Abstract
Efficient early identification of primary immunodeficiency disease (PID) is important for prognosis, but is not an easy task for non-immunologists. The Clinical Working Party of the European Society for Immunodeficiencies (ESID) has composed a multi-stage diagnostic protocol that is based on expert opinion, in order to increase the awareness of PID among doctors working in different fields. The protocol starts from the clinical presentation of the patient; immunological skills are not needed for its use. The multi-stage design allows cost-effective screening for PID within the large pool of potential cases in all hospitals in the early phases, while more expensive tests are reserved for definitive classification in collaboration with an immunologist at a later stage. Although many PIDs present in childhood, others may present at any age. The protocols presented here are therefore aimed at both adult physicians and paediatricians. While designed for use throughout Europe, there will be national differences which may make modification of this generic algorithm necessary.
Keywords: diagnostic protocol, immunological evaluation, primary immunodeficiency
Introduction
Classical primary immunodeficiency disease (PID) [1,2] is relatively rare (approximately 1 : 500–1 : 500 000 in the general population, with variable degrees of ascertainment in different countries). It has therefore not been easy to generate sufficient evidence to support diagnostic decisions. Although many PIDs present in childhood, the most common clinically significant PID, common variable immunodeficiency (CVID), has a peak onset in the second and third decades of life. Early detection of PID, before serious infections have compromised the patient’s general condition, is important for the proband’s prognosis, and for timely genetic counselling of his family [2]. Adult physicians as well as paediatricians need therefore to consider PID as a potential diagnosis. However, efficient identification of PID within the large pool of potential cases is difficult for non-immunologists. Previously published protocols for diagnosing PID (e.g. [3,4]). are founded on the traditional classification of antibody, T lymphocyte, phagocyte and complement deficiencies. They require at least some knowledge of the immune system and its defects by their users which non-immunologists often lack, making recognition of these conditions difficult [5].
Based on a Dutch initiative [6–8], the Clinical Working Party of the European Society for Immunodeficiencies (ESID) has composed a protocol for diagnosing PID. This protocol is based necessarily on expert opinion; validation will need to be obtained with clinical practice, possibly leading to amendments in the future. The protocol starts from the clinical presentation of the patient. Not only can this be recognized by all doctors, it is also the best reflection of the physiological effect of the underlying disorder [9]. The protocol is aimed at both paediatricians and adult physicians. The multi-stage design allows timely identification of potential PID in all hospitals, with simple screening tests in the initial phases of the protocol. More costly elaborate tests are reserved for definitive classification at a later stage, in collaboration with an immunologist and specialized laboratory.
Picking up the signs of PID
Infections are the hallmark of immunodeficiency [2,10]. However, other symptoms may be more prominent at first, such as the failure-to-thrive they may cause in children, the constitutional symptoms in adults such as weight loss, or concomitant syndrome-specific symptoms such as hypocalcaemia in DiGeorge syndrome. Autoimmune manifestations [11] can be a presenting feature of PID, especially in adults, as well as unusual lymphoid or granulomatous diseases. A good family history is important for the prompt recognition of genetic disorders, although many mutations may be new and the family history is not necessarily positive [2].
It is important to remember the possibility of an anatomical defect, especially when infections recur at the same anatomical site. Also, periodic fever may be difficult to distinguish from recurrent infection [12].
Relevant symptoms and signs from the history and physical examination that should alert any physician to potential PID are listed in Table 1. Any suspicion of a severe immunodeficiency, such as SCID, should result in the immediate involvement of a clinical immunologist.
Table 1.
Symptoms and signs that could point to potential PID.
