Abstract
Adverse drug reactions with an immunological basis (ADRIB) may involve activation of other concomitant, non-specific mechanisms, amplifying the specific response and contributing to the severity and duration. One concomitant mechanism could be the generation of reactive oxygen species (ROS) and/or their detoxification by anti-oxidants, including anti-oxidant enzymes. We analysed the activity of the anti-oxidant enzymes Cu/Zn-superoxide dismutase (SOD), catalase (CAT) and cellular glutathione peroxidase (GPX), as well as certain markers of oxidative damage (thiobarbituric acid reactive substances (TBARS) and carbonyl content) in peripheral blood mononuclear cells from patients with non-immediate ADRIB using spectrophotometric methods and the anti-oxidant enzymes expression by quantitative real-time reverse transcription–polymerase chain reaction. SOD activity and expression were increased in all types of non-immediate reactions (urticaria, maculopapular exanthema and toxic epidermal necrolysis). Regarding oxidative damage, TBARS were increased in urticaria and maculopapular exanthema, and carbonyl groups in all types of reactions. Our observations indicate that oxidative damage occurs in non-immediate reactions. Carbonyl stress and the inadequacy of the anti-oxidant defences are probable causes.
Keywords: anti-oxidant enzymes, carbonyl, drug allergy, lipid peroxidation, oxidative stress
Introduction
An adverse drug reaction is any noxious, unintended and undesired effect of a drug that occurs at treatment doses [1], including adverse drug reactions with an immunological basis (ADRIB). ADRIB can be classified according to the time elapsed between drug intake and the appearance of clinical manifestations as immediate, accelerated or delayed reactions [2]. Immediate reactions (IR) are those that occur within 1 h of drug intake, with urticaria and anaphylaxis being the most frequent clinical manifestations [3]. Accelerated and delayed reactions are commonly grouped as non-immediate reactions (NIR), and include mainly those occurring from 24 to 48 h to several days after drug intake with no subsequent immunological stimulation, although the reaction may occasionally appear sooner than 24 h [4]. The clinical manifestations of NIR are extremely heterogeneous, ranging from urticaria and maculopapular exanthema (MPE) to other more severe symptoms, such as Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) [3–5]. Not only is this classification useful for clinical diagnosis, it also seems to reflect the underlying immunological mechanisms [6].
IR are IgE-mediated [7]. NIR are subject to more debate, although evidence supports a T cell-mediated effector mechanism [8].
Although all the reactions described above are known to be mediated by a specific immunological mechanism, other concomitant, non-specific mechanisms may also take part, either amplifying or regulating the initial response. One of these concomitant mechanisms could be the generation of reactive oxygen species (ROS) [9]. ROS are produced by aerobic organisms in which low concentrations are beneficial and related with several cellular processes [10,11]. However, an increase in ROS levels leads to oxidative stress, which may cause oxidative modifications to lipids, nucleic acids and proteins [12]. Cells are equipped with elaborate defence systems that act in concert to detoxify ROS. These systems include scavenger enzymes, such as Cu/Zn-superoxide dismutase (SOD), catalase (CAT) and cellular glutathione peroxidase (GPX) [12]. SOD destroys the radical superoxide by producing hydrogen peroxide, which can be eliminated by CAT or GPX [13].
Our group has reported previously the existence of differences in anti-oxidant enzyme activities in several clinical entities induced by allergens [13]. In patients with adverse drug reactions [14], we observed an imbalance in the global oxidative status, although enzyme activity values were heterogenic, depending on the individual clinical manifestations generated by the particular drug. One possible explanation could be that this study included patients with both IR and NIR who presented very different symptoms.
In order to be more precise, we have now undertaken a study in patients with adverse cutaneous reactions to drugs. However, because not all IR involve the skin, e.g. anaphylaxis, in this report we focus on NIR by comparing the anti-oxidant enzyme activities and expressions (SOD, CAT and GPX), and markers of lipid peroxidation [thiobarbituric acid reactive substances (TBARS)] and of protein oxidation (carbonyl content) in peripheral blood mononuclear cells (PBMC) during the acute phase and 1 month after resolution of the reaction.
Materials and methods
Subjects
All patients who presented to the Emergency Department of Carlos Haya Hospital from January 2000 to December 2003 with a non-immediate, allergic, cutaneous reaction after drug intake were eligible for inclusion in the study. Only those patients who were confirmed as allergic after completing an allergological work-up were included. Patients were considered to have a NIR if the reaction developed more than 1 h after drug intake, including urticaria, MPE and Stevens–Johnson syndrome or toxic epidermal necrolysis (SJS/TEN). Although patients 18, 22, 28, 33, 39 and 41 were receiving several drugs at the moment of the reaction, after the allergological work-up only one was identified as the drug involved in the reaction. In those cases who suffered SJS/TEN (2, 4, 7, 15, 29, 34, 35 and 40), only one drug was administered (Table 1).
