Abstract
Critical interactions between the nervous system and the immune system during experimental autoimmune myasthenia gravis (EAMG) were examined in an animal model for human MG after immunization of adult female Lewis rats with Torpedo acetylcholine receptor (AChR) and complete Freund's adjuvant. Immunized rats depicted marked clinical severity of the disease. Using enzyme-linked immunospot (ELISPOT) assay and in situ hybridization techniques, immune responses in these animals were examined and showed elevated numbers of anti-AChR IgG secreting B cells and AChR reactive interferon (IFN)-γ-secreting cells, enhanced mRNA expression of the proinflammatory cytokines IFN-γ and tumour necrosis factor (TNF)-α as Th1 subset and the anti-inflammatory cytokines interleukin (IL)-4 and IL-10 as a Th2 subset, and transforming growth factor (TGF)-β as a Th3 cytokine. Corticosterone and prostaglandin E2 (PGE2) levels were measured by radioimmunoassay and illustrated increased production after immunization. Surgical denervation of the spleen reduced significantly the clinical severity of the disease, suppressed the numbers of IgG and IFN-γ-secreting cells, down-regulated the mRNA expression for cytokines and reduced corticosterone and PGE2 production. As controls, sham-operated rats were used and showed results as the EAMG non-denervated control rats. The data present herein, and for the first time, substantial effects of the nervous system on immune responses that may influence the outcome of EAMG. These effects were not dependent on cytokine inhibitory mediators such as prostaglandins or stress hormones. IL-10 and TGF-β, the two potent immunosuppressive cytokines, were also suppressed, indicating a general suppression by splenic denervation. More investigations are initiated at our laboratories to understand the evident neural control over the immune system during challenges leading to the break of tolerance and development of autoimmunity, which may assist in innovative therapeutic approaches.
Keywords: cytokines, denervation, proliferation, MG, spleen
Introduction
Regulatory interactions between the central nervous system (CNS) and the immune system have now become apparent. Channels of communication between these two systems include noradrenergic sympathetic innervation of the primary and secondary lymphoid organs in addition to the presence of β- and α-adrenergic receptors on lymphocyte and other immunocompetent cells [1–3]. The spleen is an important lymphoid organ, involved in immune responses against all types of antigen that appear in the circulation. It is richly innervated by noradrenergic nerve fibres that enter the spleen and remain largely associated with the splenic vasculature, also entering the parenchyma. Sympathetic nerve activity was recorded from the splenic, but not renal, nerves of adult rats after intravenous injection of lipopolysaccharide (LPS), with an onset time of 17·1–23·5 min. These findings indicated that sympathetic outflow can be directed to an immune organ in response to a stimulus known to activate the immune system [4].
Myasthenia gravis (MG) is an autoimmune disease that is mediated by antibodies against the nicotinic acetylcholine receptor (AChR) of neuromuscular junction [5]. MG has its peak incidence in adults, and occurs rarely during infancy and childhood [6]. Immunization of certain species and strains of animals with AChR emulsified in complete Freund's adjuvant (CFA) results in experimental autoimmune myasthenia gravis (EAMG) [5,7]. Cytokines produced by both CD4 and CD8 T cells play a major regulatory role in the production of anti-AChR antibodies and influence the development of EAMG [8,9]. The triggering events of these cytokines may have already started at the site of immunization in the experimental model or by an immune-challenging product such as infections or other immunostimulatory factors. Thereby, a signal may be generated and transmitted to the spleen via the nerves leading to early immune system activation that may urge the evolving late sequelae. To test this hypothesis, the EAMG model was utilized in this work to study the effects of sympathetic denervation on potential immune responses and on the clinical course of the disease.
Materials and methods
Antigen preparation
AChR was purified from electric organs of Torpedo Californica (Pacific Biomarine, Venice, CA, USA) by affinity chromatography with α-cobrotoxin-agarose (Sigma, St Louis, MO, USA) [10]. Myelin basic protein (MBP) was purified from bovine spinal cord [11]. Purity was assessed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE).
Induction of EAMG and clinical assessment
Female Lewis rats, 10 weeks old, body weight 170–180 g, were purchased from Harlan CPB, Zeist, the Netherlands. Each rat was injected subcutaneously into both hind footpads and base of tail with 50 µg Torpedo AChR emulsified in CFA in a total volume of 200 µl. The clinical severity of EAMG was graded blindly [12,13] as follows: 0, no weakness; 1+, mild decreased activity, weak grip or cry, with fatigue; 2+, marked decreased activity and body weight, hunched posture at rest, head down and forelimb digits flexed, tremulous ambulation; and 3 +, severe generalized weakness, no cry or grip. Rats were killed at day 50 post-immunization (p.i.). In every experimental group six animals were included. Furthermore, in each experiment another 12 animals were used as non-immunized control (six denervated and six non-denervated).