| 1.The hallmark of immunodeficiency: infections |
| Recurrent (proven) bacterial infections |
| Severe infections (e.g. meningitis, osteomyelitis, pneumonia) |
| Infections that present atypically, are unusually severe or chronic or fail regular treatment |
| Infections caused by an unexpected or opportunistic pathogen |
| Severe or long-lasting warts, generalized mollusca contagiosa |
| Extensive candidiasis |
| Complications of vaccination [disseminated bacille Calmette–Guérin (BCG) or vaccinia infection, paralytic polio] |
| Abscesses of internal organs; recurrent subcutaneous abscesses |
| Prolonged or recurrent diarrhoea |
| 2.Remember the family history |
| Consanguinity in the parents |
| Unexplained early infant deaths |
| Family history of possible immunodeficiency; familial occurrence of similar symptoms (affected males related by the female line, or another clear pattern of inheritance) |
| 3.Miscellaneous signs: they could point to PID, but may not |
| Abnormal hair |
| Absence of immunological tissue: a/hypoplasia of thymus, absence of lymph nodes and tonsils |
| Angioedema (without urticaria) |
| Ataxia |
| Auto-immunity |
| Auto-immune disease in several family members |
| Bleeding tendency, thrombocytopenia, small platelets |
| Congenital cardiac anomalies |
| Chronic diarrhoea (malabsorption, pancreatic insufficiency) |
| Delayed separation of umbilical cord (> 4 weeks) |
| Delayed shedding of primary teeth |
| Dental crowding or other anomalies |
| Developmental delay |
| Difficult-to-treat obstructive lung disease |
| Digital clubbing |
| Dysmorphism |
| Eczema, dermatitis (severe) |
| Eosinophilia (unexplained) |
| Facial abnormalities |
| Failure to thrive (child) or wasting (adult) |
| Giant granules in phagocytes |
| Gingivostomatitis (severe), recurrent aphthae |
| Graft-versus-host reaction after blood transfusion, or mother-to-child (infant) |
| Hypersensitivity to sunlight |
| Hypocalcaemic seizures |
| Lymphadenopathy (excessive) |
| Lymphocytopenia |
| Malignancy (mainly lymphoma) |
| Microcephaly |
| Neonatal exudative erythroderma |
| Organomegaly (spleen, liver) |
| (Partial) albinism, pale skin |
| Poor wound healing; scarring |
| Retinal lesions |
| Rib abnormalities |
| Stunted growth or disproportional growth |
| Telangiectasia |
| Thymoma |
| Unexplained bronchiectasis, pneumatoceles, interstitial lung disease |
| Vasculitis |
Recognizing the different clinical presentations of PID
It is important for all doctors to learn to recognize the different patterns of clinical presentation of PIDs (Table 2, column 1). General practitioners can use these clinical presentations to select patients for referral. Secondary immunodeficiencies (e.g. HIV infection [13]) present in a similar fashion, and occur much more frequently than PIDs in some parts of the world, so it is important to be able to eliminate any underlying condition. It is not necessary to understand immunological mechanisms (Table 2, column 2) to be able to use these different patterns for reliable early suspicion of potential PID.
Table 2.
The different clinical presentations of PID: direction indicators for the diagnostic process.
| Column 1 Clinical presentation | Column 2 Suspected immunodeficiencies | Column 3 Encountered pathogens | Column 4 Special features | Column 5 Diagnostic protocol | Column 6 Non-immunological differential diagnosis |
|---|---|---|---|---|---|
| Recurrent ENT and airway infections | Selective) antibody deficiencies [14], complement deficiencies [15], CVID [16] Sometimes phagocyte deficiency (mainly neutropenia) [17,18], WAS [19], HIV [13] | Mainly extra-cellular bacteria such as non-typable H. influenzae, pneumococcus. Sometimes: S. aureus, meningococcus, group A streptococcus, M. pneumoniae, U. urealyticum, C. jejuni, enteroviruses (Echovirus, poliovirus), giardia lamblia when gut, urinary and meningeal systems are also involved | Giardia infection may lead to a period of failure to thrive Enteroviral meningo-encephalitis is a severe complication in inadequately substituted agammaglobulinaemia Unexplained bronchiectasis; recurrent bronchitis in a non-smoker | Go to Protocol 1 Most patients do not have PID. Even if they do, it is seldom life-threatening in the short term. Exclude more frequent non- immunological problems first, except in case of a positive family history | Frequent: normal frequency of infection in infants (day-care, passive smoking), bronchial hyperreactivity, allergy, asthma, adenoidal hypertrophy, iron deficiency anaemia, gastro-oesophageal reflux. Infrequent: cystic fibrosis, inhaled foreign body, congenital anomaly, BPD;intestinal or renal protein loss. Rare: ciliary dyskinesia, α1-anti-trypsin deficiency |
