Abstract
Approximately 5% of people infected with human T lymphotropic virus type 1 (HTLV-1) develop clinical myelopathy or tropical spastic paraparesis (HAM/TSP) that is associated with high-levels of Th1 cytokines, interferon (IFN)-γ and tumour necrosis factor (TNF)-α. Chemokines are known to induce cytokine secretion and direct the trafficking of immune cells to sites of disease. The present study measured serum chemokines correlated with autonomously released IFN-γ in cell cultures. HTLV-1 infection was defined by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot. Subjects included HTLV-1 carriers (n = 56), patients with HAM/TSP (n = 31) and healthy HTLV-1 seronegative volunteer controls (n = 20). Serum chemokines and IFN-γ autonomously released by mononuclear cells in culture were quantified by ELISA. Compared to HTLV-1 carriers, serum chemokines in HAM/TSP patients showed significantly increased levels of CXCL9 and CXCL10, significantly diminished levels of CCL2 and similar amounts of CCL11 and CCL24. In contrast, CCL11 and CCL24 were significantly lower in serum of HAM/TSP patients than either control. IFN-γ was positively correlated with CXCL9 and CXCL10 when HAM/TSP and HTLV-1 carriers were used as a combined group. However, despite a large proportion of HTLV-1 carriers having high IFN-γ levels, these chemokines were not increased in carriers. This study showed that high levels of CXCL9 and CXCL10 in the systemic circulation and low serum CCL2 levels are features of HAM/TSP. HTLV-1 infection and Tax and/or additional viral encoded factor-mediated pathological processes triggering T cell activation with autogenous IFN-γ release are probably involved in regulating chemokine release.
Keywords: chemokines, CCL2, CXCL10, HAM/TSP, HTLV-1
Introduction
The human T cell lymphotropic virus (HTLV-1) infects predominantly T cells, leading to spontaneous T cell proliferation and production of cytokines [1]. The majority of HTLV-1 infected individuals are considered carriers, while only a small percentage (< 5%) of those infected develop severe disease, such as myelopathy associated with HTLV-1/tropical spastic paraparesis (HAM/TSP) or adult T cell leukaemia/lymphoma (ATLL) [2,3]. HAM/TSP is an inflammatory disease associated with circulating activated CD4+ T cells, CD8+ T cells, and high production of inflammatory cytokines, including tumour necrosis factor (TNF)-α, interferon (IFN)-γ and interleukin (IL)-2 [4,5]. The pathogenic inflammation of HAM/TSP is associated with an increased number of HTLV-1-specific CD4+ T cells and CD8+ T cells in cerebral spinal fluid (CSF), due to migration across the blood–brain barrier (BBB) or expansion within the CSF compartment [4,6]. However, the pathogenesis of HAM/TSP is incompletely understood [7]. HTLV-1 infects predominantly T cells, transactivating genes involved with the secretion of several cytokines [8]. Mononuclear cells from HTLV-1-infected subjects produce high amounts of IFN-γ and TNF-α. Although IFN-γ and TNF-α are significantly higher in HAM/TSP than HTLV-1 carriers in supernatants of blood lymphocyte cultures, a large proportion of HTLV-1 carriers produce similar amounts of IFN-γ compared to patients who have HAM/TSP [5].
Chemokines are low molecular weight cytokines with various roles, principally in migration and tissue localization of various lymphocyte subpopulations [9]. Cell lines infected with HTLV-1 express chemokines such as CCL2, CCL3, CCL4, CCL5, CXCL8 and CXCL10 [10]. Moreover, it is also known that production of cytokines may modulate (up- or down-regulation) chemokine synthesis [11]. Recently, several studies have detected chemokines in CSF of patients with autoimmune and infectious diseases and have suggested a potential pathophysiological role for these chemoattractant molecules [12,13,14]. Chemokines could induce not only secretion of other mediators of the inflammatory process, but could also directly attract activated T cells to the central nervous system (CNS) [15,16]. Increased chemokine levels in serum and CSF have been described in experimental autoimmune encephalitis (EAE), multiple sclerosis (MS) [14,17] and Sydenham’s chorea [18], and there have been two reports of elevated CSF chemokines in patients with HAM/TSP [18,19]. In the present study, the serum levels of CCL2, CCL3, CCL11, CCL24, CXCL8, CXCL9 and CXCL10 and autogenous IFN-γ production by peripheral blood mononuclear cells (PBMCs) were evaluated in healthy volunteers, HTLV-1 carriers and in patients with HAM/TSP.