Surgical denervation of the spleen and sham operation
The rats were weighed before surgery and given pentobarbital sodium (50 mg/kg) for anaesthesia. A 5-cm cut was made longitudinally in the middle of the operating area to expose the abdominal cavity. The gastrosplenic ligament was cut. Because the coeliac nerve runs along the coeliac artery, which arises from the abdominal aorta, the coeliac artery was traced back to its origin by following its branches to spleen. The coeliac nerve branches into the gastric nerve and splenic nerve. The splenic nerve was isolated from the splenic vasculature and connective tissue near the bifurcation of the coeliac artery and then the entire bundle of the nerve was cut. Immediately after surgery, each animal received an intraperitoneal injection of sulphadimethoxide (100 mg) and was then returned to the colony. After the operation, animals were kept for 7 days before the experimental immunization. For each animal, the surgical operation lasted about 20–30 min. Sham-operated control rats were manipulated similarly, but without sympathectomy. The animals were infected at 7 days after surgery. Successful denervation was confirmed by immunochemical staining of the cut-off nerve with monoclonal anti-rat tyrosine hydroxylase (TH) antibody, which is a specific marker for noradrenergic (NE) nerve fibre.
Enumeration of anti-AChR IgG antibody secreting cells
Splenocyte suspensions were prepared and then adjusted to a cell concentration of 2 × 106/ml. A solid-phase enzyme-linked immunospot (ELISPOT) assay was used with some modifications [14]. Wells were coated with 100 µl AChR or MBP [10 µg/ml in phosphate-buffered saline (PBS)]. Aliquots of 100 µl cell suspension were added in triplicate to individual wells. The wells were emptied after 24 h of incubation, followed by addition of rabbit anti-rat IgG (Sigma), biotinylated swine anti-rabbit IgG (Dakopatts, Copenhagen, Denmark) and avidin–biotin peroxidase complex (ABC, Dakopatts). After peroxidase staining, the red-brown immunospots which corresponded to cells that had secreted anti-AChR IgG were counted and standardized to numbers per 105 splenocytes.
AChR-reactive IFN-γ secreting cells
Nitrocellulose-bottomed microtitre plates were coated with 100 µl rat interferon (IFN)-γ capture antibody DB1 [15] at 15 µg/ml in PBS. Aliquots of 200 µl suspensions containing 4 × 105 splenocytes were added to individual wells in triplicate, followed by antigen (AChR or MBP) or the mitogen concanavalin A (Con A; Sigma) in 10 µl aliquots to a final concentration of 10 µg/ml AChR or MBP, or 5 µg/ml Con A. The wells were emptied after 48 h of culture. The secreted and bound IFN-γ was visualized by sequential application of polyclonal rabbit anti-rat IFN-γ[16], biotinylated swine anti-rabbit IgG (Dakopatts) and ABC (Dakopatts). After peroxidase staining, the red-brown immunospots which corresponded to cells that had secreted IFN-γ were enumerated in a dissection microscope. The data were expressed as numbers per 105 splenocytes.
Detection of cytokine mRNA expression by in situ hybridization
In situ hybridization was performed as described previously [17]. Briefly, 200 µl aliquots of suspensions containing 4 × 105 splenocytes were plated into round-bottomed microtitre plates (Nunc, Roskilde, Denmark) in triplicate. Ten-µl aliquots of AChR, MBP or Con A were added into appropriate wells at the final concentration of 10 µg/ml (AChR or MBP) or 5 µg/ml Con A. After culture for 24 h, the cells were washed, counted and applied onto restricted areas of electronically charged glass slides (ProbeOn slides; Fisher Scientific, Pittsburgh, PA, USA). Synthetic oligonucleotide probes (Scandinavian Gene Synthesis AB, Köping, Sweden) were labelled using [35S]-deoxyadenosine-5′-α-(thio)-triphosphate with terminal deoxynucleotidyl transferase (Amersham, Little Chalfont, UK). To increase the sensitivity of the method, a mixture of four different probes was employed for each cytokine. The oligonucleotide sequences were obtained from GenBank using MacVector software(Accelrys, Inc., San Diego, CA, USA) (Table 1). Cells were hybridized with 106 counts per minute (cpm) of labelled probe per 100 µl of hybridization mixture. After emulsion autoradiography, development and fixation, the coded slides were examined by dark field microscopy for positive cells containing more than 15 grains per cell in a star-like distribution. The intracellular distribution of the grains was always checked by light microscopy and expressed as numbers per 105 splenocytes. In many positive cells, the grains were so heavily accumulated within and around the cells that it was not possible to count every single grain. In cells judged negative, the numbers of grains were mainly 0–2 per cell, and the grains were scattered randomly over the cells and not distributed in a star-like fashion. There were therefore no difficulties in differentiating between positive and negative cells. Variation between duplicates was < 10%. A control probe used in parallel with the cytokine probe on cells from each rat revealed no positive cells.
Table 1.
The cytokine probes used in the study.
| Probes | Exon | GenBank accession No. | Complementary to bases |
|---|---|---|---|
| Rat IFN-γ | Exon 1 | M-29315–29317 | 298–345 |
| Exon 2 | 80–125 | ||
| Exon 3 | 303–350 | ||
| Exon 4 | 180–227 | ||
| Rat TNF-α | Exon 1 | M98820 | 639–686 |
| Exon 2 | 569–616 | ||
| Exon 3 | 278–342 | ||
| Exon 4 | 295–342 | ||
| Rat IL-4 | Exon 1 | X16058 | 83–130 |
| Exon 2 | 209–256 | ||
| Exon 3 | 270–317 | ||
| Exon 4 | 331–378 | ||
| Mouse IL-10 | Exon 1 | M37897 | 79–126 |
| Exon 2 | 134–181 | ||
| Exon 3 | 184–231 | ||
| Exon 4 | 402–449 | ||
| Human TGF-β | Exon 1 | X02812 | 1766–1813 |
| Exon 2 | 1953–2000 |
IFN: interferon; IL: interleukin; TGF: transforming growth factor; TNF: tumour necrosis factor.