| Failure to thrive from early infancy | T-lymphocyte deficiency [19] (remember HIV) [13]. STAT1 deficiency [20]. Hypermorphic mutations in IκBα[21]. | Mainly viruses (CMV, EBV, VZV, HSV), fungi (superficial candida, aspergillus, Cryptococcus, histoplasma, pneumocystis jiroveci/carinii), protozoa (toxoplasma, microsporidium, cryptosporidium) and intracellular bacteria such as mycobacterium spp. and salmonella | Intractable diarrhoea. Unusual infections or unusually severe course of infections. Graft- versus-host reaction from maternal T lymphocytes or blood transfusion. Eczema | Go to Protocol 2 Only a few patients have PID, but delay in diagnosis and treatment by SCT greatly impairs survival. Perform immunological tests in parallel with tests for other causes of failure to thrive | A variety of gastrointestinal, renal, cardiopulmonary, endocrine, neurological, metabolic, and congenital causes. Malignancy. Chronic lead poisoning. Perinatal infection. See appropriate textbooks |
| Recurrent pyogenic infections | Phagocyte deficiency; rare: defects in phagocyte function [17], more common: neutropenia [18] | Mainly S. aureus, sometimes klebsiella, E. coli, enterobacter, serratia, pseudomonas, salmonella. Invasive fungal infection (disseminated candida, aspergillus, nocardia) | Infections of body surface areas (skin, mouth, mucous membranes), internal organs (lung, liver, lymph nodes) and bones. Unexplained granulomatous inflammation. Poor wound healing | Go to Protocol 3 Defects in phagocyte function are rare and seldom immediately life-threatening. Neutropenia is more common and easily detected | Neutropenia: iatrogenic, haematological malignancy, aplastic anaemia Disrupted skin (eczema, burns) |
| Unusual infections or unusually severe course of infections | T lymphocyte deficiency [19] (remember HIV) [13]. WAS [19]. STAT1 deficiency [20]. Hypermorphic mutations in IκBα[21], X–linked lymphoproliferative syndrome [25] | Mainly intracellular bacteria such as mycobacterium spp. and salmonella, viruses (CMV, EBV, VZV, HSV), fungi (superficial candida, aspergillus, Cryptococcus, histoplasma, pneumocystis jiroveci/carinii) and protozoa (toxoplasma, microsporidium, cryptosporidium) | Might present later in life | Go to Protocol 2 An uncommon presentation of a common disease is more common than an uncommon disease (such as immunodeficiency). Perform screening immunological investigations at an early stage; however, because underlying immunodeficiency may be life-threatening | Virulent strain of pathogen, reduced general condition of patient leading to secondary immunodeficiency (malignancy, malnutrition, chronic disease) Immunosuppressive therapy |
| Recurrent infections with the same type of pathogen | Dependent on type of pathogen Intracellular bacteria: T lymphocyte–macrophage interaction for cytokine production; auto-antibodies to γ-interferon [20]. Meningococci: complement deficiency [15], sometimes antibody deficiency [14,16]. Candida: T-lymphocyte deficiency [19], CMC [22]. Encapsulated bacteria: antibody deficiencies [14,16]. Pneumococci: IRAK4 deficiency [21]. No/delayed fever/raise in CRP: deficiency in NFκB signalling (IRAK4, NEMO, IκBα deficiency) [21]. Encapsulated bacterial sepsis: asplenia [23]. Excessive warts: epidermodysplasia verruciformis, WHIM. Herpesviruses: NK-cell deficiency. X–linked lymphoproliferative syndrome [25] | Normally no other recurrent infectious problems | Dependent on (type of) pathogen, go to: Intracellular bacteria (e.g. salmonella, mycobacteria): Protocol 2, Step 3. Meningococci: Protocol 1. Candida: Protocols 2 and 3. Encapsulated bacteria: Protocol 1; perform splenic ultrasound in case of sepsis. Viruses: Protocol 2. Many have no PID, but the recurrent infections may be life-threatening. Screening is therefore warranted | Increased exposure, coincidence Inadequate treatment of first infection Anatomical defect (e.g. fistula) | |