Materials and methods
Participants of the present study included 56 HTLV-1 carriers and 31 patients with HAM/TSP, all from the HTLV-1 multi-disciplinary clinic of the Hospital Universitário Prof Edgard Santos. Twenty healthy people without HTLV-1 infection were used as controls. A clinical history was taken and a physical examination performed in all patients. The diagnosis of HTLV-1 infection was made by antibody detection by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot (Genelabs HTLV 2·3–2·4, Singapore). Patients admitted to the clinic are evaluated twice a year by a neurologist and divided into three groups according to Osame's motor disability score (OMDS) and expanded disability status scale (EDSS): HAM/TSP patients, HTLV-1 carriers without neurological symptoms and HTLV-1 carriers with mild neurological or urological symptoms (‘oligosymptomatic’ patients) who did not fulfil the WHO criteria for HAM/TSP [20,21]. We evaluated HTLV-1 carriers without neurological symptoms (referred to hereafter as HTLV-1 carriers). Informed consent was obtained for all participants and human experimentation guidelines of the Hospital Universitário Prof Edgard Santos were followed in the conduction of this clinical research. All patients had negative stool exams for helminths.
The age of the 31 patients with HAM/TSP ranged from 17 to 80 years (mean 53 ± 13) and 65% were women. The age of the 56 HTLV-I carriers ranged from 22 to 64 years (mean 45 ± 10) and 55% were women. The age of the 20 healthy controls ranged from 22 to 50 years (mean 34 ± 9) and 70% were women.
Serum processing for chemokines analysis
Blood was collected aseptically in lipopolysaccharide-free siliconized tubes and serum prepared and stored at − 70 ° until required. For analysis samples were thawed and excess proteins were removed by acid/salt precipitation. Briefly, equal volumes of serum and 1·2% trifluoracetic acid/1·35 M NaCl were mixed and left at room temperature for 10 min. After the samples were centrifuged for 5 min at 3000 g, the supernatants were then adjusted for salt content (0·14 M sodium chloride and 0·01 M sodium phosphate) and pH 7·4 for determination of chemokine concentrations [22].
Mononuclear cell separation and determination of IFN-γ
PBMCs were obtained by density gradient centrifugation using lymphocyte separation media (LSM; Organon Tecnika Corporation, Duham, NC, USA). After washing in saline, the cells were adjusted to 3 × 106/ml in RPMI-1640 (Gibco, Grand Island, NY, USA) containing 100 U penicillin G and 10 µ/ml of streptomycin and supplemented with 10% AB serum. All cultures were incubated without stimulus at 37°C in 5% CO2 for 72 h. Supernatants were collected and stored at − 20 ° C.
Chemokine and IFN-γ measurements
Chemokines were measured by sandwich ELISA with matched antibody pairs for CXCL8, CXCL9 and CXCL10 (PharMingen, San Diego, CA, USA) and for CCL2, CCL3, CCL11 and CCL24 (R&D Systems, Minneapolis, MN, USA). All samples were assayed in duplicate and on the same plate. The detection limits for these assays were 5 pg/ml for CCL2 and CCL3 and 20 pg/ml for CCL11, CCL24, CXCL8, CXCL9 and CXCL10. IFN-γ was measured by commercial ELISA following the manufacturers’ instructions (PharMingen).
Statistical analysis
Chemokines in the controls and in the two patient groups were analysed by the Mann–Whitney U-test. Correlation between IFN-γ and cytokine levels CXCL9, CXCL10 and CCL2 was performed with Spearman’s rank correlation test.