[125I]-Radioimmune assay for prostaglandin E2 (PGE2)
Splenocytes were prepared and each sample was added in triplicate in 96-well plastic plates. Two hundred µl of culture medium per well containing 105 cells were incubated for 24 h and supernatants were collected thereafter. PGE2 in 100 µl of supernatant from each sample was measured using a PGE2[125I]-radioimmunoassay (RIA) kit (NEK-020, Du Medical Scandinavia, Sollentuna, Sweden), according to the manufacturer's instructions. The normalized percentage bound for each standard and sample was determined and each standard versus the corresponding amounts of PGE2 was plotted using logarithmic graph paper in picograms (pg).
Measurement of plasma corticosterone
Blood was collected from the rats at day 7 after the operation and just prior to the immunization and also on the following time-points after immunization. It was taken immediately after scarification by heart puncture and serum was separated for subsequent corticosterone determination. Corticosterone was determined by means of corticosterone radioimmunoassay kit according to protocols provided by the manufacturer (Amersham). Briefly, serum samples were incubated with the labelled hormone and the antibodies for 2 h at room temperature. The complex formed was precipitated by addition of second antibodies. Separation of the free and bound tracer was carried out by centrifugation for 15 min at 1500 g. The supernatant was aspirated and the resulting pellet was counted in a gamma counter. Corticosterone concentrations were calculated using a computer program and expressed as nanogram/millilitre (ng/ml).
Statistical analysis
Analysis of variance (anova) was used for multiple comparisons. Mann–Whitney's U-test was used to calculate levels of significance at **P< 0·001 and ***P< 0·0001.
Results
Confirmation of successful denervation
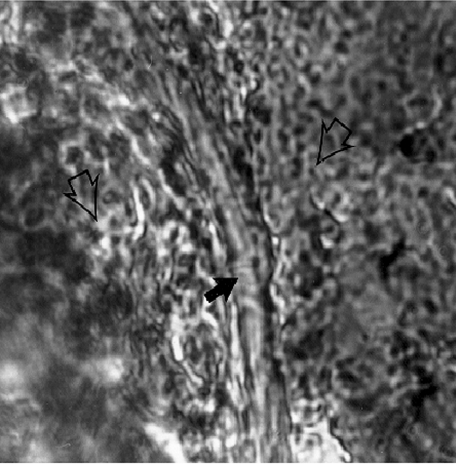
This was confirmed by immunochemical staining of the cut-off nerve with the anti-rat tyrosine hydroxylase-specific monoclonal antibody, as described in Materials and methods and shown in Fig. 1.
Fig. 1.
Confirmation of successful denervation by histological studies and immunostaining. Light microscopy (1000×) shows immunochemical staining of the cut-off nerve with monoclonal anti-rat tyrosine hydroxylase (TH) antibody, which is a specific marker for noradrenergic (NE) nerve fibre (solid arrow). Open arrow shows surrounding connective tissue from splenic vasculature.
Clinical signs of EAMG and effects of surgical denervation
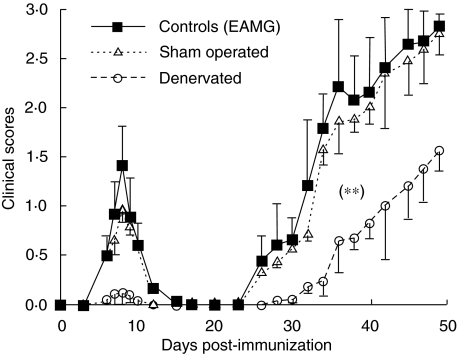
Nine of 10 adult Lewis rats exhibited early signs of muscular weakness around day 9 p.i. The weakness was moderately severe (2·0), transient, and disappeared after 12 days. Weakness recurred from 25 days p.i., and was progressive. Three of 10 adult Lewis rats died of severe generalized weakness over the observation period up to 7 weeks p.i. In contrast, splenic denervation abrogated the muscular weakness around day 9 p.i. However, 14 of 20 denervated rats began to develop slight muscular weakness around 35 days p.i., with very mild clinical scores (> 1·0) followed by a slight progression after day 40 p.i. The clinical scores on the denervated rats were significantly lower than those of the controls (non-denervated rats; P < 0·001) throughout the observation period up to 7 weeks p.i. Sham-operated rats did not show a significant difference in the clinical scores compared to the EAMG non-denervated controls (Fig. 2). Rats immunized with CFA only remained normal.
Fig. 2.
Clinical signs of experimental autoimmune myasthenia gravis (EAMG) and effects of surgical denervation. Clinical score changes in non-denervated (solid squares); denervated (open circles) and sham-operated (open triangles) female Lewis rats after immunization with acetylcholine receptor (AChR) + complete Freund's adjuvant (CFA). The clinical severity of EAMG was graded blindly as described in the Materials and methods section. Symbols refer to mean values, and bars to standard deviation. Denervated immunized rats had less clinical severity scores at all points when compared with non-denervated immunized controls (**P< 0·001). There was no statistical difference between sham-operated immunized rats and non-denervated immunized controls.