| Autoimmune or chronic inflamma-tory disease; lymphopro-liferation | Immunodysregulation in the context of antibody deficiency (CVID, IgA deficiency) [14,16]; complement deficiency (early components of classical pathway) [15], or defective cell-mediated immunity (WAS, CMC) [19]. Defect in apoptosis: caspase 8/10, FAS/FASL [24]. XLP [25]. Polyendocrinopathy ± CMC (APECED; AIRE gene) [26], with enteropathy (IPEX; FOXP3 gene) [27]. Periodic fever syndromes [12] | – | – | Follow appropriate Protocol guided by first work-up. First work-up may comprise: immunoglobulins, CH50, blood count and differential, lymphocyte subpopulations, acute phase proteins during fever, organ-specific autoantibody screen | Most cases of autoimmune disease, chronic inflammatory disease, and lymphoproliferation are not associated with recurrent infections. If the combination occurs, or if the case presents atypically, immunodeficiency is more likely. See appropriate textbooks |
| Characteristic combinations of clinical features in eponymous syndromes | Variable. Evaluate the degree of immunodeficiency | Different syndromes are associated with particular forms of immunodeficiency and concomitant infectious problems [28] | Identify syndrome by clinical features (e.g. DiGeorge, AT, Nijmegen breakage syndrome, EDA–ID) [19,21] | Follow appropriate Protocol guided by first work-up First work-up: immunoglobulins, blood count and differential, lymphocyte subpopulations Perform appropriate tests for the particular syndrome | See appropriate textbooks for syndrome characteristics |
| Angioedema | C1 inhibitor deficiency [29] | – | Related to triggering factors (e.g. stress, trauma, menses) Symptoms typically last > 24 h. May mimic acute abdomen | Go to Protocol 1, Step 2b | Allergy, malignancy, auto-immunity ACE-inhibitor therapy |
Columns 1 and 5 are the core of the Table, and can be used to go directly to the appropriate diagnostic protocol, guided solely by the clinical presentation of the patient. Columns 2, 3, 4 and 6 contain extra information that can be useful, but does not necessarily have to be used. ACE, angiotensin-converting enzyme; AIRE, autoimmune regulator; APECED, autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy; AT, ataxia telan giectasia; BPD; bronchopulmonary dysplasia; C, complement component; CH50, haemolytic assay of classical pathway of complement; CMC, chronic mucocutaneous candidiasis; CVID, common variable immunodeficiency; EDA-ID, X-linked anhidrotic ectodermal dysplasia with immunodeficiency; FAS(L), tumour necrosis factor receptor associated protein (ligand); FOXP3, forkhead box P3; HIV, human immunodeficiency virus; Ig, immunoglobulin; IPEX; immune dysregulation–polyendocrinopathy–enteropathy–X-linked; PID, primary immunodeficiency disease; SCT, stem cell transplantation; WAS, Wiskott–Aldrich syndrome; XLP; X-linked lymphoproliferative syndrome; WHIM, warts, hypogammaglobulinaemia, infections, myelokathexis syndrome.
Other features can be useful to distinguish between the different clinical presentations of PIDs. In children, the time of onset of symptoms can help to elucidate the underlying aetiology. For instance, as long as maternal immunoglobulin is present in the first months of life, antibody deficiency in the child may remain unnoticed [14]. Recurrent bacterial infections will only become prominent later. If only certain pathogens cause clinical problems, as in undue mycobacterial sensitivity, some time is generally needed to encounter these pathogens. However, if ubiquitous opportunistic pathogens cannot be combated, problems will start very early in life. If the immunodeficiency develops later in life, as in common variable immunodeficiency disorder (CVID), the infections will also start later.
The type of pathogen encountered (Table 2, column 3) is causally related to the underlying immunodeficiency [10]. For instance, extracellular encapsulated bacteria that cause ear, nose and throat (ENT) and airway infections are normally cleared by opsonization with specific antibody and complement, and subsequent elimination by phagocytosis. Fungi and bacteria that are normally present on the skin and mucosal surfaces are kept at bay by local phagocytosis. Cytokines and cytotoxic substances secreted by activated T lymphocytes need to interact with functional macrophages for the elimination of intracellular and slow-growing pathogens. Therefore, specific immunological defects will lead to particular patterns of infection which, together with other special features (Table 2, column 4), help to differentiate reliably between the different clinical presentations of PID.
