2570. [DOI] [PubMed] [Google Scholar]
- 13.Nooteboom A, van der Linden CJ, Hendriks T. Tumor Necrosis Factor-α and Interleukin-1β mediate endothelial permeability induced by lipopolysaccharide stimulated whole blood. Crit Care Med. 2002;30:2063–8. doi: 10.1097/00003246-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Nooteboom A, van der Linden CJ, Hendriks T. Modulation of adhesion molecule expression on endothelial cells after induction by lipopolysaccharide-stimulated whole blood. Scand J Immunol. 2004;59:440–8. doi: 10.1111/j.0300-9475.2004.01413.x. [DOI] [PubMed] [Google Scholar]
- 15.Joshi AR, Chung CS, Song GY, Lomas J, Priester RA, Ayala A. NF-κB activation has tissue-specific effects on immune cell apoptosis during polymicrobial sepsis. Shock. 2002;18:380–6. doi: 10.1097/00024382-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Paterson RL, Galley HF, Dhillon JK, Webster NR. Increased nuclear factor κB activation in critically ill patients who die. Crit Care Med. 2000;28:1047–51. doi: 10.1097/00003246-200004000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Liu SF, Ye X, Malik AB. Pyrrolidine dithiocarbamate prevents I-κB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol Pharmacol. 1999;55:658–67. [PubMed] [Google Scholar]
- 18.Nooteboom A, Hendriks T, Otteholler I, van der Linden CJ. Permeability characteristics of human endothelial monolayers seeded on different extracellular matrix proteins. Med Inflamm. 2000;9:235–41. doi: 10.1080/09629350020025755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pijpers AH, van Setten PA, van den Heuvel LP, et al. Verocytotoxin-induced apoptosis of human microvascular endothelial cells. J Am Soc Nephrol. 2001;12:767–78. doi: 10.1681/ASN.V124767. [DOI] [PubMed] [Google Scholar]
- 20.Royall JA, Berkow RL, Beckman JS, Cunningham MK, Matalon S, Freeman BA. Tumor necrosis factor and interleukin 1α increase vascular endothelial permeability. Am J Physiol. 1989;257:L399–L410. doi: 10.1152/ajplung.1989.257.6.L399. [DOI] [PubMed] [Google Scholar]
- 21.Foca A, Berlinghieri MC, Barreca GS, et al. Kinetics of IL-8, MIP-1α, TNFα, IL-1β, IL-1ra and IL-10 in human whole blood samples triggered by smooth and rough LPS. New Microbiol. 1998;21:123–30. [PubMed] [Google Scholar]
- 22.Goldblum SE, Brann TW, Ding X, Pugin J, Tobias PS. Lipopolysaccharide (LPS)-binding protein and soluble CD14 function as accessory molecules for LPS-induced changes in endothelial barrier function, in vitro. J Clin Invest. 1994;93:692–702. doi: 10.1172/JCI117022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–80. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- 24.Boughton-Smith NK, Evans SM, Laszlo F, Whittle BJ, Moncada S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br J Pharmacol. 1993;110:1189–95. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrell R. Human responses to bacterial endotoxin. Circ Shock. 1994;43:137–53. [PubMed] [Google Scholar]
- 26.Goldblum SE, Ding X, Brann TW, Campbell WJ. Bacterial lipopolysaccharide induces actin reorganization, intercellular gap formation, and endothelial barrier dysfunction in pulmonary vascular endothelial cells. concurrent F-actin depolymerization and new actin synthesis. J Cell Physiol. 1993;157:13–23. doi: 10.1002/jcp.1041570103. [DOI] [PubMed] [Google Scholar]
- 27.Messmer UK, Briner VA, Pfeilschifter J. Basic fibroblast growth factor selectively enhances TNF-α-induced apoptotic cell death in glomerular endothelial cells: effects on apoptotic signaling pathways. J Am Soc Nephrol. 2000;11:2199–211. doi: 10.1681/ASN.V11122199. [DOI] [PubMed] [Google Scholar]
- 28.Orthner CL, Rodgers GM, Fitzgerald LA. Pyrrolidine dithiocarbamate abrogates tissue factor (TF) expression by endothelial cells: evidence implicating nuclear factor-κB in TF induction by diverse agonists. Blood. 1995;86:436–43. [PubMed] [Google Scholar]
- 29.Meisner M, Schmidt J, Schywalsky M, Tschaikowsky K. Influence of pyrrolidine dithiocarbamate on the inflammatory response in macrophages and mouse endotoxin shock. Int J Immunopharmacol. 2000;22:83–90. doi: 10.1016/s0192-0561(99)00071-5. [DOI] [PubMed] [Google Scholar]
- 30.Rahman A, Kefer J, Bando M, Niles WD, Malik AB. E-selectin expression in human endothelial cells by TNF-α-induced oxidant generation and NF-κB activation. Am J Physiol. 1998;275:L533–L544. doi: 10.1152/ajplung.1998.275.3.L533. [DOI] [PubMed] [Google Scholar]
- 31.Chan EL, Murphy JT. Reactive oxygen species mediate endotoxin-induced human dermal endothelial NF-κB activation. J Surg Res. 2003;111:120–6. doi: 10.1016/s0022-4804(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Kim JH, Lee J, Hsu CY, Ahn YS. Thiol antioxidant reversal of pyrrolidine dithiocarbamate-induced reciprocal regulation of AP-1 and NF-κB. Biol Chem. 2003;384:143–50. doi: 10.1515/BC.2003.015. [DOI] [PubMed] [Google Scholar]