Results
Th2-associated chemokines, CCL11 and CCL24
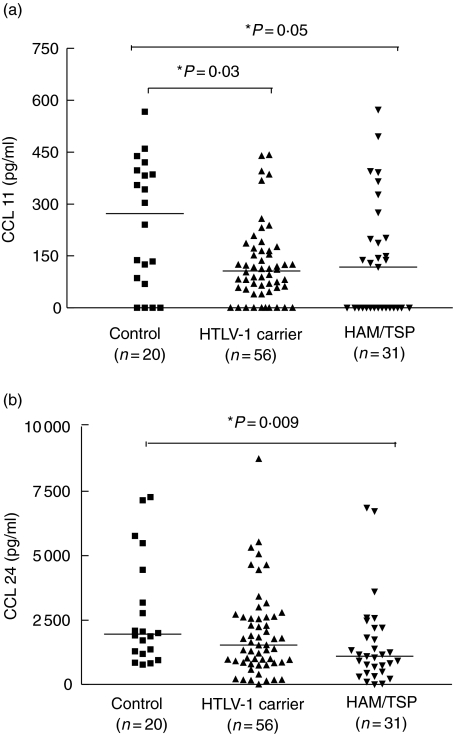
Ligands of the chemokine CCR3 receptor were evaluated. Serum levels of CCL11 were significantly lower in HTLV-I carriers (median 106 pg/ml ranging from 0–84) than in healthy controls (median 272 pg/ml, range 0–567 pg/ml), P = 0·03, Mann–Whitney U-test (Fig. 1a). A strong trend towards lower serum levels of CCL11 was observed between patients with HAM/TSP (median 118 pg/ml, range 0–572 pg/ml) and healthy controls (P = 0·05). Serum levels of CCL24 were significantly lower in HAM/TSP patients (median 1095 pg/ml range 0–6857) when compared to serum levels to healthy controls (1951 pg/ml range 769–7267), P = 0·009, Mann–Whitney U-test (Fig. 1b). Serum levels of CCL24 were not significantly different between healthy controls and HTLV-1 carriers (1527 pg/ml range 0–8753), P = 0·12.
Fig. 1.
Serum levels of type 2 chemokines, CXCL11 (a) and CXCL24 (b) are shown for healthy volunteers (n = 20) in human T cell lymphotropic virus (HTLV-1) carriers (n = 56) and in patients with HTLV-1/tropical spastic paraparesis (HAM/TSP) (n = 31). Mann–Whitney U-test was used for statistical analysis.
CCL2, CCL3 and CXCL8
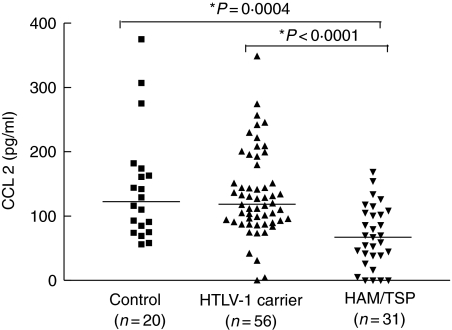
Serum levels of CCL2 were lower in HAM/TSP patients (median 67 pg/ml range 0–169) than HTLV-1 carriers (118 pg/ml ranging 0–348) or healthy controls (123 pg/ml range 56–375), P < 0·0001 and P = 0·0004, respectively. No significant differences were found in serum levels of CCL2 between healthy controls and HTLV-1 carriers (Fig. 2). No correlation was found between IFN-γ and CCL2 in patients with HAM/TSP. Levels of CCL3 and CXCL8 detected were similar in all groups studied. CCL3 levels in HAM/TSP, HTLV-1 carriers and controls ranged from 0 to 1158 pg/ml, 0–2364 pg/ml and 0–492 pg/ml, respectively (data not shown). The median for all groups was 0. CXCL8 levels in HAM/TSP patients, HTLV-1 carriers and controls ranged from 0 to 4993 pg/ml, 0–2422 pg/ml and 0–793 pg/ml, respectively. The median for all groups was 0 (data not shown).
Fig. 2.
Serum levels of CCL2 in patients with different clinical forms of human T cell lymphotropic virus (HTLV-1) infection [carrier, n= 56 and HTLV-1/tropical spastic paraparesis (HAM/TSP), n= 31] and in healthy subjects (n = 20). Mann–Whitney U-test was used for statistical analysis.
