Abstract
Summary
The present study is aimed at investigating the effect of curcumin (CMN) in salvaging endotoxin-induced hepatic dysfunction and oxidative stress in the liver of rodents. Hepatotoxicity was induced by administering lipopolysaccharide (LPS) in a single dose of 1 mg/kg intraperitoneally to the animals, which were being treated with CMN daily for 7 days. Liver enzymes serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST) and alkaline phosphatase (ALP), total bilirubin and total protein were estimated in serum. Oxidative stress in liver tissue homogenates was estimated by measuring thiobarbituric acid reactive substances (TBARS), glutathione (GSH) content and superoxide dismutase (SOD) activity. Serum and tissue nitrite was estimated using Greiss reagent and served as an indicator of NO production. A separate set of experiments was performed to estimate the effect of CMN on cytokine levels in mouse serum after LPS challenge. LPS induced a marked hepatic dysfunction evident by rise in serum levels of ALT, AST, ALP and total bilirubin (P < 0·05). TBARS levels were significantly increased, whereas GSH and SOD levels decreased in the liver homogenates of LPS-challenged rats. CMN administration attenuated these effects of LPS successfully. Further CMN treatment also regressed various structural changes induced by LPS in the livers of rats and decreased the levels of tumour necrosis factor-α and interleukin-6 in mouse plasma. In conclusion, these findings suggest that CMN attenuates LPS-induced hepatotoxicity possibly by preventing cytotoxic effects of NO, oxygen free radicals and cytokines.
Keywords: curcumin, free radicals, hepatotoxicity, lipopolysaccharide, nitrosative stress, oxidative stress
Introduction
Despite current medical and surgical advances, septic hepatic failure is still associated with a high mortality rate [1,2]. Hepatic damage is mediated mainly by endotoxin of Gram-negative bacteria, which is primarily a lipopolysaccharide (LPS). Given that Gram-negative bacteria normally colonize the colon, the body has developed strong defensive mechanisms that tightly regulate the entry and processing of LPS [3]. The liver plays a central role in this process by virtue of its dual ability not only to clear LPS, but to respond energetically to LPS [4]. Within the liver, the LPS binds to LPS binding proteins which then facilitate its transfer to CD14 receptors on the surface of Kupffer cells, the resident macrophages of liver. Signalling of LPS through CD14 receptors is mediated by the downstream Toll-like receptor-4 and results in the production of two classes of potentially disastrous mediators: proinflammatory cytokines such as interleukins (IL-1, IL-6), tumour necrosis factor (TNF)-α and oxygen free radicals [5]. Most of the toxicities of LPS, both in the liver and in the systemic circulation, have been related to the release of these toxic mediators [6].
LPS-induced increase in lipid peroxidation, which is an index of oxidative stress, has been described in several studies and is reported to be both time- and dose-dependent. Yoshikawa et al. [7] observed enhanced lipid peroxidation in rats as early as 45 min post-LPS infusion [100 mg/kg body weight (b.w.)] in many tissues including the liver, small intestine, stomach and abdominal aorta [7]. Hepatic levels of malonaldehyde (MDA, a product of lipid peroxidation) increased fivefold within 16 h after LPS administration (15 mg/kg b.w.) and 3·4-fold 8 h after 30 mg/kg LPS administration [8]. Several studies have shown that LPS can cause liver glutathione (GSH) depletion in a dose-dependent manner [9]. This is thought to be secondary to enhanced efflux of GSH from the liver or acute depression of liver GSH synthesis. It has been found that the precursor of GSH synthesis, N-acetylcysteine (NAC), protects against LPS toxicity and the inhibitor of GSH synthesis, DL-buthionine-SR-sulfoxime, has the opposite effect. Also oxidized glutathione (GSSG) is released from liver and other tissues during oxidative damage. Sewerynek et al. [10] have shown an increase in GSSG levels after LPS injection, which is decreased by the anti-oxidant melatonin [10].
Current traditional Indian medicine claims the use of Curcuma longa L. (Zingiberaceae) powder against biliary disorders, anorexia, coryza, cough, diabetic wounds, hepatic disorder, rheumatism and sinusitis [11]. Curcumin (CMN) is a major component in curcuma/turmeric, being responsible for its biological action. An increasing number of studies now show that CMN exhibits anti-inflammatory [12], anti-human immunodeficiency virus [13,14], anti-bacterial [15] and nematocidal activities [16]. Various in-vitro and in-vivo studies have established the anti-oxidant properties of CMN [17,18]. It is well documented that CMN scavenges superoxide anions [19] and peroxynitrite radicals [20], and quenches singlet oxygen [21]. CMN has also been shown to inhibit hydrogen peroxide-induced cell damage [22]. Due to its anti-oxidant activity, it reduces the oxidative stress induced by ethanol and protects the liver cells in vitro[23]. It has also been shown to reduce lipid peroxidation on cadmium-induced oxidative damage in the liver of rats and mice [24]. CMN induces glutathione biosynthesis and inhibits oxidant- and cytokine-induced NF-kappaB [22,25]. Hence, the present study was aimed at investigating the modulation of LPS-induced hepatic dysfunction, cytokine production effect and oxidative stress in rodent livers by CMN.