Table 1.
Clinical data and results of the allergological work-up of patients.
| Patient | Sex | Age | Drug involved | Clinical symptoms | Skin test | DPT |
|---|---|---|---|---|---|---|
| 1 | F | 50 | Amoxicillin | Urticaria | AX | n.d. |
| 2 | F | 75 | Diphenylidantoine | SJS/TEN | n.d. | n.d. |
| 3 | F | 36 | Amoxicillin | MPE | – | + |
| 4 | F | 27 | Allopurinol | SJS/TEN | n.d. | n.d. |
| 5 | M | 23 | Cephalexin | Urticaria | – | + |
| 6 | F | 65 | Ebastine | MPE | – | + |
| 7 | F | 48 | Diphenylidantoine | SJS/TEN | n.d. | n.d. |
| 8 | M | 19 | Amoxicillin | Urticaria | AX | n.d. |
| 9 | F | 44 | Carbamazepine | MPE | – | + |
| 10 | F | 32 | Diphenylidantoine | SJS/TEN | n.d. | n.d. |
| 11 | F | 50 | Amoxicillin | MPE | AX | n.d. |
| 12 | F | 26 | Tetrazepam | Urticaria | – | + |
| 13 | M | 60 | Amoxicillin | MPE | – | + |
| 14 | F | 33 | Phenobarbital | MPE | – | + |
| 15 | F | 57 | Allopurinol | SJS/TEN | n.d. | n.d. |
| 16 | F | 42 | Cefaclor | Urticaria | – | + |
| 17 | F | 37 | Cephalexin | Urticaria | – | + |
| 18 | F | 43 | Amoxicillin | Urticaria | – | + |
| 19 | F | 46 | Ibuprofen | Urticaria | – | + |
| 20 | M | 50 | Spiramicin | Urticaria | – | + |
| 21 | F | 44 | Tetrazepam | Urticaria | n.d. | + |
| 22 | F | 26 | Ebastine | Urticaria | n.d. | + |
| 23 | M | 21 | Amoxicillin | Urticaria | – | + |
| 24 | F | 29 | Amoxicillin | Urticaria | – | + |
| 25 | F | 60 | Spiramicin | Urticaria | – | + |
| 26 | F | 26 | Amoxicillin | MPE | – | + |
| 27 | F | 37 | Amoxicillin | MPE | – | + |
| 28 | F | 57 | Amoxicillin | MPE | AX | n.d. |
| 29 | F | 73 | Diphenylidantoine | SJS/TEN | n.d. | n.d. |
| 30 | F | 26 | Phenobarbital | MPE | – | + |
| 31 | M | 39 | Carbamazepine | MPE | – | + |
| 32 | F | 56 | Metamizol | Urticaria | – | + |
| 33 | F | 53 | Paracetamol | Urticaria | – | + |
| 34 | F | 50 | Allopurinol | SJS/TEN | n.d. | n.d. |
| 35 | F | 38 | Diphenylidantoine | SJS/TEN | n.d. | n.d. |
| 36 | M | 51 | Metamizol | Urticaria | – | + |
| 37 | F | 32 | Amoxicillin | MPE | – | + |
| 38 | F | 18 | Paracetamol | MPE | – | + |
| 39 | F | 56 | Ibuprofen | Urticaria | – | + |
| 40 | F | 36 | Diphenylidantoine | SJS/TEN | n.d. | n.d. |
| 41 | F | 37 | Spiramicin | MPE | – | + |
| 42 | M | 55 | Amoxicillin | Urticaria | AX | n.d. |
| 43 | F | 33 | Cefaclor | Urticaria | – | + |
DPT: drug provocation test; AX: amoxicillin; SJS/TEN: Stevens–Johnson syndrome/toxic epidermal necrolysis; MPE: maculopapular exanthema; n.d.: not done. MPE: maculopapular exanthema; SJS/TEN: Stevens–Johnson syndrome/toxic epidermal necrolysis.
To assess whether the drug itself could induce changes in the variables studied, we included two age- and sex-matched control groups, formed by non-atopic healthy subjects with good tolerance to drugs and no cutaneous disease, who were divided into two groups: one group of 10 non-exposed subjects and the other of 14 exposed subjects undergoing similar standardized treatment to the group of patients: amoxicillin (four), cefaclor (one), diphenylidantoine (two), phenobarbital (one), allopurinol (one), ebastine (one), spiramicin (one), ibuprofen (two) and metamizol (one). The study was approved by the institutional review board, and informed consent for all the diagnostic procedures was obtained from all the participants.