Th1-associated CXCL9 and CXCL10
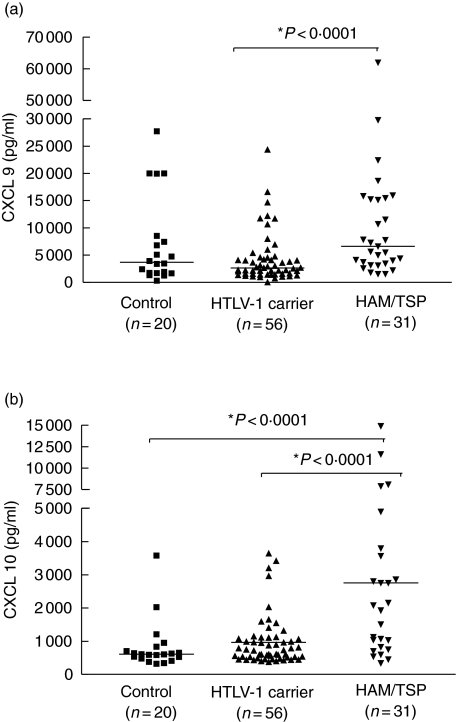
The serum levels of CXCL9 were significantly higher in HAM/TSP patients (P < 0·0001) than in HTLV-I carriers and healthy controls. The median serum levels of CXCL9 for HAM/TSP patients and HTLV-1 carriers were 6605 pg/ml (range 1556–66 052) and 2660 pg/ml (range 6–31 220), respectively (Fig. 3a). In contrast, levels of CXCL9 were similar between HTLV-1 carriers and controls. CXCL9 values were variable and approximately 40% of HTLV-1 carriers had levels of CXCL9 that were similar to those observed in patients with HAM/TSP. High levels of CXCL9 were also observed in a few healthy controls.
Fig. 3.
Serum levels of CXCL9 (a) and CXCL10 (b) are shown for healthy volunteers (n = 20), in human T cell lymphotropic virus (HTLV-1) carriers (n = 56) and in patients with HTLV-1/tropical spastic paraparesis (HAM/TSP) (n = 31). Mann–Whitney U-test was used for statistical analysis.
The serum levels of CXCL10 were significantly higher (P < 0·0001) in HAM/TSP patients than in HTLV-1 carriers and healthy controls (Fig. 3b). The median serum levels of CXCL10 in HAM/TSP patients versus HTLV-1 carriers and controls were 2777 pg/ml (range 339–14 881), pg/ml versus 719 pg/ml (range 371–3640) and 608 pg/ml (range 320–3576), respectively. The CXCL10 level was significantly associated with HAM/TSP and 93% of HTLV-1 carriers had CXCL10 levels lower than the median observed in the patients with HAM/TSP. Serum levels of CXCL9 and CXCL10 were similar in healthy controls and HTLV-1 carriers.
Correlation between IFN-γ and chemokines
IFN-γ is produced spontaneously by PBMCs from HAM/TSP patients and HTLV-1 carriers [1]. An association between levels of IFN-γ released in vitro and some chemokines, e.g. CXCL9 and CXCL10, have been reported [23]. We therefore measured amounts of IFN-γ produced by unstimulated PBMC cultured in vitro from HTLV-1 carriers and HAM/TSP patients. In this study, the median IFN-γ levels in HTLV-1 carriers and HAM/TSP patients were, respectively, 567 pg/ml (range 0–6895) and 2515 pg/ml (range 58–10 750). Despite higher IFN-γ levels in HAM/TSP compared with HTLV-1 carriers, a large percentage (∼42%) of HTLV-1 carriers produced IFN-γ as high as patients with HAM/TSP. This result was similar to previous findings, that ∼50% of HTLV-1 carriers had IFN-γ levels that were as high as patients with HAM/TSP [1,5]. When HAM/TSP and HTLV-1 carriers were analysed as a combined group, levels of IFN-γ directly correlated with CXCL9 and CXCL10 (Table 1), Spearman’s rank correlation test. However, when HTLV-1 infection was segregated into HAM/TSP or HTLV-1 carriers, the correlation between IFN-γ and CXCL9 was lost and there was a tendency for a direct correlation between IFN-γ and CXCL10 in HAM/TSP. Moreover, although a large proportion of HTLV-1 carriers had IFN-γ levels similar to that observed in HAM/TSP and higher (P < 0·0001) than that observed in controls, the chemokines CXCL9 and CXCL10 were not increased in serum of HTLV-1 carriers.
Table 1.
Correlation between interferon (IFN)-γ and chemokine levels CXCL9 and CXCL10 in the whole group of individuals infected with human T cell lymphotropic virus (HTLV-1) in patients with HTLV-1/tropical spastic paraparesis (HAM/TSP) and in HTLV-1 carriers.
| Groups | Variables | R | P-value* |
|---|---|---|---|
| HTLV-1 infected individuals | IFN-γ × CXCL9 | 0·27 | 0·009 |
| n = 86 | IFN-γ × CXCL10 | 0·33 | 0·001 |
| Patients with HAM/TSP | IFN-γ × CXCL9 | 0·27 | 0·14 |
| n = 31 | IFN-γ × CXCL10 | 0·34 | 0·06 |
| HTLV-1 carriers | IFN-γ × CXCL9 | 0·05 | 0·68 |
| n = 55 | IFN-γ × CXCL10 | 0·08 | 0·52 |
Spearman’s rank correlation test.