Methods
Animals
Male Wistar rats (150–200 g), bred in the central animal house of Panjab University (Chandigarh, India), were used. The animals were housed under standard conditions of light : dark cycle with free access to food (Hindustan Lever Products, Kolkata, India) and water. The experimental protocols were approved by the Institutional Ethical Committee of Panjab University, Chandigarh.
Drugs
CMN (Sigma Aldrich Chemicals Private Ltd, New Delhi, India) was suspended in 0·5% carboxy methyl cellulose (CMC) and administered orally. KDO estimation (2-keto-3-deoxyoctonate) [26] was carried out to detect the presence of LPS in CMN [27] and was also confirmed by Limulus amoebocyte lysate test (LAL; Sigma Aldrich Chemicals Private Ltd). LPS (serotype Escherichia coli 0111:B4 containing not less than 500 000 EU/mg) Sigma Aldrich Chemicals Private Ltd) was prepared in pyrogen-free water for injection. The drug solutions were made freshly at the beginning of each experiment.
Experimental groups and protocol
Induction of liver damage
LPS, prepared in pyrogen-free water for injection, was injected on the seventh day in a single dose of 1 mg/kg intraperitoneally (i.p.) to the rats, which were being administered CMN daily for 6 days. This particular dose of LPS has been shown to induce hepatic damage in preliminary studies performed in our laboratory.
Treatment schedule
At the beginning of the experiment, rats were divided into the following four groups, each consisting of six to seven animals.
Control group: the animals were treated with equivalent volumes of CMC (vehicle of CMN) for 1 week and were administered water for injection i.p. on the seventh day.
LPS group: the animals were administered CMC for 7 days and were challenged with LPS (1 mg/kg) i.p. on the seventh day.
CMN per se group: the animals were treated with CMN 60 mg/kg per os (p.o.) for 7 days.
CMN + LPS groups: the animals received various doses of CMN (5, 30, 60 mg/kg) p.o. for 7 days and received LPS on the seventh day.
The doses of CMN were selected on the basis of previous studies performed in our laboratory.
Assessment of liver function
After 6 h of LPS injection, all the animals were killed and blood was collected. A midline abdominal incision was performed, their livers were harvested, perfused with cold isotonic saline, dried carefully on filter papers, weighed and were deep-frozen until further enzymatic analysis and histological studies.
Serum alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST) were estimated using the method recommended by the International Federation of Clinical Chemistry [28] (Erba test kits). Total bilirubin was estimated by the Diazo method of Pearlman and Lee [29] (Erba test kits). Alkaline phosphatase (ALP) was estimated by the p-nitrophenyl phosphate (p-NPP) method using the recommendations of the German Society for Clinical Chemistry [30] (Enzopak diagnostic kit). Total protein in the serum was also estimated by the Biuret method (Erba test kits). Serum nitrite was estimated using Griess reagent [31]. All the readings were taken on a semi-autoanalyser (Erba Chem-5 plus, Transasia, New Delhi, India)
Assessment of oxidative stress
Post-mitochondrial supernatant preparation (PMS)
On the day of biochemical estimation, the entire liver was homogenized with 10% (w/v) cold phosphate-buffered saline (0·1 mol/l, pH 7·4) using a homogenizer. The homogenates were used to estimate liver thiobarbituric acid reactive substances (TBARS) and reduced glutathione (GSH) after centrifugation at 800 g for 5 min at 4°C to separate the nuclear debris. The supernatant obtained was centrifuged further at 10 500 g for 20 min at 4°C to obtain the PMS, which was used to assay superoxide dismutase (SOD) activity.
Estimation of lipid peroxidation
The quantitative measurement of lipid peroxidation in liver was performed according to the method of Wills [32]. The MDA content, a measure of lipid peroxidation, was assayed in the form of TBARS. In brief, the reaction mixture consisted of 0·5 ml of tissue homogenate and 0·5 ml Tris HCl (pH 7·4) and was incubated at 37°C for 2 h. The reaction mixture was brought to 2 ml with 1 ml of 10% ice-cold trichloroacetic acid. After centrifugation (5000 g, 10 min), 1 ml of 0·67% of thiobarbituric acid was added to 1 ml of supernatant. The tubes were then kept in a boiling water-bath for 10 min and after cooling under tap water, 1 ml of distilled water was added. Absorbance was measured at 540 nm.
Estimation of reduced glutathione and SOD
Reduced GSH in the liver was assayed by the method of Jollow et al. [33] and SOD activity was assayed by the method of Kono et al. [34]. In our earlier studies we had used these methods to report the levels of oxidative stress and endogenous anti-oxidants in rat liver and kidneys [35].