Effects of denervation on B cell responses assessed by anti-AChR IgG antibody secreting cells
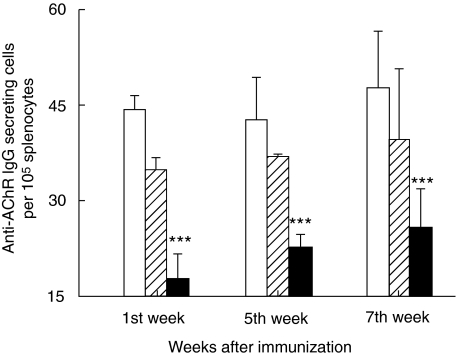
Gradually increasing levels of anti-AChR IgG antibody-secreting B cells were detected by ELISPOT on both EAMG non-denervated and sham-operated controls. The levels were significantly lower in the denervated rats when examined at 1, 5 and 7 weeks p.i. (P > 0·0001; Fig. 3). No significant statistical differences were found in experiments when cells were cultured with MBP or left without antigen (data not shown). Also, there was no correlation of disease with the anti-AChR antibody levels in the 14 (of 20) denervated rats (which had mild clinical disease) when compared to those remaining four disease-free rats.
Fig. 3.
Enumeration of anti-acetylcholine receptor (AChR) IgG antibody-secreting cells. The figure shows AChR IgG antibody-secreting cells per 105 splenocytes as measured by enzyme-linked immunospot (ELISPOT) assay. Splenocyte suspensions were prepared from non- denervated, denervated immunized and sham-operated female Lewis rats after immunization with AChR + complete Freund's adjuvant (CFA). The cells were then adjusted to a concentration of 2 × 106/ml. Wells were coated with 100 µl AChR [10 µg/ml in phosphate-buffered saline (PBS)]. Aliquots of 100 µl cell suspension were added in triplicate to individual wells and cultured for 24 h followed by immunostaining (for details see Materials and methods). At each time-point six animals were included in each group. Mean and standard deviations are shown. Stars denote statistical difference between denervated immunized rats (solid bars) and non-denervated immunized controls [open bars; experimental autoimmune myasthenia gravis (EAMG)] (P > 0·0001). There was no statistical difference between sham-operated immunized rats (hatched bars) and non-denervated immunized controls (open bars).
Effects of denervation on AChR-induced T cell responses assessed by IFN-γ production
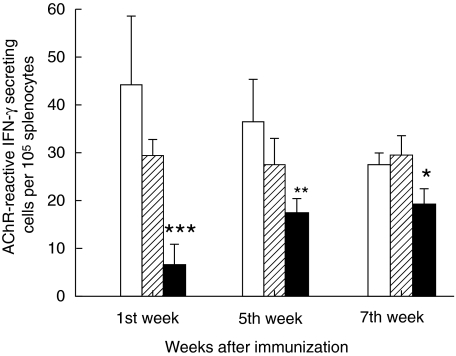
As shown in Fig. 4, denervated rats immunized with AChR + CFA had lower numbers of AChR-reactive IFN-γ-secreting T cells compared with adult EAMG non-denervated and sham-operated control rats (P= 0·0001 week 1, P > 0·001 week 2 and P > 0·01 week 3, respectively). Con-A was used as positive control and exhibited a high induction of IFN-γ when incubated in non-denervated, non-immunized, denervated non-immunized and sham-operated non-immunized cells. However, Con-A stimulation of immunized cells did not show statistical significance compared to similar stimulation of non-immunized cells. No differences were found in experiments when cells were cultured with MBP or left without antigen (data not shown).
Fig. 4.
Acetylcholine receptor (AChR)-reactive interferon (IFN)-γ-secreting cells. The figure illustrates AChR-reactive IFN-γ-secreting cells per 105 splenocytes as measured by enzyme-linked immunospot (ELISPOT) assay. Splenocyte suspensions were prepared from non- denervated, denervated and sham-operated female Lewis rats after immunization with AChR + complete Freund's adjuvant (CFA). Aliquots of 200 µl suspensions containing 4 × 105 splenocytes were added to individual wells in triplicate followed by antigen (AChR) in 10 µl aliquots to a final concentration of 10 µg/ml AChR. The wells were emptied after 48 h of culture. The secreted and bound IFN-γ was visualized and enumerated in a dissection microscope (for details see). At each time-point six animals were included in each group. Mean and standard deviations are shown. Stars denote statistical difference between denervated rats (solid bars) and non- denervated controls [open bars; experimental autoimmune myasthenia gravis (EAMG)] (*P< 0·01, **P > 0·001, ***P > 0·0001). There was no statistical difference between sham-operated rats (hatched bars) and non-denervated controls (open bars).