Allergological work-up
Depending on the drug taken and the reaction, and once this had subsided, we undertook a skin test as recommended by the European Network for Drug Allergy (ENDA) [15]. Intradermal tests were carried out with soluble drugs and patch testing with both soluble and insoluble drugs. Skin testing was not performed in severe delayed reactions (Table 1). If skin testing was negative and the reaction was not severe a drug provocation test (DPT) was performed, as recommended by the ENDA [15]. When the culprit drug was a non-steroidal anti-inflammatory drug (NSAID), only those patients with selective reactions were included. To establish selectivity, indomethacin or acetyl salicylic acid was administered, provided that they were not the drug that had elicited the reaction.
Sample collection
Venous peripheral blood was obtained in ethylenediamine tetraacetic acid (EDTA)-coated tubes at two different times: during the acute phase of the reaction, in the 24 h following the symptoms appearance (T1), and 30 days after resolution of the reaction (T2). All the patients’ samples at T1 were obtained before starting any treatment for relief of symptoms. For the exposed control group two samples were also obtained: 2 days after the initiation of the treatment (T1) and 30 days after its finalization (T2). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient from 5 ml for reverse transcription–polymerase chain reaction (RT–PCR) and from 10 ml for obtain cell lysates. Cell lysates were centrifuged at 50 000 g for 20 min at 4°C and the supernatant collected for measuring of anti-oxidant enzymes and lipid peroxidation products. The plasma fraction was used to determine carbonyl content.
Anti-oxidant enzyme activity, lipid peroxidation and protein oxidation
The activity of SOD, CAT and GPX was determined from cell lysates using commercial kits (SOD-525, Catalase-520 and GPx-340 from OxisResearchTM, Portland, OR, USA) following the manufacturer’s instructions. Final SOD, CAT and GPX activities were expressed as units/mg of protein.
We determined lipid peroxidation from cell lysates by the TBARS method [16], whereas protein oxidation was determined by measuring the carbonyl content in plasma proteins by the method described by Reznick and Racker [17]. Results were expressed as nmol TBARS and nmol carbonyl per mg of protein, respectively.
Protein concentrations were determined by a modified Bradford method, using Coomassie protein assay reagent (Pierce, Rockford, IL, USA) according to the manufacturer’s recommendations.
Semi-quantification of anti-oxidant enzyme mRNA
Total RNA was extracted in TriPure (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions and treated with DNAse I (Sigma, Madrid, Spain). One µg of total RNA was reverse-transcribed with random hexamers and Moloney murine leukaemia virus–reverse transcriptase at a final volume of 20 µl. Specific cDNA amplification of human SOD, CAT and GPX was carried out with 2 µl of cDNA in a Light Cycler by using FastStart DNA Master SYBR Green I (Roche). The specific primers used were (5′−3′): CATCATCAATTTCGAGCAGA and GCCACACCA TCTTTGTCAGCAG for SOD; GCGGTCAAGAACTTCA CTGA and GCTAAGCTTCGCTGCACAGGT for CAT; and TGGCTTCTTGGACAATTGCG and GATGCCCAAACTG GTTGCACGGGAA for GPX. Primers for the housekeeping gene porphobilinogen deaminase (PBGD) were (5′−3′): TCCAAGCGGAGCCATGTCTG and AGAATCTTGTCCC CTGTGGTGGA. The quantification was performed by crossing-point extrapolation into a standard curve with known cDNA concentrations by using Light Cycler system software. Relative units represent the ratio of concentration between the specific mRNA and the housekeeping mRNA.
Statistical analysis
Comparisons for quantitative variables (enzyme activities, oxidative damage and enzyme expression) were performed by non-parametric analysis (Mann–Whitney and Kruskall–Wallis tests for non-related samples). Comparisons at two different times (T1 and T2) of these quantitative variables were carried out with the Wilcoxon test for related samples. All reported P-values represented two-tailed tests, with values ≤ 0·05 considered statistically significant. The statistical analysis was performed using the spss program, version 11·5.
Results
Forty-three patients diagnosed as having a NIR to drugs were finally included. Table 1 shows the clinical characteristics (sex, age, drug involved and clinical symptoms) and the results of the allergological work-up (skin test and DPT) of the patients.
Anti-oxidant enzyme activity and expression and oxidative damage in the acute phase of the reactions (T1) and after symptoms subsided (T2)
We first compared the results obtained at two different times (T1 and T2) in the exposed control. Data showed no differences in CAT activity or TBARS and carbonyl content, whereas SOD activity was significantly increased and GPX activity significantly decreased at T1 (P = 0·004 and P = 0·045, respectively). Accordingly, we analysed the results obtained with SOD and GPX with caution. The median and interquartile ranges are presented in Table 2.
Table 2.