Discussion
Chemokines perform several immunological functions, of which gradient directed cell recruitment was the first defined action. Chemokines have also been noted to influence CD4 Th0 T cell polarization to type 1 or type 2 cells and to retain cells in the inflamed tissue [15,24]. HTLV-1 infection is associated with an enhanced autonomous type 1 immune response [25] and the disease, HAM/TSP, is thought to be mediated by local host immunity triggered by HTLV-1 [7]. This study showed that high levels of CXCL9 and CXCL10 and low levels of CCL2 were features of patients with HAM/TSP.
HTLV-1 infection is characterized by activation of Th1 cells, and specific sets of chemokines have selective activity towards type 1 or type 2 T cells. CXCL9 and CXCL10 are chemokines known to recruit and support Th1 cells and are increased in MS, a disease with an enhanced Th1 immune response [11,26]. In contrast, high levels of CCL11 and CCL24 are elevated in patients with helminth infections, diseases associated with a type 2 of the immune response [22]. Our finding of diminished levels of CCL11 and CCL24 in HAM/TSP patients is compatible with the hypothesis that the polarization to a type 1 immune response may down-modulate type 2 T cell activity, represented here by CCL11 and CCL24. Our previous finding, that in patients co-infected with HTLV-1 and Strongyloides stercoralis or Schistosoma mansoni there is decreased IL-4 and IL-5 production in response to parasite antigens [27–29], supports this hypothesis.
Many types of cells produce chemokines and their levels are variable, even in healthy subjects. In this study, CCL3 and CXCL8 were detected in only a few individuals of the three groups studied. CCL-2 was higher in healthy subjects and in HTLV-1 carriers than in patients with HAM/TSP. The role of CCL2 in central nervous inflammatory disease differs in experimental models and humans. While CCL2-deficient mice are resistant to EAE [30], the CSF of patients with active MS has a decreased amount of CCL2 [31]. It is likely that decreased CSF CCL2 in MS and the decreased serum levels of CCL2 observed in patients with HAM/TSP in this study reflects a polarization of the immune response toward a type 1 cytokine profile, as CCL2 is probably associated with type 2 responses [24].
The most striking finding of the present study was increased levels of CXCL9 and CXCL10 in patients with HAM/TSP compared to HTLV-1 carriers. IFN-γ was positively correlated with CXCL9 and CXCL10 when HAM/TSP patients and HTLV-1 carriers were used as a combined group. However, when HAM/TSP patients and HTLV-1 carriers were analysed alone the correlation with CXCL9 and CXCL10 was lost. It is likely that, in addition of the role of IFN-γ in inducing chemokine secretion, shared but not necessarily synonymous viral-mediated processes are involved in up-regulating CXCL9, CXCL10 and IFN-γ.
Our finding of high serum levels of CXCL9 and CXCL10 raises the question of where these chemokines are produced and by what cell type. We speculate that in HAM/TSP patients one compartment may be the CNS, in which recruited immune cells and resident cells participate in the production of these chemokines. Several lines of data support this speculation. HAM/TSP is characterized by T cell infiltration and lesion of the spinal cord [7]. It is known that activated T cells can penetrate an intact BBB and enter the CNS perivascular or subarachnoid space to initiate and perpetuate the inflammatory process in the presence of antigen that maintains T cell stimulation [32,33]. Migration of inflammatory cells within the CNS parenchyma may be directed by a chemotactic gradient created by chemokines that diffuse from sites of production at foci of inflammation [30,34,35]. Resident glial cells, including astrocytes, express chemokines in great abundance in both autoimmune and anti-viral inflammatory response [13,36] and high levels of CXCL10 have been documented in immunologically mediated diseases of the CNS such as MS [17] and in EAE [14]. Recently, two studies reported increased CXCL10 levels in the CSF of a small number of patients with HAM/TSP [18,19]. Increased concentrations of CXCL10 in MS are observed during flares of disease activity but not in periods of disease remission [37]. In addition to HAM/TSP, HTLV-1-infected patients have also other associated diseases, and other sites may also participate in the release of CXCL9 and CXCL10 into the systemic circulation. We also cannot exclude the possibility that circulating immune cells produce CXCL9 and CXCL10 because we did not perform their measurements in in vitro cultured PBMCs. On the other hand, as chemokines function by attracting cells in response to a chemotactic gradient, it is unlikely that a systemic production of these mediators makes more biological sense.