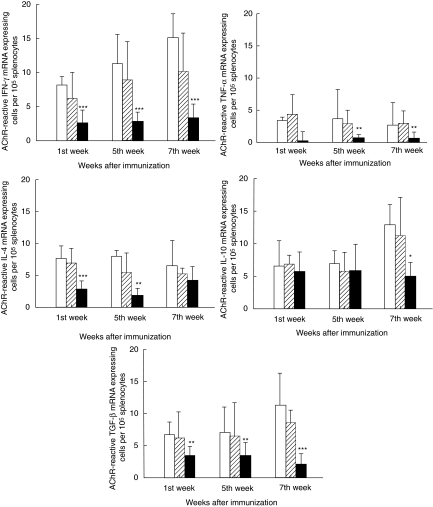
AChR-reactive cytokine mRNA expressing cells
Levels of splenocytes expressing mRNA for IFN-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-4 and transforming growth factor (TGF)-β after stimulation with AChR were lower in the denervated rats immunized with AChR + CFA, compared with non-denervated and sham-operated control rats (P< 0·001) for all comparisons except IL-4 at week 7, where no statistical difference was observed. In contrast, there were no differences for numbers of AChR-reactive IL-10 mRNA expressing cells in denervated versus non-denervated rats except at week 7 (P > 0·01) (Figs 5 and 6). There was no difference between the groups for numbers of cytokine mRNA-expressing cells after culture with MBP or without antigen (data not shown). Con-A was used a positive control and its effects on mRNA expression for cytokines was in line with those of IFN-γ secreting cells described above.
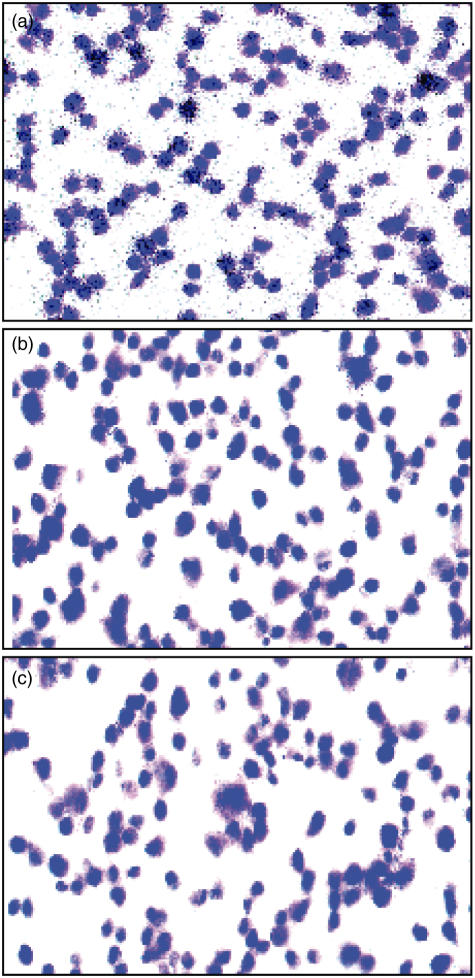
Fig. 5.
Illustration of cytokine mRNA expression as detected by in situ hybridization. Note increased expression of mRNA for interferon (IFN)-γ in immunized non-denervated rats (a), while the signal for this cytokine is very weak in immunized denervated rats (b). Sense control is shown in the micrograph (c). The pictures were taken in bright field microscopy (×630).
Fig. 6.
Detection of cytokine mRNA expression by in situ hybridization. The figure demonstrates numbers of acetylcholine receptor (AChR)-reactive mRNA expressing cells for the cytokines: interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-4, IL-10 and transforming growth factor (TGF)-β per 105 splenocytes by in situ hybridization technique. Splenocyte suspensions were prepared from non-denervated, denervated and sham-operated female Lewis rats after immunization with AChR + complete Freund's adjuvant (CFA). Two hundred-µl aliquots of suspensions containing 4 × 105 splenocytes were plated into round-bottomed microtitre plates in triplicate. Ten µl aliquots of AChR were added into appropriate wells at the final concentration of 10 µg/ml. After culture for 24 h, the cells were washed, counted and applied onto restricted areas of electronically charged glass slides. Synthetic oligonucleotide probes were labelled using [35S]-deoxyadenosine-5′-α-(thio)-triphosphate with terminal deoxynucleotidyl transferase. Cells were hybridized with 106 counts per minute of labelled probe per 100 µl of hybridization mixture. After emulsion autoradiography, development and fixation, the coded slides were examined by dark field microscopy for positive cells containing more than 15 grains per cell in a star-like distribution. A control probe used in parallel with the cytokine probe on cells from each rat revealed no positive cells (for details see Materials and methods). At each time-point six animals were included in each group. Mean and standard deviations are shown. Stars denote statistical difference between denervated rats (solid bars) and non-denervated controls [open bars; experimental autoimmune myasthenia gravis (EAMG)] (*P< 0·01, **P > 0·001, ***P > 0·0001). There was no statistical difference between sham-operated rats (hatched bars) and non-denervated controls (open bars).
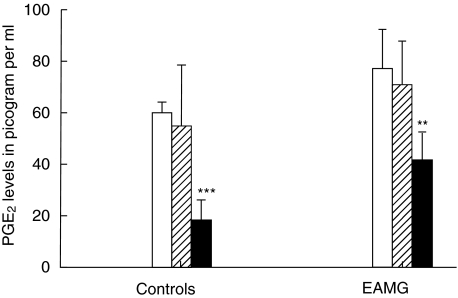
Induction of PGE2
Because few numbers of IFN-γ-secreting cells were recorded during denervation the levels of PGE2, which is known to suppress IFN-γ[18], were detected. The experiments were designed to measure PGE2 in denervated and non-denervated rats to examine whether denervation alone induces release of PGE2, which can then affect the levels of IFN-γ. The results showed that denervation of non-immunized rats decreased PGE2 levels significantly compared to normal controls (P< 0·0001). PGE2 release in immunized animals was also inhibited markedly by denervation (P< 0·001) (Fig. 7).