Anti-oxidant enzyme activities, lipid peroxidation and protein oxidation markers in patients and healthy exposed controls taking drugs at T1 and T2. All data are represented as median and interquartile range (IR) (25–75).
| SOD activity (U/mg protein) | CAT activity (U/mg protein) | GPX activity (U/mg protein) | TBARS (nmo/mg protein) | Carbonyl (nmol/mg protein) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Median | IR (25–75) | Median | IR (25–75) | Median | IR (25–75) | Median | IR (25–75) | Median | IR (25–75) |
| Patient | ||||||||||
| T1 | 46·61 | 15·56–53·69 | 98·77 | 21·22–172·14 | 210·22 | 139·42–312·97 | 0·59 | 0·47–1·11 | 11·94 | 9·71–24·89 |
| T2 | 8·65 | 6·62–16·83 | 21·98 | 8·84–40·27 | 114·95 | 81·35–114·95 | 0·53 | 0·42–0·81 | 3·94 | 2·75–9·11 |
| Exposed controls | ||||||||||
| T1 | 7·56 | 4·38–13·19 | 45·56 | 13·91–108·95 | 306·6 | 174·42–476·97 | 0·35 | 0·28–0·49 | 4·44 | 3·28–5·04 |
| T2 | 3·61 | 3·01–5·53 | 50·75 | 34·19–53·63 | 434·36 | 363·45–659·64 | 0·43 | 0·31–0·52 | 4·16 | 3·83–4·44 |
SOD: superoxide dismutase; CAT: catalase; GPX: cellular glutathione peroxidase; TBARS: thiobarbituric acid reactive substances; IR: interquartile range.
Patients’ comparisons at two different times (T1 and T2) showed that SOD and CAT activities were significantly increased at T1 (P = 0·029 and P = 0·05, respectively), as well as carbonyl content (P = 0·001). No significant differences were noted in GPX activity or TBARS (Table 2).
When we analysed the anti-oxidant enzyme gene expression at two different times (T1 and T2), in the exposed control we found no differences in any of the variables analysed. In patients, the SOD expression was increased at T1 compared with T2 (P = 0·008), but no differences were detected in the expression of the other two enzymes (Table 3).
Table 3.
Anti-oxidant enzyme expression in patients and healthy exposed controls at T1 and T2. Anti-oxidant enzyme expressions were normalized to the porphobilinogen deaminase housekeeping gene (PBGD) levels and expressed as relative units. Results are represented as median and interquartile range (IR) (25–75).
| Ratio SOD/PBGD (× 103) | Ratio CAT/PBGD (× 103) | Ratio GPX/PBGD (× 103) | ||||
|---|---|---|---|---|---|---|
| Group | Median | IR (25–75) | Median | IR (25–75) | Median | IR (25–75) |
| Patient | ||||||
| T1 | 16 | 9–16 | 13·17 | 5·5–26·16 | 28·83 | 3·95–87·32 |
| T2 | 4·33 | 3·53–5·03 | 15·41 | 6·21–22·41 | 10·57 | 3·95–76·31 |
| Exposed controls | ||||||
| T1 | 3·64 | 1·88–9·62 | 9·47 | 4·62–16·64 | 17·38 | 12·16–24·64 |
| T2 | 4·3 | 2·29–6·8 | 10·65 | 6·35–27·23 | 26·54 | 3·75–72·4 |
SOD: superoxide dismutase; CAT: catalase; GPX: cellular glutathione peroxidase; PBGD: porphobilinogen deaminase; IR: interquartile range.
Anti-oxidant enzyme activity and expression and oxidative damage at the acute phase of the reactions (T1) compared to controls
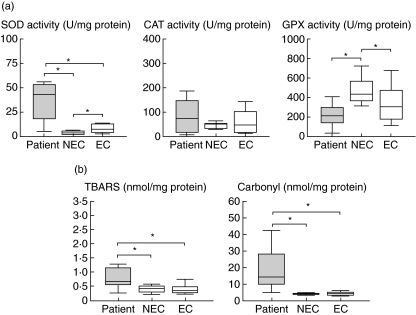
Comparisons at the acute phase of the reaction (T1) showed a significant increase in SOD activity in patients compared to the two control groups (P = 0·001 for both), whereas no differences in CAT activity were found. GPX activity was decreased, compared only with non-exposed controls (P = 0·001) (Fig. 1). TBARS values were significantly increased compared to the two controls (non-exposed controls: P = 0·005; exposed controls: P = 0·009), as were the carbonyl levels (P = 0·001 for both) (Fig. 1).
Fig. 1.
Box plots of the enzymatic activities of superoxide dismutase (SOD), catalase (CAT) and cellular glutathione peroxidase (GPX) (a), and levels of thiobarbituric acid-substances (TBARS) and carbonyl (b). The study was performed in patients with non-immediate reactions during the acute phase and in two control groups: non-exposed (NEC) and exposed controls (EC). Statistical differences (*) are considered significant with P < 0·05.