Assessment of serum/tissue nitrite concentration
Serum and tissue nitrite was estimated using Greiss reagent and served as an indicator of nitrogen (NO) production. Greiss reagent, 500 µl (1 : 1 solution of 1% sulphanilamide in 5% phosphoric acid and 0·1% napthaylamine diamine dihydrochloric acid in water) was added to suitably diluted 100 µl of plasma and absorbance was measured at 546 nm [31]. Nitrite concentration was calculated using a standard curve for sodium nitrite. Nitrite levels were expressed as µmol/ml in serum and as µmol/mg protein in liver homogenates.
Cytokine assays
A separate set of experiments was performed to estimate the levels of cytokines in mouse serum after LPS challenge. Three group of animals (controls, LPS and CMN 60 mg/kg) were used (n = 6). These animals were treated similarly as per the above given schedule. Assays for IL-1α, IL-6 and TNF-α were performed by enzyme-linked immunosorbent assay (ELISA) in the plasma by commercially available cytokine assay kits (Chemicon, Temecule, CA, USA) according to the manufacturer’s instructions. The ELISA was sensitive to 0·2 pg/ml of the cytokine released [36].
Histopathological examination
For microscopic evaluation, livers were fixed in 10% neutral phosphate-buffered formalin solution. Following dehydration in ascending series of ethanol (70, 80, 96, 100%), tissue samples were cleared in xylene and embedded in paraffin. Tissue sections of 5 µm were stained with haematoxylin and eosin (H&E). A minimum of 10 fields for each liver slide were examined and assigned for severity of changes by an observer blinded to the treatments. The liver sections were examined in blinded fashion for (a) Kupffer cell hyperplasia; (b) PMN infiltration; (c) necrosis; and (d) pyknotic nuclei.
Statistical analysis
Results were expressed as mean ± s.e.m. The intergroup variation was measured by one-way analysis of variance (anova) followed by Fisher's least squares difference (LSD) test. Statistical significance was considered at P < 0·05. The statistical analysis was performed using the Jandel Sigma Stat Statistical Software version 2·0(San Rafel, CA, USA).
Results
Effect of CMN on LPS-induced liver dysfunction
LPS caused a marked rise in serum levels of ALT (125 IU/l versus 75 IU/l), AST (300 IU/l versus 170 IU) and AP (600 IU/l versus 240), demonstrating marked liver damage. LPS also caused significant rise in serum bilirubin (0·909 mg%versus 0·303 mg%) and decrease in serum total protein (3 mg/dl versus 10 mg/dl) compared to control. Treatment with CMN significantly and dose-dependently decreased the elevated levels of AST, ALT, AP and bilirubin in serum. It also prevented LPS-induced decrease in serum protein. However, CMN per se had no effect on AST, ALT, AP, bilirubin and protein (Table 1).
Table 1.
Effect of oral administration of different doses of curcumin (CMN) on liver function test after lipopolysaccharide (LPS) challenge.
| AST (IU/1) | ALT (IU/1) | ALP (IU/1) | Srm Prt (mg/dl) | T bil (mg%) | |
|---|---|---|---|---|---|
| Control | 170 ± 8·50 | 75 ± 3·75 | 240 ± 12·00 | 10 ± 0·50 | 0·30 ± 0·01 |
| LPS | 300 ± 15·00a | 125 ± 6·25a | 600 ± 30·00a | 3 ± 0·15a | 0·90 ± 0·04a |
| CMN (60) | 160 ± 8·00 | 72 ± 3·60 | 220 ± 11·00 | 10·4 ± 0·52 | 0·29 ± 0·01 |
| LPS + CMN (5) | 270 ± 13·50a | 118 ± 5·90a | 505 ± 25·25a,b | 4 ± 0·20a | 0·75 ± 0·03a,b |
| LPS + CMN (30) | 199 ± 9·95a,b | 100 ± 5·00a,b | 355 ± 17·75a,b | 5·5 ± 0·27a,b | 0·54 ± 0·02a,b |
| LPS + CMN (60) | 160 ± 8·00b | 85 ± 4·25a,b | 202 ± 10·10b | 8 ± 0·40a,b | 0·31 ± 0·01b |
AST = serum aspartate aminotransferase; ALT = serum alanine aminotransferase; ALP = alkaline phosphatase; Srm Prt = serum protein, T bil = total bilirubin. Values are expressed mean ± s.e.m.
P< 0·05 compared to vehicle and saline-treated group.
P< 0·05 compared to vehicle and lipopolysaccharide (LPS)-challenged rats.
Effect of CMN on LPS-induced nitrosative stress
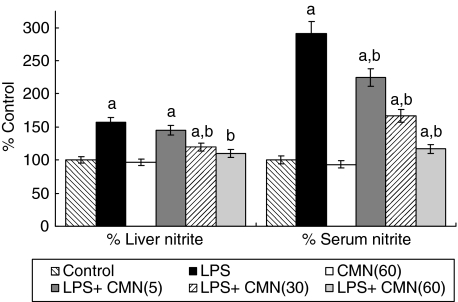
LPS caused a marked increase in nitrite level both in serum (350 µmol/ml versus 120 µmol/ml) and liver homogenate (425 µmol/mg protein versus 370 µmol/mg of protein) compared to control. CMN significantly attenuated this increase in nitrite levels. However, CMN per se had no effect on either serum or liver nitrite levels (Fig. 1).