Fig. 7.
Detection of prostaglandin E2 (PGE2). The figure shows levels of isotope-labelled PGE2 antigen-antibody registered in pg/ml at 4 h post-immunization as detected by [125I]-radioimmune assay (RIA). Splenocyte suspensions were prepared from non-denervated, denervated and sham-operated female Lewis rats after immunization with acetylcholine receptor (AChR) + complete Freund's adjuvant (CFA). Two hundred µl aliquots of suspensions containing 4 × 105 splenocytes were plated into 96-well plastic plates in triplicate. Two hundred µl of culture medium per well containing 105 cells were incubated for 24 h and supernatants were collected thereafter. Prostaglandin E2 (PGE2) in 100 µl of supernatant from each sample was measured using a PGE2[125I]-RIA kit (for details see Materials and methods). Six animals were included in each group. Note low PGE2 induction in the denervated group. Mean and standard deviations are shown. Stars denote statistical difference between denervated rats (solid bars) and non-denervated controls [open bars; experimental autoimmune myasthenia gravis (EAMG)] (**P > 0·001, ***P > 0·0001). There was no statistical difference between sham-operated rats (hatched bars) and non-denervated controls (open bars).
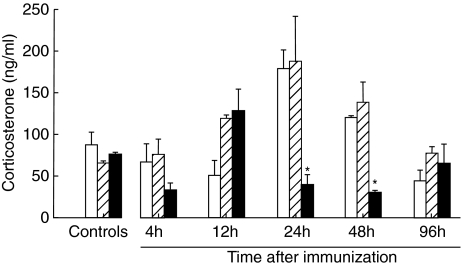
Levels of plasma corticosterone
In order to demonstrate that the differences observed after splenic denervation were due to the splenic denervation per se and not to stress-induced systemic immunosuppression, we measured the levels of corticosterone in non-immunized, denervated and sham-operated rats prior to the immunization. Furthermore, levels of plasma cortisone were measured during the course of the disease in this study and the effects of splenic denervation on these levels were also examined. As shown in Fig. 8, the animals were not under stress as the levels of this hormone were suppressed by the surgical denervation and immunization had increased the plasma cortisone levels significantly at 24 h and 48 h p.i. (P< 0·01), reaching normal values at 96 h.
Fig. 8.
Levels of serum cortisone in ng/ml as determined by the corticosterone radioimmunoassay. Blood was obtained from non-denervated, denervated and sham-operated rats at day 7 after the surgery and prior to the immunization and 12 h, 24 h, 48 h and 96 h after immunization. Bars denote means ± standard deviation. Six rats were examined in each group. Triplicate wells were used from each rat. Note that corticosterone levels were suppressed significantly by surgical denervation of the spleen (solid bars) compared to the non-denervated (opened bars) and sham-operated (hatched bars) control groups at the same time-points (e.g. 24 h, 48 h) (P< 0·01).
Discussion
The data of the present work illustrate communications between the central nervous system (CNS) and the immune system during the course of EAMG in rats, which is affected through the autonomic innervation of the spleen. The interactions demonstrated may have a great impact in the late-ensuing immunopathology. This is supported by data where denervation of the spleen was shown to reduce significantly the progression of the disease. Because the immune system recognize a foreign antigen immediately and deal with it, it should be guided in a rectified and rapid manner. This is achieved initially by non-specific mechanisms of the innate immunity, and subsequently with the extensively studied specific immune response. The first signals are of vital importance, as they may determine the delayed specific responses. Bearing these in mind, we hypothesized that elementary signals are conducted via nerves for rapid communication between cells of the immune system during different immune challenges. Thus, we examined the effects of sympathetic denervation on immune responses during the course of EAMG in rats. The splenic nerves were chosen because of their previously reported early selective electrical activity after intravenous injection of bacterial endotoxin [4] and due also to the fact that the spleen is an important lymphoid organ, which is involved in immune responses against all types of antigens that appear in the circulation. The nervous system can usually generate outflow capable of signalling cells of the immune system via two routes: (a) hormonal influences via hypothalamo–pituitary target organ activation [1], and (b) neurotransmitter influences via direct innervation of the parenchyma of both primary and secondary lymphoid organs [2,19]. Noradrenergic fibres target specifically lymphocyte-rich areas in mammalian lymphoid tissue and their ablation, or the administration of adrenergic agents, can significantly alter immune responses [20]. Furthermore, immune-active molecules such as cytokines and active agents and their components may induce profound alterations in several neurotransmitters in the CNS such as dopamine, GABA/benzodiazepine, acetylcholine and serotonin, suggesting reciprocal interactions between cytokines and neurotransmitters in the CNS [21].