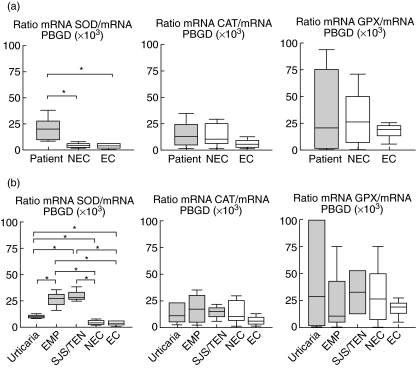
With respect to the anti-oxidant enzyme gene expression, we found that SOD mRNA was increased during the acute phase of the reactions compared to controls (non-exposed: P = 0·05; exposed: P = 0·01), whereas no differences in CAT or GPX expression were found (Fig. 2a).
Fig. 2.
(a) Relative superoxide dismutase (SOD), catalase (CAT) and cellular glutathione peroxidase (GPX) mRNA levels in patients during the acute phase of a non-immediate drug reaction compared with the two control groups: non-exposed (NEC) and exposed controls (EC). (b) Relative SOD, CAT, and GPX mRNA levels during the acute phase of a non-immediate drug reaction in patients who developed urticaria, maculopapular exanthema (MPE) or Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), compared with the two control groups (exposed and non-exposed). Results were normalized to the levels of porphobilinogen deaminase (PBGD) and expressed as arbitrary units. Statistical differences (*) are considered significant with P < 0·05.
No statistical correlations between enzymatic activity and the different anti-oxidant enzyme expression were found by Pearson test.
Anti-oxidant enzyme activity and expression and oxidative damage in the different types of NIR at the acute phase
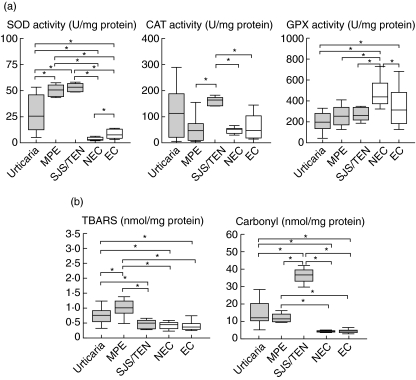
Patients with NIR were classified as having urticaria, MPE or SJS/TEN (Fig. 3). Comparisons at the acute phase (T1) of NIR showed that CAT activity was only increased significantly in SJS/TEN when compared with the two control groups (P = 0·001 for both), with an additional increase in SJS/TEN when compared to MPE (P = 0·001). SOD activity was increased in all types of clinical manifestations compared to controls: urticaria (non-exposed: P = 0·001; exposed: P = 0·005), MPE (non-exposed: P = 0·001; exposed: P = 0·001) and SJS/TEN (non-exposed: P = 0·001; exposed: P = 0·001), with MPE and SJS/TEN also being higher when compared to urticaria (P = 0·002 and P = 0·001, respectively). GPX activity was decreased in urticaria compared to controls (non-exposed: P = 0·023; exposed: P = 0·001).
Fig. 3.
Box plots of the activities of superoxide dismutase (SOD), catalase (CAT) and cellular glutathione peroxidase (GPX) (a), and levels of TBARS and carbonyl (b). The study was performed in patients during the acute phase of a non-immediate reaction to drugs who developed urticaria, maculopapular exanthema (MPE) or Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), and in two control groups: non-exposed (NEC) and exposed controls (EC). Statistical differences (*) are considered significant with P < 0·05.
Regarding oxidative damage and compared to controls, the TBARS content was significantly increased in urticaria (non-exposed: P = 0·001; exposed: P = 0·002) and in MPE (non-exposed: P = 0·001; exposed: P = 0·001), with MPE also being higher when compared to urticaria (P = 0·042) and both urticaria and MPE when compared to SJS/TEN (P = 0·014 and P = 0·03, respectively). Concerning carbonyl content, significant increases were found when compared to the two control groups in urticaria, MPE and SJS/TEN (P = 0·001 for all three clinical entities). However, the most important increase was observed in SJS/TEN when compared to urticaria and MPE (P = 0·001 for both).
Concerning the different enzyme expressions (Fig. 2b), SOD mRNA was increased in all types of clinical manifestations compared to the controls: urticaria (non-exposed: P = 0·029; exposed: P = 0·03), MPE (non-exposed: P = 0·034; exposed: P = 0·05) and SJS/TEN (non-exposed: P = 0·034; exposed: P = 0·05). There was also an increase in the expression of this enzyme in MPE and SJS/TEN compared to patients with urticaria (P = 0·034 for both). CAT and GPX mRNA levels remained unchanged in the different clinical entities when compared to the two controls.
Discussion
Although specific immunological mechanisms are known to be involved in adverse drug reactions [3], concomitant non-specific processes, such as oxidative stress, can also play a role, whether amplifying the specific response or influencing its severity and duration [12]. Oxidative stress involves an imbalance in the levels of ROS, which are not adequately scavenged by anti-oxidant enzymes such as SOD, CAT and GPX [12].