Fig. 1.
Effect of different doses of curcumin (CMN) administration on serum and liver nitrite after lipopolysaccharide (LPS) challenge. Values are expressed as percentage response compared to control rats. aP< 0·05 compared to vehicle and saline-treated group. bP< 0·05 compared to vehicle and LPS-challenged rats.
Effect of CMN on LPS-induced lipid peroxidation
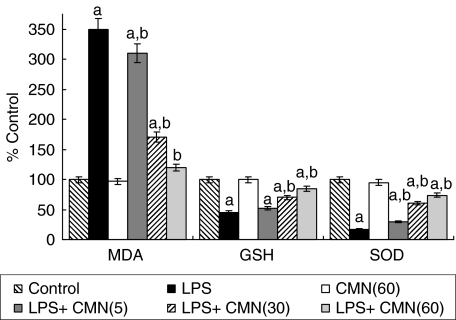
LPS challenge caused a marked lipid peroxidation in liver (3·5 µmol/mg protein versus 1·22 µ mol/mg protein) compared to control rats. Treatment with CMN produced a significant and dose-dependent attenuation in LPS-induced increase in lipid peroxidation. Seven days of oral feeding of CMN per se did not result in a significant alteration of lipid peroxidation (Fig. 2).
Fig. 2.
Effect of different doses of curcumin (CMN) administration on lipopolysaccharide (LPS)-induced lipid peroxidation (MDA), reduced glutathione (GSH) levels and superoxide dismutase (SOD) activity in liver homogenates. Values are expressed as percentage response compared to control rats. aP< 0·05 compared to vehicle and saline-treated group. bP< 0·05 compared to vehicle and LPS-challenged rats.
Effect of CMN on LPS-induced changes in hepatic anti-oxidant profile
LPS administration induced a marked reduction in hepatic GSH (18 µmol/mg protein versus 40 µmol/mg protein) and SOD (6·8 U/mg protein versus 40 U/mg protein) compared to control. This reduction was significantly and dose-dependently attenuated by CMN per se but had no affect on these endogenous anti-oxidants (Fig. 2).
Effect of CMN on LPS-induced changes in IL-1α, IL-6 and TNF-α
LPS challenge caused a marked rise in the levels of TNF-α and IL-6 compared to normal mouse. We were not able to detect the presence of IL-1α in any of the sample assayed. Seven-day oral feeding of CMN (60 mg/kg) decreases this rise of cytokines in LPS-challenged mice (Table 2).
Table 2.
Effect of curcumin (CMN, 60 mg/kg orally) on lipopolysaccharide (LPS)-induced changes in interkeukin (IL)-6 and tumour necrosis factor (TNF)-α levels in mouse serum.
| TNF-α (pg/ml) | IL-6 (pg/ml) | |
|---|---|---|
| Vehicle | 360 ± 5 | 190 ± 3 |
| LPS | 670 ± 10a | 460 ± 9a |
| LPS + CMN | 420 ± 7a,b | 230 ± 7a,b |
Values are expressed mean ± mean.
P< 0·05 compared to vehicle (control);
P< 0·05 compared to LPS.
Effect of CMN and on LPS-induced changes in hepatic morphology
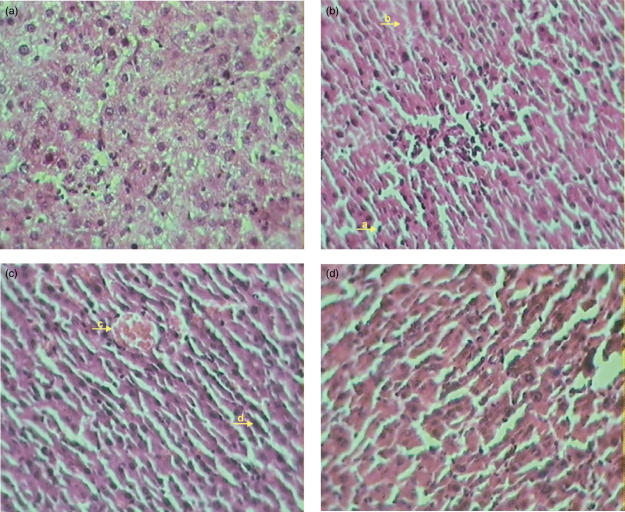
Histopathological evaluation did not reveal any morphological alterations in control group. In contrast, livers of LPS-administered rats showed marked morphological disruption such as Kupffer cell hyperplasia, PMN infiltration, necrosis and pyknotic nuclei. Treatment with CMN resulted in regression of these morphological changes (Fig. 3).