Our present results indicate that splenic denervation markedly reduces the induction of the AChR specific IgG response, IFN-γ production and mRNA expression of the examined Th1/Th2/Th3 cytokines in response to immunization with AChR. Denervated rats also showed markedly reduced clinical severity of the disease, which might be related to regression of the cytokines that drive the development of the disease. The development of EAMG requires both Th1 and Th2 cytokines. The involvement of Th2 cytokines in EAMG and MG is expected on the basis of strong disease-promoting B cell responses in EAMG and MG. IL-4 is a driving force for Ig synthesis and class switches [22], and provides help for the production of anti-AChR antibodies in both EAMG and MG [21,23]. IL-10 also has stimulatory effects on B cell proliferation and differentiation into cells secreting large amounts of IgM, IgG and IgA [24]. Administration of recombinant human IL-10 accelerated and enhanced EAMG in Lewis rats by augmenting Th2 as well as B cell responses to AChR [25]. The low levels of anti-AChR IgG antibody-secreting cells in the denervated rats p.i. with AChR + CFA are in line with the low levels of IL-4 and IL-10 mRNA-expressing cells, indicating the important role for IL-4 and IL-10 in the promotion of AChR-specific B cell responses and the progression of EAMG. However, denervation modulated the IL-4 and IL-10 responses in a kinetic manner. The data suggest that the action of IL-10 occurs late as the disease progresses, because a significant difference between denervated and non-denervated immunized rats was noted only at week 7 p.i. In contrast, IL-4 effects occur early during the disease where significant differences were registered at weeks 1 and 5 p.i.; the levels then became more or less equal between the different immunized groups by week 7, the time-point where significant IL-10 suppression by splenic denervation was demonstrated. The Th3-related TGF-β has been suggested to play a role in strain associated-resistance to EAMG [26], which fits very well with significant suppression of the AChR-induced mRNA expression for this cytokine by denervation and the resulting reduced clinical severity. The Th1 cytokine IFN-γ is required during the initiation and development of an organ-specific autoimmune disease [26], and its role in inducing B cell maturation [27] might be important in the induction of EAMG [5,21]. Expression of IFN-γ in neuromuscular junctions induces humoral autoimmunity [28]. In the present study, IFN-γ was lower at both mRNA and protein levels in the denervated rats, thereby probably contributing to EAMG resistance that occurred after surgical denervation of the spleen. The Th1 cytokine TNF-α was induced minimally by immunization compared to IFN-γ. However, this induction was abrogated by splenic denervation.
To analyse further whether these responses upon splenic denervation were induced directly or through yields of other mediators, the production of PGE2, which is known to down-regulate IFN-γ production [18], was measured. However, PGE2 was also inhibited by denervation, suggesting that PGE2 may not be related to the repression of antibody production and cytokine expression in this regard. Because PGE2 is an important early immune mediator of the innate immune response, its suppression by splenic denervation provides further evidence that the activation of early immune responses is mediated by the autonomic nervous system. Inhibition of PGE2 may be due to the lack of innervation of NE fibres to the spleen after denervation because NE is a stimulator of PGE2 release [29]. However, the present data do not necessarily exclude the effects of suppressed PGE2 on the expression of cytokines such as IFN-γ or TNF-α produced by macrophages in the EAMG model. Also, data from the levels of corticosterone excluded the possibility that the differences observed after splenic denervation were not stress-induced by, for example, cortisone, which down-regulates certain immunoreactivity. A novel observation in this study is the increased induction of plasma cortisone levels at 24 h and 48 h after immunization. This induction of corticosterone demonstrates a role of the endocrine system in this disease, which opens a new area for further investigation. The suppression of corticosterone by splenic denervation at these time-points may represent a link between the nervous, endocrine and immune systems that may cooperate during autoimmune reactions.
In conclusion, immune responses and clinical severity after immunization with AChR in the early stages of EAMG were modulated through the sympathetic nervous system. The triggering mechanism of sympathetic nerve activity is not known, but such interactions between the CNS, endocrine and immune systems may involve a network of mediators, and can be of pivotal significance in regulation of the consequential development of the autoimmune-specific response.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council, the Swedish MS Society (NHR) and the Karolinska Institute. Ethical approval was obtained from Stockholm South Committee for Animal Ethics, Sweden.