Many processes can modify the anti-oxidant enzymes, including ageing, nutrition and drugs. As our study was focused on patients with an ADRIB, we were initially interested in determining whether the activity and the expression of anti-oxidant enzymes are affected by drugs. Thus, we evaluated these parameters in two groups of healthy controls, either taking or not taking drugs, and found a significant decrease in GPX activity and an increase in SOD activity in those controls taking drugs, with no differences in CAT activity or oxidative damage for all enzyme mRNA levels. These drug-associated effects have also been reported with GPX activity in patients taking paracetamol [18] and with SOD activity in asthmatic patients taking corticosteroids [19]. Moreover, these same effects in the activity of these two enzymes have also been described at the same time in myocardium from a single experimental model after treatment with anti-hypertensive drugs [20]. These findings indicate that the drug itself can have some effect on these enzyme activities, probably because their metabolic pathways. Comparative results with these enzymes were therefore taken into account only in those cases where the patient data were significantly different to the two control groups.
The anti-oxidant enzymes have been shown to modulate the expression of a variety of inflammatory molecules in several immunological diseases, such as atopic dermatitis, psoriasis and asthma [21,22]. In asthma, which is considered a classical inflammatory disease, increases in oxidative damage and anti-oxidant enzyme activity have been described, with an increase in SOD with or without changes in CAT activity [23,24]. ADRIB may also be influenced by these enzymes, and to this extent an earlier study by our group showed an imbalance in anti-oxidant enzyme activities, as well as an increase in both TBARS and carbonyl contents [14], although we are unaware of any further similar reports. Accordingly, we continued our study of anti-oxidant enzyme activity in a large group of patients with non-immediate cutaneous ADRIB, with a better characterization of the clinical reactions and including the study of gene expression at mRNA levels. We focused the study on PBMC, because these are the cells most involved in immunological processes.
In the present work, not only did we confirm our previously published results [14] but, additionally, we showed that in NIR SOD activity was increased, GPX decreased and CAT unchanged when compared to controls. This imbalance in the activity of different anti-oxidant enzymes can lead to oxidative damage of proteins, as reflected especially by the high levels of carbonyl groups.
Although all the NIR had, by definition, cutaneous manifestations, these reactions involved very different clinical entities with varying pathophysiological mechanisms. For instance, MPE is produced by CD4 cytotoxic lymphocytes, whereas in SJS/TEN a massive keratinocyte apoptosis is produced [25]; these differences were not taken into account in previous work [14]. Accordingly, we determined whether there were differences in anti-oxidant enzyme activity depending on the clinical entity. Data showed that although SOD activity was significantly increased in all types of clinical entities, especially in MPE and SJS/TEN, CAT activity was significantly increased only in SJS/TEN, with no changes detected in GPX activity. When carbonylation of proteins (a marker of protein damage) was used as an index of the oxidative damage, all the patient groups showed significant increases compared with the two control groups, with carbonylation being highest in SJS/TEN. However, the level of lipid peroxidation measured by the TBARS content (a marker of lipid damage) remained unaltered in SJS/TEN. This discrepancy in the oxidative damage has also been shown at high-altitude training conditions in animal models [24], indicating that lipid peroxidation and protein oxidation may have different mechanisms in vivo. Moreover, proteins seem to be more sensitive to oxidative modifications and these oxidized proteins are generally more stable, over time, than lipids in peripheral blood [26], probably making the measurement of carbonyl groups more useful as a marker of oxidative damage. Another probable reason for the high increase in carbonyl groups in SJS/TEN is the massive apoptosis produced in this disease [25], via caspase activation with subsequent proteolytic cellular disintegration [27,28]. On the other hand, CAT has recently been reported to have a pro-apoptotic role in human cells [29]. All these circumstances could explain the high levels of carbonyl contents in SJS/TEN.
When anti-oxidant enzyme gene expression was determined by quantifying the specific mRNA we obtained a good correspondence with the SOD activity, both by comparing patients with controls and comparing the different clinical entities themselves. However, no changes in the mRNA levels were detected for CAT or GPX in patients compared to controls.
Anti-oxidant enzymes are known to be regulated by multiple factors and changes in anti-oxidant enzyme activity do not parallel the changes uniformly in the corresponding mRNA levels [30–33]. Some authors have found that these enzymes are importantly regulated at a post-transcriptional level by alterations in the degradation rate of mRNA (mRNA stability), protein synthesis (translation) and post-translational modifications (specific activity) [34]. In the case of RNA stability, proteins that bind the RNA may function as a translational enhancer [35]. Generally, the gene expression of anti-oxidant enzymes with the respective activities either increases to a greater or lesser extent [36,37], remains unchanged, even when messenger levels are elevated [33–36] or even falls [38]. Although more difficult to explain, elevations in enzyme activities with no change in messenger production have also been reported [39]. Post-transcriptional effects, the presence of preformed anti-oxidant enzymes or conformational changes could have led to increased anti-oxidant enzyme activity [40]. These data therefore show the danger of measuring only mRNA levels when looking for a particular response when post-transcriptional mechanisms are exerting a controlling effect.