Fig. 3.
(a) Haemotoxylin and eosin (H&E)-stained longitudinal section of liver of saline-treated rats (40×). (b) H&E-stained longitudinal section of liver of rats, 6 h after lipopolysaccharide (LPS) challenge (40×). (a). Kupffer cell hyperplasia (b) necrosis. (c) H&E-stained longitudinal section of liver of LPS-treated rats 6 h after LPS challenge (40×) (c). PMN infiltration (d) pyknotic nucleus. (d) H&E-stained longitudinal section of liver of curcumin-treated rats and 6 h after LPS challenge (40×).
Discussion
Bacterial LPS (endotoxin) induces extensive damage to a variety of organs, including liver, due to the increased production of reactive oxygen intermediates and a resultant rise in lipid peroxidation [37,38]. Endotoxin accumulates in tissues rich in cells of the reticuloendothelial system such as liver and spleen [8]. In the liver, Kupffer cells are the major targets of LPS, which produce excessive amounts of O2.− on activation by LPS [39]. Additionally, LPS induces migration of activated PMNs into the liver [40], which constitutes another source of free radicals. Thus, severe oxidative stress is induced in liver by LPS.
Anti-oxidants and drugs from herbal origins prove to be beneficial in reversing the hepatotoxicity and oxidative stress produced by LPS [41,42]. In the present study administration of CMN for 7 days before endotoxin challenge markedly attenuated LPS-induced hepatic dysfunction and accompanying oxidative stress in liver. LPS contamination was not detectable in CMN by either of the two methods used (LAL and KDO) in contrast to 0·02% LPS contamination detected in the OMPs (Salmonella typhi outer membrane proteins) observed in our earlier studies [43]. This shows that the CMN sample used in the present study was free of any of LPS contamination and the beneficial effect of CMN seen was not due to the LPS tolerance.
LPS elevated serum levels of AST, ALT, AP and bilirubin which are the circulating markers of hepatocyte injury. This elevation of hepatic enzymes was coupled with a marked increase in hepatic oxidative stress as evident by a marked increase in TBARS levels and decrease in GSH and SOD levels in the liver. The tripeptide GSH is an important endogenous anti-oxidant which has a major role in restoring other free radical scavengers and anti-oxidants, such as vitamins C and E, to their reduced state. In our study, CMN significantly and dose-dependently attenuated hepatic dysfunction along with lipid peroxidation and restored the levels of GSH and SOD in LPS-administered rats. This observation is in line with various previous reports, which show that CMN decreases lipid peroxidation possibly by its anti-oxidant mechanism [44]. Vajragupta et al. [19] have reported that CMN–manganese complex and acetylcurcumin–manganese complex, a low molecular weight synthetic compound, showed much greater SOD mimetic activity and an inhibitory effect on lipid peroxidation. Sreejayan et al. claimed that the CMN inhibit iron-catalysed lipid peroxidation in rat brain tissue homogenates by chelation of iron [45]. An increasing number of studies have now established the ability of CMN to mainly eliminate the hydroxyl radical [46], superoxide radical [47], singlet oxygen [48], nitrogen dioxide [49] and NO [50]. It has also been demonstrated that CMN inhibits the generation of the superoxide radical [51]. Rukkumani et al. [52] reported a protective effect of CMN on circulating lipids in plasma and lipid peroxidation products in alcohol and polyunsaturated fatty acid-induced toxicity. In-vitro findings support the hypothesis that CMN inhibits free radical-induced apoptosis in cell lines [53].
LPS is a potent stimulator of nitric oxide (NO) [54,55]. It is reported that endotoxaemia for 6 h resulted in a 4·5-fold rise in the serum levels of nitrite, an end-product of NO metabolism. The increased levels of NO after endotoxin challenge can react with O2.− leading to formation of the peroxynitrite anion (ONOO–), which oxidizes sulphydryl groups and generates ·OH [56]. LPS has been demonstrated to cause iNOS expression in Kupffer cells and hepatocytes [57,58]. Consequently, there is a potential for large amounts of NO to be generated in the liver during sepsis, which could impair hepatic function by direct injury to hepatocytes [59]. L-NIL, a selective inhibitor of iNOS, has been shown to protect LPS- induced hepatic dysfunction [60]. In the present study, serum levels of nitrite were increased threefold after 6 h of LPS administration.
CMN inhibits iNOS gene expression in isolated BALB/C mouse peritoneal macrophages and also in the livers of LPS-injected mice [61]. Very recently, Sumanont et al.[62] have studied that CMN and its analogue show potent peroxynitrite anion scavenging activity in vitro using the sodium nitroprusside-generating nitric oxide system [62]. The present study also shows that LPS-induced nitrosative stress was significantly and dose-dependently attenuated by CMN in serum and liver tissue homogenates.