Proviral load has a clear correlation with HTLV-1 disease state. The proviral load of these patients was not assessed. We therefore cannot exclude the possibility that these differences in chemokine levels were not due, in part, to the expected difference in proviral load between HTLV-1 carriers and HAM/TSP patients.
This study shows that chemokine levels differ in healthy controls, HTLV-1 carriers and HAM/TSP. In contrast to low CCL2 levels in HAM/TSP patients, serum levels of CXCL9 and CXCL10 were significantly higher in HAM/TSP patients than healthy volunteers and HTLV-1 carriers. As IFN-γ levels alone could not justify the increase in chemokines in HAM/TSP, this suggests that, in addition to the predominant type 1 response, a distinct HTLV-1-mediated process may be involved. Furthermore, elevated levels of CXCL9 and CXCL10 and lowered levels of CCL2 probably modulate selective leucocyte recruitment to initiate local pathology seen in HAM/TSP.
Acknowledgments
This work was supported financially by the Brazilian National Research Council (CNPq), Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), NIH research grants no. D43 TW007127 funded by the Fogarty International Center and no. R03 AI060830. E. M. C. is a senior investigator of the CNPq. D. J. M. was supported by NIH grant no. T32 AI07613.
References
- 1.Carvalho EM, Bacellar O, Porto AF, Braga S, Galvao-Castro B, Neva F. Cytokine profile and immunomodulation in asymptomatic human T-lymphotropic virus type 1-infected blood donors. J Acquir Immune Defic Syndr. 2001;27:1–6. doi: 10.1097/00126334-200105010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I) J Emerg Med. 2000;18:109–19. doi: 10.1016/s0736-4679(99)00173-0. [DOI] [PubMed] [Google Scholar]
- 3.Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Biddison WE, Kubota R, Kawanishi T, et al. Human T cell leukemia virus type I (HTLV-I)-specific CD8+ CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. J Immunol. 1997;159:2018–25. [PubMed] [Google Scholar]
- 5.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004;4:7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey WF. Migration of hematogenous cells through the blood–brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1:97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson S. Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J Infect Dis. 2002;186(Suppl. 2):S187–92. doi: 10.1086/344269. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen TH, Paludan SR. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65:131–50. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 10.Baba M, Imai T, Yoshida T, Yoshie O. Constitutive expression of various chemokine genes in human T-cell lines infected with human T-cell leukemia virus type 1: role of the viral transactivator Tax. Int J Cancer. 1996;66:124–9. doi: 10.1002/(SICI)1097-0215(19960328)66:1<124::AID-IJC21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Salmaggi A, Gelati M, Dufour A, et al. Expression and modulation of IFN-inducible chemokines (IP-10, Mig, and I-TAC) in human brain endothelium and astrocytes: possible relevance for the immune invasion of the central nervous system and the pathogenesis of multiple sclerosis. J Interferon Cytokine Res. 2002;22:631–40. doi: 10.1089/10799900260100114. [DOI] [PubMed] [Google Scholar]
- 12.Ransohoff RM. The chemokine system in neuroinflammation: an update. J Infect Dis. 2002;186(Suppl. 2):S152–6. doi: 10.1086/344266. [DOI] [PubMed] [Google Scholar]
- 13.Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–12. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 14.Fife BT, Kennedy KJ, Paniagua MC, et al. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7617–24. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- 15.Biernacki K, Prat A, Blain M, Antel JP. Regulation of Th1 and Th2 lymphocyte migration by human adult brain endothelial cells. J Neuropathol Exp Neurol. 2001;60:1127–36. doi: 10.1093/jnen/60.12.1127. [DOI] [PubMed] [Google Scholar]
- 16.Biernacki K, Prat A, Blain M, Antel JP. Regulation of cellular and molecular trafficking across human brain endothelial cells by Th1 and Th2-polarized lymphocytes. J Neuropathol Exp Neurol. 2004;63:223–32. doi: 10.1093/jnen/63.3.223. [DOI] [PubMed] [Google Scholar]