In summary, these results demonstrate the occurrence of oxidative stress in the different types of NIR examined. Moreover, although we, like others for asthma [23], found no general overall association between oxidative stress and the severity of the cutaneous manifestations, we did find a certain correlation between stress and severity in the carbonyl content. Although excessive production of ROS initiates oxidative stress and multiple pathological states are related to this situation, it is unclear whether this oxidative stress is the cause or the consequence of the allergic reaction to drugs. Further understanding of the mechanisms involved in oxidative stress, as well as their relationship with the immunological response, will aid the development of new therapeutic strategies in this and other related fields.
Acknowledgments
We thank Ian Johnstone for help with the English language version of the manuscript. This study was partly supported by the Spanish Ministry of Health (FIS 01/0014, FIS 01/3031 and FIS PIO20640) and the Junta de Andalucía (134/01).
References
- 1.World Health Organization (WHO). International drug monitoring: the role of the hospital. Geneva: WHO; 1966. [Google Scholar]
- 2.Levine BB. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med. 1966;275:1115–25. doi: 10.1056/NEJM196611172752009. [DOI] [PubMed] [Google Scholar]
- 3.Adkinson NF. Drug allergy. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FE, editors. Middleton’s allergy: principles and practice. Philadelphia: Mosby Inc.; 2003. pp. 1679–94. vol. 2. [Google Scholar]
- 4.Gadde J, Spence M, Wheeler B, Adkinson NF., Jr Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456–63. [PubMed] [Google Scholar]
- 5.Roujeau JC, Stern RS. Severe cutaneous adverse reactions to drugs. N Engl J Med. 1994;331:1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 6.Mauri-Helweg D, Bettens F, Mauri D, Brander C, Hunziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin and carbamazepine. J Immunol. 1995;155:462–72. [PubMed] [Google Scholar]
- 7.Weiss ME, Adkinson NF. Immediate hypersensitivity reactions to penicillins and related antibiotics. Clin Allergy. 1998;18:515–40. doi: 10.1111/j.1365-2222.1988.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 8.Pichler WJ, Snyder B, Zanni MP, Hari Y, von Greyerz S. Role of T cells in drug allergies. Allergy. 1998;53:22–32. doi: 10.1111/j.1398-9995.1998.tb03881.x. [DOI] [PubMed] [Google Scholar]
- 9.Matés JM. Effects of anti-oxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;53:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 10.Nemoto S, Takeda KYuZX, Ferrans VJ, Finkel T. A role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol. 2000;20:7311–8. doi: 10.1128/mcb.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cemersky S, Cantagrel A, van Meerwijk J, Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem. 2002;227:19585–93. doi: 10.1074/jbc.M111451200. [DOI] [PubMed] [Google Scholar]
- 12.Matés JM, Pérez-Gómez C, Blanca M. Chemical and biological activity of free radical scavengers in allergic diseases. Clin Chem Acta. 2000;296:1–15. doi: 10.1016/s0009-8981(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 13.Matés JM, Segura JM, Pérez-Gómez C, et al. Anti-oxidant enzymatic activities in human blood cells after an allergic reaction to pollen or house dust mite. Blood Cells Mol Dis. 1999;25:103–9. doi: 10.1006/bcmd.1999.0234. [DOI] [PubMed] [Google Scholar]
- 14.Matés JM, Pérez-Gómez C, Olalla L, Segura JM, Blanca M. Allergy to drugs: anti-oxidant enzymic activities, lipid peroxidation and protein oxidative damage in human blood. Cell Biochem Funct. 2000;18:77–84. doi: 10.1002/(SICI)1099-0844(200006)18:2<77::AID-CBF851>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Brockow K, Romano A, Blanca M, Ring J, Pichler WJ, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002;57:45–51. [PubMed] [Google Scholar]
- 16.Esterbauer H, Cheeseman KH. Determination of aldehyde lipid peroxidation products: malondyaldehyde and 4-hydroxynonenal. Meth Enzymol. 1984;105:457–64. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 17.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Meth Enzymol. 1994;233:357–63. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 18.Kozer E, Evans S, Barr J, et al. Glutathione, glutathione-dependent enzymes and anti-oxidant status in erythrocytes from children treated with high-dose paracetamol. Br J Clin Pharmacol. 2003;55:234–40. doi: 10.1046/j.1365-2125.2003.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Raeve HR, Thunnissen FB, Kaneko FT, et al. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroids in vivo. Am J Physiol. 1997;272:L148–54. doi: 10.1152/ajplung.1997.272.1.L148. [DOI] [PubMed] [Google Scholar]