Various potent inflammatory cytokines are released by macrophages and neutrophils in response to a variety of stimuli, including endotoxin, and some Gram-positive bacteria [63]. Activated Kupffer cells were shown to increase proinflammatory cytokines such as TNF-α and IL-6 [64]. Antibodies of TNF-α are protective in some animal models of endotoxaemia and Gram-negative bacteraemia [65]. The role of IL-6 in inflammatory injury remains controversial. Some studies show that IL-6-deficient mice were more prone to LPS damage [66], whereas in other studies IL-6 increase in liver was found to be detrimental [67,68]. Administration of clenbuterol, although a β2 blocker, was shown to protect liver damage by decreasing proinflammatory cytokines such as TNF-α, IL-1β and IL-6 [69]. Further depletion of reduced glutathione can sensitize cultured mouse hepatocytes to TNF-α-induced cell death in the absence of transcription inhibitor, suggesting that reactive oxygen species (ROS) play a critical role in TNF-α-induced liver injury [70]. The critical role of ROS in TNF-α-induced liver injury is supported further by evidence that over-expression of thioredoxin, a small redox-active protein with anti-oxidant effects, significantly attenuates LPS/GalN-induced liver injury in mice [71].
We also observed an abrupt rise in the serum levels of TNF-α and IL-6 after LPS challenge. This rise was blocked effectively by CMN treatment. The most recent studies show that CMN can inhibit haemorrhage/resuscitation-induced IL-6 production and TNF-α-mediated activation of nuclear factor kappa-B (NF-κB), cell proliferation and IL-8 release [22,72]. Further, Wang and colleagues have shown that CMN can improve cardiac function by inhibition of collagen remodelling associated with suppression of the myocardial expression of TNF-α [73].
Histological sections of the endotoxaemic livers showed a high number of neutrophils, Kupffer cell hyperplasia, necrosis and pyknotic nuclei 6 h after LPS administration. CMN not only protected hepatic function, oxidative stress and cytokines production but also reduced PMN infiltration in the liver. This is important, because the number of PMNs is proportional to the extent of liver damage following LPS administration [74]. The recruiting neutrophils and lymphocytes have been shown to produce large amounts of proinflammatory cytokines such as IFN-γ and IL-6, which are involved in hepatotoxicity. This is consistent with in vitro studies showing that CMN inhibits phagocytosis and ROS production by neutrophils [75].
In conclusion, the study reported here shows that pretreatment of CMN effectively protects LPS-induced liver damage. This effect might be due to the effective blocking of oxidative stress, cytokine production and PMN infiltration in livers. Further studies are ongoing in our laboratory to establish whether CMN will be able to show a similar effect when it is administered after an infection sets in.
Acknowledgments
Grants from the Indian Council of Medical Research (ICMR) for conducting the study are gratefully acknowledged. The authors would like to express their thanks to Ms Saraswati Gupta, Senior Technical officer, University Institute of Pharmaceutical Sciences Panjab University Chandigarh for her help in conducting the spectrophotometric analyses. The authors also like to thank Dr (Mrs) Anju Bhandari MBBS, MD (pathology), of the Navjeevan Clinical Laboratory Chandigarh, for her help in performing the histological studies.
References
- 1.Hartung T, Tiegs G, Wendel A. The role of leukotriene D4 in septic shock models. Eicosanoids. 1992;5(Suppl):S42–4. [PubMed] [Google Scholar]
- 2.James PE, Madhani M, Roebuck W, Jackson SK, Swartz HM. Endotoxin-induced liver hypoxia: defective oxygen delivery versus oxygen consumption. Nitric Oxide. 2002;6:18–28. doi: 10.1006/niox.2001.0383. [DOI] [PubMed] [Google Scholar]
- 3.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–65. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 4.Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310–4. doi: 10.1152/ajpgi.00094.2004. [DOI] [PubMed] [Google Scholar]
- 5.Luster MI, Germolec DR, Yoshida T, Kayama F, Thompson M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology. 1994;19:480–8. [PubMed] [Google Scholar]
- 6.Hartung T, Wendel A. Endotoxin-inducible cytotoxicity in liver cell cultures − I. Biochem Pharmacol. 1991;42:1129–35. doi: 10.1016/0006-2952(91)90298-j. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T, Takano H, Takahashi S, Ichikawa H, Kondo M. Changes in tissue antioxidant enzyme activities and lipid peroxides in endotoxin-induced multiple organ failure. Circ Shock. 1994;42:53–8. [PubMed] [Google Scholar]
- 8.Sugino K, Dohi K, Yamada K, Kawasaki T. The role of lipid peroxidation in endotoxin-induced hepatic damage and the protective effect of antioxidants. Surgery. 1987;101:746–52. [PubMed] [Google Scholar]