Following the appropriate diagnostic protocol
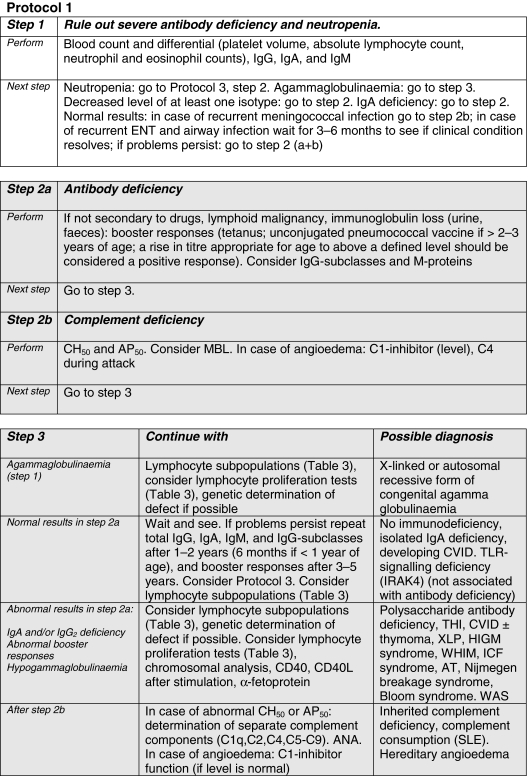
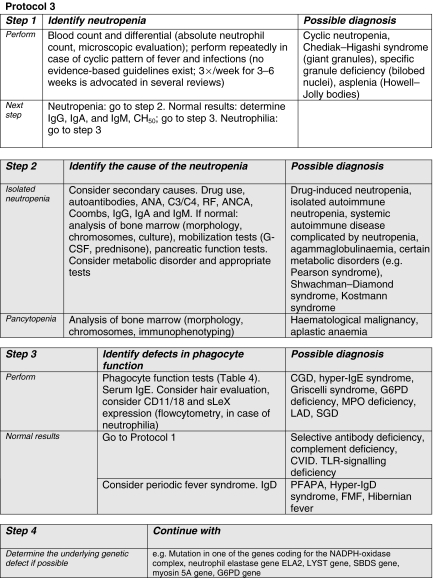
In column 5 of Table 2, the user is directed towards the appropriate multi-stage diagnostic protocol for each clinical presentation for the analysis of possible underlying immunodeficiency. These Protocols 1, 2 and 3 (see Figs 1–3) in fact represent the traditional division into humoral (Ig + C), cellular and phagocyte deficiencies, respectively.
Fig. 1.
Grey shading: collaboration with an immunologist is highly recommended for this step. ANA, anti-nuclear antibodies; AP50, haemolytic assay of alternative pathway of complement; AT, ataxia telangiectasia; CD, cluster of differentiation; CH50, haemolytic assay of classical pathway of complement; CVID, common variable immunodeficiency; ENT, ear, nose and throat; HIGM, hyper-IgM syndrome; ICF, syndrome of immunodeficiency, centromeric instability and facial dysmorphism; Ig, immunoglobulin; IRAK4, interleukin-1 receptor-associated kinase 4; L, ligand; MBL, mannan binding lectin; SLE, systemic lupus erythematosus; THI, transient hypogammaglobulinaemia of infancy; TLR, Toll-like receptor; XLP, X-linked lymphoproliferative syndrome.
Fig. 3.
Grey shading: collaboration with an immunologist or hematologist is highly recommended for this step. ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic autoantibodies; C, complement component; CD, cluster of differentiation; CGD, chronic granulomatous disease; CVID, common variable immunodeficiency; FMF, familial Mediterranean fever; G–CSF, granulocyte–colony-stimulating factor; G6PD, glucose-6-phosphate dehydrogenase; MPO, myeloperoxidase; NADPH, nicotinamide adenine dinucleotide phosphate; PFAPA, periodic fever–aphthous stomatitis–pharyngitis–cervical adenopathy; RF, rheumatoid factor; SBDS, Schwachman–Bodian–Diamond syndrome; SGD, neutrophil-specific granule deficiency; sLeX, sialyl Lewis X; TLR, Toll-like receptor.
The Protocols comprise several steps. Severe defects are ruled out first, with widely available screening tests that should be accessible to the whole range of hospital doctors. Less severe forms of PID can be diagnosed later, after more frequent non-immunological diseases (Table 2, column 6) have been ruled out. The help of specialized immunology laboratories will be needed for definitive classification using the more elaborate tests presented downstream in the Protocols. Age-related reference values, or controls run in parallel, are needed for correct interpretation of the results.