- 17.Trebst C, Ransohoff RM. Investigating chemokines and chemokine receptors in patients with multiple sclerosis: opportunities and challenges. Arch Neurol. 2001;58:1975–80. doi: 10.1001/archneur.58.12.1975. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira AL, Jr, Cardoso F, Souza AL, Teixeira MM. Increased serum concentrations of monokine induced by interferon-gamma/CXCL9 and interferon-gamma-inducible protein 10/CXCL-10 in Sydenham’s chorea patients. J Neuroimmunol. 2004;150:157–62. doi: 10.1016/j.jneuroim.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Narikawa K, Fujihara K, Misu T, et al. CSF-chemokines in HTLV-I-associated myelopathy: CXCL10 up-regulation and therapeutic effect of interferon-alpha. J Neuroimmunol. 2005;159:177–82. doi: 10.1016/j.jneuroim.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Osame M. Review of WHO Kagoshima meeting and diagnosis guidelines from HAM/TSP. In: Blatter WA, editor. Human retrovirology: HTLV. New York: Rayen Press; 1990. pp. 191–7. [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 22.Falcao PL, Correa-Oliveira R, Fraga LA, et al. Plasma concentrations and role of macrophage inflammatory protein-1alpha during chronic Schistosoma mansoni infection in humans. J Infect Dis. 2002;186:1696–700. doi: 10.1086/345370. [DOI] [PubMed] [Google Scholar]
- 23.Narumi S, Wyner LM, Stoler MH, Tannenbaum CS, Hamilton TA. Tissue-specific expression of murine IP-10 mRNA following systemic treatment with interferon gamma. J Leukoc Biol. 1992;52:27–33. doi: 10.1002/jlb.52.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–11. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 25.Goon PKC, Hanon E, Igakura T, et al. High frequencies of Th1-type CD4+ T cells specific to HTLV-1 Env and Tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood. 2002;99:3335–41. doi: 10.1182/blood.v99.9.3335. [DOI] [PubMed] [Google Scholar]
- 26.Mahad DJ, Howell SJL, Woodroofe MN. Expression of chemokines in the CSF and correlation with clinical disease activity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;72:498–502. doi: 10.1136/jnnp.72.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neva FA, Filho JO, Gam AA, et al. Interferon-gamma and interleukin-4 responses in relation to serum IgE levels in persons infected with human T lymphotropic virus type I and Strongyloides stercoralis. J Infect Dis. 1998;178:1856–9. doi: 10.1086/314507. [DOI] [PubMed] [Google Scholar]
- 28.Porto AF, Neva FA, Bittencourt H, et al. HTLV-1 decreases Th2 type of immune response in patients with strongyloidiasis. Parasite Immunol. 2001;23:503–7. doi: 10.1046/j.1365-3024.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 29.Porto AF, Santos SB, Alcantara L, et al. HTLV-1 modifies the clinical and immunological response to schistosomiasis. Clin Exp Immunol. 2004;137:424–9. doi: 10.1111/j.1365-2249.2004.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang D, Han Y, Rani MR, et al. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol Rev. 2000;177:52–67. doi: 10.1034/j.1600-065x.2000.17709.x. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen TL, Trebst C, Kivisakk P, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 32.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–60. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 33.Wekerle H, Sun D, Oropeza-Wekerle RL, Meyermann R. Immune reactivity in the nervous system. modulation of T-lymphocyte activation by glial cells. J Exp Biol. 1987;132:43–57. doi: 10.1242/jeb.132.1.43. [DOI] [PubMed] [Google Scholar]
- 34.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–24. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 35.Umehara F, Izumo S, Takeya M, Takahashi K, Sato E, Osame M. Expression of adhesion molecules and monocyte chemoattractant protein-1 (MCP-1) in the spinal cord lesions in HTLV-I-associated myelopathy. Acta Neuropathol (Berl) 1996;91:343–50. doi: 10.1007/s004010050435. [DOI] [PubMed] [Google Scholar]
- 36.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;150:617–30. [PMC free article] [PubMed] [Google Scholar]
- 37.Franciotta D, Martino G, Zardini E, et al. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J Neuroimmunol. 2001;115:192–8. doi: 10.1016/s0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]