- 9.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264:G801–9. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 10.Sewerynek E, Melchiorri D, Reiter RJ, Ortiz GG, Lewinski A. Lipopolysaccharide-induced hepatotoxicity is inhibited by the antioxidant melatonin. Eur J Pharmacol. 1995;293:327–34. doi: 10.1016/0926-6917(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 11.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 12.Joe B, Lokesh BR. Effect of curcumin and capsaicin on arachidonic acid metabolism and lysosomal enzyme secretion by rat peritoneal macrophages. Lipids. 1997;32:1173–80. doi: 10.1007/s11745-997-0151-8. [DOI] [PubMed] [Google Scholar]
- 13.Taher MM, Lammering G, Hershey C, Valerie K. Curcumin inhibits ultraviolet light induced human immunodeficiency virus gene expression. Mol Cell Biochem. 2003;254:289–97. doi: 10.1023/a:1027393719610. [DOI] [PubMed] [Google Scholar]
- 14.De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev. 2000;20:323–49. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Pal A, Pal AK. Studies on the genotoxicity of turmeric extracts in bacterial system. Int J Antimicrob Agents. 2000;16:415–7. doi: 10.1016/s0924-8579(00)00268-5. [DOI] [PubMed] [Google Scholar]
- 16.Jurgens TM, Frazier EG, Schaeffer JM, et al. Novel nematocidal agents from Curcuma comosa. J Nat Prod. 1994;57:230–5. doi: 10.1021/np50104a006. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal M, Sharma SD, Okazaki Y, Fujisawa M, Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92:33–8. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramanyam M, Koteswari AA, Kumar RS, Monickaraj SF, Maheswari JU, Mohan V. Curcumin-induced inhibition of cellular reactive oxygen species generation: novel therapeutic implications. J Biosci. 2003;28:715–21. doi: 10.1007/BF02708432. [DOI] [PubMed] [Google Scholar]
- 19.Vajragupta O, Boonchoong P, Watanabe H, Tohda M, Kummasud N, Sumanont Y. Manganese complexes of curcumin and its derivatives: evaluation for the radical scavenging ability and neuroprotective activity. Free Radic Biol Med. 2003;35:1632–44. doi: 10.1016/j.freeradbiomed.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Kim JE, Kim AR, Chung HY, Han SY, Kim BS, Choi JS. In vitro peroxynitrite scavenging activity of diarylheptanoids fromCurcuma longa. Phytother Res. 2003;17:481–4. doi: 10.1002/ptr.1179. [DOI] [PubMed] [Google Scholar]
- 21.Das KC, Das CK. Curcumin (diferuloylmethane), a singlet oxygen((1)O(2)) quencher. Biochem Biophys Res Commun. 2002;295:62–6. doi: 10.1016/s0006-291x(02)00633-2. [DOI] [PubMed] [Google Scholar]
- 22.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxide Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 23.Naik RS, Mujumdar AM, Ghaskadbi S. Protection of liver cells from ethanol cytotoxicity by curcumin in liver slice culture in vitro. J Ethnopharmacol. 2004;95:31–7. doi: 10.1016/j.jep.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Eybl V, Kotyzova D, Bludovska M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol Lett. 2004;151:79–85. doi: 10.1016/j.toxlet.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 25.van’t Land B, Blijlevens NM, Marteijn J, et al. Role of curcumin and the inhibition of NF-kappaB in the onset of chemotherapy-induced mucosal barrier injury. Leukemia. 2004;18:276–84. doi: 10.1038/sj.leu.2403233. [DOI] [PubMed] [Google Scholar]
- 26.Morrison DC, Leive L. Fractions of lipopolysaccharide from Escherichia coliO111: B4 prepared by two extraction procedures. J Biol Chem. 1975;250:2911–19. [PubMed] [Google Scholar]
- 27.Piotrowicz BI, Edlin SE, McCartney AC. A sensitive chromogenic Limulus amoebocyte lysate micro-assay for detection of endotoxin in human plasma and in water. Zentralbl Bakteriol Mikrobiol Hyg [A] 1985;260:108–12. doi: 10.1016/s0176-6724(85)80105-x. [DOI] [PubMed] [Google Scholar]
- 28.International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Methods for the measurement of catalytic concentrations of enzymes. Part 3. IFCC method for alanine aminotransferase (1-alanine 2 -oxoglutarate aminotransferase, ec 2.6.1.2) Clin Chim Acta. 1980;105:F145–72. [PubMed] [Google Scholar]
- 29.Pearlman FC, Lee RT. Detection and measurement of total bilirubin in serum, with use of surfactants as solubilizing agents. Clin Chem. 1974;20:447–53. [PubMed] [Google Scholar]
- 30.Recommendations of the German Society for Clinical Chemistry. Standardisation of methods for the estimation of enzyme activities in biological fluids. Experimental basis for the optimized standard conditions. Z Klin Chem Klin Biochem. 1972;10:281–91. [PubMed] [Google Scholar]