The advice of an immunologist is extremely important, even during the diagnostic process. In Table 2, the degree of urgency for performing the various tests is outlined in column 5. Areas in italics in the Protocols demarcate the test phases for which collaboration with an immunologist is highly recommended. Detailed information about the various PIDs can also be found by checking the references to current review articles, which are mentioned in Table 2 (column 2).
Anticipating future developments
In the past two decades, there has been an explosion of knowledge concerning PIDs. This will probably be followed by the identification of many more disease entities in the near future, especially of those with a less overt clinical phenotype [9]. This implies that the multi-stage diagnostic protocol presented here will need to be revised from time to time. It will probably even turn out that PIDs − albeit with varying severity − are more common than we think at present.
Contributors to the study
E. de Vries, Department of Paediatrics, Jeroen Bosch Hospital (loc GZG), ’s-Hertogenbosch, the Netherlands, D. S. Kumararatne, Department of Clinical Immunology, Addenbrooke’s Hospital, Cambridge, UK, A. Al-Ghonaium, Department of Paediatrics, Paediatric Allergy and Immunology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia, J.-L. Casanova, Unité d’Immunologie et d’Hématologie Pédiatriques, Hôpital Necker-Enfants Malades, Paris, France, H. Chapel, Nuffield Department of Medicine, John Radcliffe Hospital, Headington, Oxford, UK, K. C. Gilmour, Department of Immunology, Great Ormond Street Hospital, London, UK, B. Eley, Department of Paediatrics and Child Health, Red Cross Children’s Hospital, Rondebosch, Cape Town, South Africa, A. Ferster, Hemato-Oncology Unit, Hôpital des Enfants Reine Fabiola, Belgium, A. S. Grumach, Outpatient Group of PID and Laboratory of Medical Investigation in Dermatology and Immunodeficiencies, Department Dermatology, University of São Paulo Medical School, São Paulo, Brazil, M. Helbert, Department of Immunology, Manchester Royal Infirmary, Manchester, UK, M. Helminen, Department of Paediatrics, Paediatric Research Center, Tampere University Hospital, Tampere, Finland, T. W. Kuijpers, Department of Paediatric Immunology, Emma Children’s Hospital, Academic Medical Centre, Amsterdam, the Netherlands, B. Martire, Department of Biomedicine, Eta′ Evolutiva Universita′ di Bari, Bari, Italy, F. Mascart, Laboratory Immunology, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium, D. de Mattia, Department of Paediatrics, Policlinico, Università di Bari, Bari, Italy, A. Stray-Pedersen, Department of Medical Genetics, Rikshospitalet University Hospital, Oslo, Norway, S. Velbri, Tallinn Childrens’ Hospital, Tallinn, Estonia, P. Wood, Department of Clinical Immunology, Leeds Teaching Hospitals, Leeds, UK, H. B. Gaspar, Molecular Immunology Unit, Institute of Child Health, London, UK, and A. J. Cant, Children’s Bone Marrow Transplant Unit, Newcastle General Hospital, Newcastle Upon Tyne, UK.
Fig. 2.
Grey shading: collaboration with an immunologist is highly recommended for this step. ADA, adenosine deaminase; AIDS, acquired immunodeficiency syndrome; BAL, bronchoalveolar lavage; CD, cluster of differentiation; CMC, chronic mucocutaneous candidiasis; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; Ig, immunoglobulin; IFN, interferon; IL, interleukin; JAK, janus kinase; L, ligand; PCR, polymerase chain reaction; PNP, purine nucleoside phosphorylase; RAG, recombination activating gene; SCID, severe combined immunodeficiency; SCT, stem cell transplantation; STAT, signal transducer and activator of transcription; WAS, Wiskott–Aldrich syndrome; Zap, zeta-associated protein.
Table 3.