References
- 1.Hadden JW, Handden EM, Middleton EJR. Lymphocyte blast transformation. I. Demonstration of adrenergic receptors in human peripheral lymphocytes. Cell Immunol. 1970;1:583–95. doi: 10.1016/0008-8749(70)90024-9. [DOI] [PubMed] [Google Scholar]
- 2.Madden KS, Livnat S. Catecholamine action and immunologic reactivity. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. New York: Academic Press; 1991. pp. 283–310. [Google Scholar]
- 3.Sanders VM, Munson AE. Norepinephrine and the antibody response. Pharmacol Rev. 1985;37:229–48. [PubMed] [Google Scholar]
- 4.MacNeil BJ, Jansen A, Greenbery AH, Nance DM. Activation and selectivity of splenic sympathetic nerve electrical activity response to bacterial endotoxin. Am J Physiol. 1996;270:264–70. doi: 10.1152/ajpregu.1996.270.1.R264. [DOI] [PubMed] [Google Scholar]
- 5.Drachman DB. Myasthnia gravis. N Engl J Med. 1994;330:1797–807. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 6.Dubowitz V. Muscle disorders in childhood. 2. London: Saunders; 1995. [Google Scholar]
- 7.Lindstrom J, Shelton D, Fujii Y. Myasthenia gravis. Adv Immunol. 1988;42:233–85. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 8.Asthana D, Fujii Y, Huston Y, Lindstrom J. Regulation of antibody production by helper T cell clones in experimental myasthenia gravis is mediated by IL-4 and antigen-specific T cell factors. Clin Immunol Immunopathol. 1993;67:240–8. doi: 10.1006/clin.1993.1071. [DOI] [PubMed] [Google Scholar]
- 9.Zhang GX, Xiao BG, Bakhiet M, et al. Both CD4+ and CD8+ T cells are essential to induce experimental autoimmune myasthenia gravis. J Exp Med. 1996;148:349–56. doi: 10.1084/jem.184.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindstrom J, Einarson B, Tzartos S. Production and assay of antibodies to acetylcholine receptors. Meth Enzymol. 1981;74:432–60. doi: 10.1016/0076-6879(81)74031-x. [DOI] [PubMed] [Google Scholar]
- 11.Deibler GE, Martenson RE, Kies MV. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2:139–65. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- 12.Lennon VA, Lambert EH, Leiby KR, Okarma TB, Talib S. Recombinant human acetylcholine receptor α-subunit induces chronic experimental autoimmune myasthenia gravis. J Immunol. 1991;146:2245–8. [PubMed] [Google Scholar]
- 13.Loutrari H, Kokla A, Tzartos SJ. Passive transfer of experimental autoimmune myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur J Immunol. 1992;22:2449–52. doi: 10.1002/eji.1830220939. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZY, Link H, Qiao J, Olsson T, Huang WX. B cell autoimmunity to acetylcholine receptor and its subunits in Lewis rats over the course of experimental autoimmune myasthenia gravis. J Neuroimmunol. 1993;45:103–12. doi: 10.1016/0165-5728(93)90169-y. [DOI] [PubMed] [Google Scholar]
- 15.Czerkinsky C, Andersson G, Ekre HP, Nilsson LÅ, Klareskog L, Ouchterlony Ö. Reverse ELI-SPOT assay for clonal analysis of cytokine production. I. Enumeration of γ interferon-secreting cells. J Immunol Meth. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 16.Van der Meide P, Dubbeld HM, Vijverberg K, Kos T, Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986;67:1059–71. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]
- 17.Bakhiet M, Olsson T, Van der Meide P, Kristensson K. Depletion of CD8+ T cells suppresses growth of Trypanosoma brucei brucei and interferon-γ production in infected rats. Clin Exp Immunol. 1990;81:195–9. doi: 10.1111/j.1365-2249.1990.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cesario TC, Yousefi S, Carandang G. The regulation of interferon production by aspirin, other inhibitors of the cyclooxygenase pathway and agents influencing calcium channel flux. Bull NY Acad Med. 1989;65:26–35. [PMC free article] [PubMed] [Google Scholar]
- 19.Felten DL. Direct innervation of lymphoid organs: substrate for neurotransmitter signaling of cells of the immune system. Neuropsychobiology. 1993;28:1–2. doi: 10.1159/000119011. [DOI] [PubMed] [Google Scholar]
- 20.Pipili E, Poyser NL. Release of prostaglandin I2 and E2 from the perfused mesenteric arterial bed of normotensive and hypertensive rats. The effects of sympathetic nerve stimulation and norepinephrin administration. Prostaglandin. 1982;23:543–9. doi: 10.1016/0090-6980(82)90114-9. [DOI] [PubMed] [Google Scholar]
- 21.De Simoni MG, Imeri L. Cytokine–neurotransmitter interactions in the brain. Biol Signals. 1998;7:33–44. [PubMed] [Google Scholar]
- 22.Link J, Söderström M, Ljungdahl Å, et al. Organ-specific autoantigens induce IFN-γ and IL-4 mRNA expression in mononuclear cells in multiple sclerosis and myasthenia gravis. Neurology. 1994;44:728–34. doi: 10.1212/wnl.44.4.728. [DOI] [PubMed] [Google Scholar]
- 23.Howard M, O'Garra A. Biological properties of interleukin-10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 24.Zhang GX, Xiao BG, Shi FD, Yu LY, Link H. Enhancement of experimental autoimmune myasthenia gravis by IL-10 is associated with augumented Th2 and B cell responses to AChR. V; International Conference on Cytokines; Florence, Italy. 1996. p. 111. [Abstract] [Google Scholar]
- 25.Zhang GX, Ma CG, Xiao BG, van der Meide P, Link H. Autoreactive T cell responses and cytokine pattern reflect resistance against experimental autoimmune myasthenia gravis in Wistar Furth rats. Eur J Immuunol. 1996;26:2552–8. doi: 10.1002/eji.1830261103. [DOI] [PubMed] [Google Scholar]
- 26.Barrett SP, Gleeson PA, de Silva H, Toh BH, van Driel IR. Interferon-γ is required during the initiation of an organ-specific autoimmune disease. Eur J Immunol. 1996;26:1642–55. doi: 10.1002/eji.1830260737. [DOI] [PubMed] [Google Scholar]
- 27.Sidman CL, Marshall JD, Shultz LD, Gray PW, Johnson HM. γ-Interferon is one of several direct B cell-maturing lymphokines. Nature. 1984;309:801–3. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- 28.Gu D, Wogensen L, Calcult NA, et al. Myasthenia gravis-like syndrome induced by expression of interferon-γ in the neuromuscular junction. J Exp Med. 1995;181:547–57. doi: 10.1084/jem.181.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–9. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]