- 20.Cabell KS, Ma L, Johnson P. Effects of antihypertensive drugs on rat tissue anti-oxidant enzymes activities and lipid peroxidation levels. Biochem Pharmacol. 1997;46:133–41. doi: 10.1016/s0006-2952(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 21.Briganti S, Picardo M. Anti-oxidant activity, lipid peroxidation and skin diseases. What’s new? J Eur Acad Dermatol Venereol. 2003;17:663–9. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 22.Nadeem A, Chhabre SK, Masood A, Raj HG. Increased oxidative stress and altered levels of anti-oxidants in asthma. J Allergy Clin Immunol. 2003;111:72–8. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 23.Mak JC, Leung HC, Ho SP, et al. Systemic oxidative and oxidative status in Chinese patients with asthma. J Allergy Clin Immunol. 2004;114:260–4. doi: 10.1016/j.jaci.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Radak Z, Asano K, Lee KC, et al. High altitude training increases reactive carbonyl derivatives but not lipid peroxidation in skeletal muscle of rats. Free Radic Biol Med. 1997;22:1109–14. doi: 10.1016/s0891-5849(96)00350-4. [DOI] [PubMed] [Google Scholar]
- 25.Paul C, Wolkenstein P, Adle H, et al. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol. 1996;134:710–4. doi: 10.1111/j.1365-2133.1996.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 26.Pantke U, Volk T, Schmutzler M, Kox WJ, Sitte N, Grune N. Oxidized proteins as a marker of oxidative stress during coronary heart surgery. Free Radic Biol Med. 1999;27:1080–6. doi: 10.1016/s0891-5849(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 27.Hildeman DA, Mitchell T, Teague TK, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–44. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 28.England K, O’Driscoll C, Cotter TG. Carbonylation of glycolytic proteins is a key response to drug-induced oxidative stress and apoptosis. Cell Death Diff. 2004;11:252–60. doi: 10.1038/sj.cdd.4401338. [DOI] [PubMed] [Google Scholar]
- 29.Sancho P, Troyano A, Fernández C, De Blas E, Aller P. Differential effects of catalase on apoptosis induction in human promonocytic cells. Relationships with heat-shock protein expression. Mol Pharmacol. 2003;63:581–9. doi: 10.1124/mol.63.3.581. [DOI] [PubMed] [Google Scholar]
- 30.Ho YS, Dey MS, Crapo JD. Anti-oxidant enzyme expression in rat lungs during hyperoxia. Am J Physiol. 1996;270:L810–8. doi: 10.1152/ajplung.1996.270.5.L810. [DOI] [PubMed] [Google Scholar]
- 31.Rohrdanz E, Kahl R. Alterations of anti-oxidant enzyme expression in response to hydrogen peroxide. Free Radic Biol Med. 1998;24:27–38. doi: 10.1016/s0891-5849(97)00159-7. [DOI] [PubMed] [Google Scholar]
- 32.Tsam MF, White JE, Treanor C, Shaffer JB. Molecular basis for tumor necrosis factor-induced increase in pulmonary superoxide dismutase activities. Am J Physiol. 1990;259:L506–12. doi: 10.1152/ajplung.1990.259.6.L506. [DOI] [PubMed] [Google Scholar]
- 33.Gómez M, Esparza JL, Nogués MR, Giral M, Cabré M, Domingo JL. Pro-oxidant activity of aluminum in the rat hippocampus: gene expression of anti-oxidant enzymes after melatonin administration. Free Radic Biol Med. 2005;38:104–11. doi: 10.1016/j.freeradbiomed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Clerch LB. Post-transcriptional regulation of lung anti-oxidant enzyme gene expression. Ann NY Acad Sci. 2000;899:103–11. doi: 10.1111/j.1749-6632.2000.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 35.Chung DJ, Wright AE, Clerch LB. The 3′ untranslated region of manganese superoxide dismutase RNA contains a translational enhancer element. Biochemistry. 1998;37:16298–306. doi: 10.1021/bi980935g. [DOI] [PubMed] [Google Scholar]
- 36.Yoshioka T, Homma T, Meyrick B, et al. Oxidants induce transcriptional activation of manganese superoxide dismutase in glomerular cells. Kidney Int. 1994;46:405–13. doi: 10.1038/ki.1994.288. [DOI] [PubMed] [Google Scholar]
- 37.Cowan DB, Weisel RD, Williams WG, Mickle DAG. The regulation of glutathione peroxidase gene expression by oxygen tension in cultured human cardiomyocytes. J Mol Cell Cardiol. 1992;24:423–33. doi: 10.1016/0022-2828(92)93196-q. [DOI] [PubMed] [Google Scholar]
- 38.Li T, Danelisen I, Bello-Klein A, Singal PK. Effects of probucol on changes of anti-oxidant enzymes in adriamycin-induced cardiomyopathy in rats. Cardiovasc Res. 2000;46:523–30. doi: 10.1016/s0008-6363(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 39.Franco AA, Odom RS, Rando TA. Regulation of anti-oxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27:1122–32. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- 40.Harris ED. Regulation of anti-oxidant enzymes. FASEB J. 1992;6:2675–83. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]