Basic protocol for in vitro determination of lymphocyte subpopulations and function.
| (a) Determine the absolute count of the following lymphocyte subpopulations, and compare the results with age-matched reference values | |||
| CD3+ | T lymphocytes | ||
| CD3+/CD4+ | Helper-T lymphocytes | ||
| CD3+/CD8+ | Cytotoxic T lymphocytes | ||
| CD3+/HLA-DR+ | Activated T lymphocytes | ||
| CD3+/CD4–/CD8– | ‘Double-negative’ T cells | ||
| CD3+/TCR-γδ+ | Subset of T lymphocytes | ||
| CD19+ or CD20+ | B lymphocytes | ||
| CD3–/CD16+ and/or CD56+ | NK cells | ||
| (b) Determine the uptake of [3H]-thymidine (or CFSE or activation markers) and compare the results with − preferably − age-matched controls after stimulation with: | Mitogens (e.g. PHA, PMA + ionomycin, PWM) | ||
| Consider monoclonal antibodies (e.g. CD2 ± CD28, CD3 ± CD28) | |||
| Antigens (e.g. tetanus, after booster vaccination) | |||
| Consider allogeneic cells | |||
(a) Can be performed in many hospitals; for correct interpretation of the results, the advice of an immunologist is highly recommended. (b) Collaboration with an immunologist and specialized laboratory is recommended.
Abbreviations: CD = cluster of differentiation, CFSE = carboxyfluorescein succinimidyl ester, HLA = human leucocyte antigen, NK = natural killer, PHA = phytohaemagglutinin, PMA = phorbol myristate acetate, PWM = pokeweed mitogen, TCR = T-cell receptor.
Table 4.
Protocol for determination of granulocyte function.
| (a) Oxidative burst and flow cytometry |
| Nitroblue tetrazolium test (NBT) to a stimulant (PMA, LPS) |
| Chemoluminescence test |
| Flow cytometric analysis using dihydrorhodamine (DHR) |
| Immunophenotyping (CD18, CD11) |
| (b) Chemotaxis, granule contents, bacterial killing, phagocytosis |
| Migration to a chemoattractant (e.g. FMLP) |
| Immunohistochemistry of granule contents, electron microscopy |
| Bacterial killing (e.g. of Staphylococcus aureus) |
| Phagocytosis (e.g. zymosan uptake) |
(a) Can be performed in many hospitals; for correct interpretation of the results, the advice of an immunologist is highly recommended. (b) Collaboration with an immunologist and specialized laboratory is recommended. FMLP, formyl-met-leu-phe, a bacterial peptide; LPS, lipopolysaccharide; PMA, phorbol myristate acetate.
Acknowledgments
Dr Helen Chapel gratefully acknowledges the grant from the European Community 6th Framework Programme ‘Policy-orientated and harmonizing research activities in the field of primary immunodeficiency diseases (PIDs)’ (EURO-POLICY-PID), which enabled her to spend time on the ESID multi-stage diagnostic protocol for suspected immunodeficiency.
References
- 1.Notarangelo L, Casanova JL, Fischer A, et al. Primary immunodeficiency diseases: an update. J Allergy Clin Immunol. 2004;114:677–87. doi: 10.1016/j.jaci.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Stiehm ER, Ochs HD, Winkelstein JA. Immunodeficiency disorders: general considerations. In: Stiehm ER, Ochs HD, Winkelstein JA, editors. Immunologic disorders in infants and children. 5. Philadelphia; 2004. pp. 289–355. [Google Scholar]
- 3.Folds JD, Schmitz JL. Clinical and laboratory assessment of immunity. J Allergy Clin Immunol. 2003;111(2 Suppl):S702–11. doi: 10.1067/mai.2003.122. [DOI] [PubMed] [Google Scholar]
- 4.Bonilla FA, Bernstein IL, Khan DA, et al. American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl. 1):S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 5.Spickett GP, Askew T, Chapel HM. Management of primary antibody deficiency by consultant immunologists in the United Kingdom: a paradigm for other rare diseases. Qual Health Care. 1995;4:263–8. doi: 10.1136/qshc.4.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vries E, Kuijpers TW, van Tol MJ, van der Meer JW, Weemaes CM, van Dongen JJ. [Immunology in medical practice. XXXIV. Screening for suspected immunodeficiency. Introduction] Ned Tijdschr Geneeskd. 2000;144:2192–6. [PubMed] [Google Scholar]
- 7.De Vries E, Kuijpers TW, van Tol MJ, van der Meer JW, Weemaes CM, van Dongen JJ. [Immunology in medical practice. XXXV. Screening of suspected immunodeficiency: diagnostic protocols for patients with opportunistic or recurrent severe infections, wasting and failure to thrive] Ned Tijdschr Geneeskd. 2000;144:2197–203. [PubMed] [Google Scholar]
- 8.De Vries E, Kuijpers TW. The Dutch multistage laboratory protocol for the diagnosis of immunodefiency. In: Etzioni A, editor. Proceedings of the European Society for Immunodeficiencies. Bologna: Monduzzi Editore; 2000. pp. 165–71. [Google Scholar]
- 9.Casanova JL, Fieschi C, Bustamante J, Reichenbach J, Remus N, von Bernuth H, Picard C. From idiopathic infectious diseases to novel primary immunodeficiencies. J Allergy Clin Immunol. 2005;116:426–30. doi: 10.1016/j.jaci.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 10.Stiehm ER, Chin TW, Haas A, Peerless AG. Infectious complications of the primary immunodeficiencies. Clin Immunol Immunopathol. 1986;40:69–86. doi: 10.1016/0090-1229(86)90070-x. [DOI] [PubMed] [Google Scholar]
- 11.Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99:2694–702. doi: 10.1182/blood.v99.8.2694. [DOI] [PubMed] [Google Scholar]
- 12.Long SS. Distinguishing among prolonged, recurrent, and periodic fever syndromes: approach of a pediatric infectious diseases subspecialist. Pediatr Clin North Am. 2005;52:811–35. doi: 10.1016/j.pcl.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrook R. The AIDS epidemic in 2004. N Engl J Med. 2004;351:115–7. doi: 10.1056/NEJMp048156. [DOI] [PubMed] [Google Scholar]
- 14.Ballow M. Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol. 2002;109:581–91. doi: 10.1067/mai.2002.122466. [DOI] [PubMed] [Google Scholar]
- 15.Sjoholm AG, Jonsson G, Braconier JH, Sturfelt G, Truedsson L. Complement deficiency and disease: an update. Mol Immunol. 2006;43:78–85. doi: 10.1016/j.molimm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency. clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 17.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–84. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 18.Berliner N, Horwitz M, Loughran TP., Jr Congenital and acquired neutropenia. Hematology (Am Soc Hematol Educ Prog) 2004;2004:63–79. doi: 10.1182/asheducation-2004.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Buckley RH. Primary cellular immunodeficiencies. J Allergy Clin Immunol. 2002;109:747–57. doi: 10.1067/mai.2002.123617. [DOI] [PubMed] [Google Scholar]
- 20.Remus N, Reichenbach J, Picard C, et al. Impaired interferon gamma-mediated immunity and susceptibility to mycobacterial infection in childhood. Pediatr Res. 2001;50:8–13. doi: 10.1203/00006450-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Puel A, Picard C, Ku CL, Smahi A, Casanova JL. Inherited disorders of NF-kappaB-mediated immunity in man. Curr Opin Immunol. 2004;16:34–41. doi: 10.1016/j.coi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick CH. Chronic mucocutaneous candidiasis. Pediatr Infect Dis J. 2001;20:197–206. doi: 10.1097/00006454-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7:657–60. doi: 10.1046/j.1198-743x.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira JB, Fleisher T. Autoimmune lymphoproliferative syndrome. Curr Opin Allergy Clin Immunol. 2004;4:497–503. doi: 10.1097/00130832-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Sneller MC, Dale JK, Straus SE. Autoimmune lymphoproliferative syndrome. Curr Opin Rheumatol. 2003;15:417–21. doi: 10.1097/00002281-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Betterle C, Greggio NA, Volpato M. Autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1998;83:1049–55. doi: 10.1210/jcem.83.4.4682. [DOI] [PubMed] [Google Scholar]
- 27.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Ming JE, Stiehm ER, Graham JM., Jr Syndromes associated with immunodeficiency. Adv Pediatr. 1999;46:271–351. [PubMed] [Google Scholar]
- 29.Gompels MM, Lock RJ, Abinun M, et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139:379–94. doi: 10.1111/j.1365-2249.2005.02726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen FS. Severe combined immunodeficiency: a pediatric emergency. J Pediatr. 1997;130:345–6. [PubMed] [Google Scholar]