- 31.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 32.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jollow D, Mitchell L, Zampaglione N, Gillete J. Bromobenze induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzenoxide as the hepatotoxic intermediate. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 34.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 35.Tirkey N, Pilkhwal S, Kuhad A, Chopra K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005;5:2. doi: 10.1186/1471-2210-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Wei W, Shen YX, et al. Protective effect of melatonin against liver injury in mice induced by bacillus Calmette–Guerin plus lipopolysaccharide. World J Gastroenterol. 2004;10:2690–6. doi: 10.3748/wjg.v10.i18.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kono H, Asakawa M, Fujii H, et al. Edaravone, a novel free radical scavenger, prevents liver injury and mortality in rats administered endotoxin. J Pharmacol Exp Ther. 2003;307:74–82. doi: 10.1124/jpet.103.053595. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda H, Ishikado A, Nishida N, et al. Hepatoprotective, superoxide scavenging, and antioxidative activities of aromatic constituents from the bark of Betula platyphylla var. japonica. Bioorg Med Chem Lett. 1998;8:2939–44. doi: 10.1016/S0960-894X(98)00528-9. [DOI] [PubMed] [Google Scholar]
- 39.Bautista AP, Meszaros K, Bojta J, Spitzer JJ. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol. 1990;48:123–8. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- 40.Levy E, Ruebner BH. Hepatic changes produced by a single dose of endotoxin in the mouse. Light microscopy and histochemistry. Am J Pathol. 1967;51:269–85. [PMC free article] [PubMed] [Google Scholar]
- 41.Aniya Y, Koyama T, Miyagi C, et al. Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol Pharm Bull. 2005;28:19–23. doi: 10.1248/bpb.28.19. [DOI] [PubMed] [Google Scholar]
- 42.Kao ES, Wang CJ, Lin WL, Yin YF, Wang CP, Tseng TH. Anti-inflammatory potential of flavonoid contents from dried fruit of Crataegus pinnatifida in vitro and in vivo. J Agric Food Chem. 2005;53:430–6. doi: 10.1021/jf040231f. [DOI] [PubMed] [Google Scholar]
- 43.Sood S, Rishi P, Dhawan V, Sharma S, Ganguly NK. Protection mediated by antibodies to iron-regulated outer membrane proteins of S. typhi in a mouse peritonitis model. Mol Cell Biochem. 2005;273:69–78. doi: 10.1007/s11010-005-7756-8. [DOI] [PubMed] [Google Scholar]
- 44.Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002;9:232–8. doi: 10.1078/0944-7113-00119. [DOI] [PubMed] [Google Scholar]
- 45.Sreejayan Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46:1013–6. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 46.Reddy AC, Lokesh BR. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol Cell Biochem. 1994;137:1–8. doi: 10.1007/BF00926033. [DOI] [PubMed] [Google Scholar]
- 47.Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung. 1996;46:169–71. [PubMed] [Google Scholar]
- 48.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–66. [PubMed] [Google Scholar]
- 49.Unnikrishnan MK, Rao MN. Curcumin inhibits nitrogen dioxide induced oxidation of hemoglobin. Mol Cell Biochem. 1995;146:35–7. doi: 10.1007/BF00926878. [DOI] [PubMed] [Google Scholar]
- 50.Sreejayan Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–7. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 51.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 52.Rukkumani R, Sri Balasubashini M, Menon VP. Protective effects of curcumin and photo-irradiated curcumin on circulatory lipids and lipid peroxidation products in alcohol and polyunsaturated fatty acid-induced toxicity. Phytother Res. 2003;17:925–9. doi: 10.1002/ptr.1254. [DOI] [PubMed] [Google Scholar]
- 53.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–75. [PubMed] [Google Scholar]
- 54.Tsuji K, Kwon AH, Yoshida H, et al. Free radical scavenger (edaravone) prevents endotoxin-induced liver injury after partial hepatectomy in rats. J Hepatol. 2005;42:94–101. doi: 10.1016/j.jhep.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi Y, Abe M, Murai A, et al. Comparison of effects of nitric oxide synthase (NOS) inhibitors on plasma nitrite/nitrate levels and tissue NOS activity in septic organs. Microbiol Immunol. 2005;49:139–47. doi: 10.1111/j.1348-0421.2005.tb03713.x. [DOI] [PubMed] [Google Scholar]
- 56.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–50. [PubMed] [Google Scholar]
- 57.Duval DL, Miller DR, Collier J, Billings RE. Characterization of hepatic nitric oxide synthase: identification as the cytokine-inducible form primarily regulated by oxidants. Mol Pharmacol. 1996;50:277–84. [PubMed] [Google Scholar]
- 58.Roland CR, Naziruddin B, Mohanakumar T, Flye MW. Gadolinium chloride inhibits Kupffer cell nitric oxide synthase (iNOS) induction. J Leukoc Biol. 1996;60:487–92. doi: 10.1002/jlb.60.4.487. [DOI] [PubMed] [Google Scholar]
- 59.Billiar TR, Curran RD, West MA, Hofmann K, Simmons RL. Kupffer cell cytotoxicity to hepatocytes in coculture requires 1-arginine. Arch Surg. 1989;124:1416–20. doi: 10.1001/archsurg.1989.01410120062013. discussion 20-1. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C, Walker LM, Hinson JA, Mayeux PR. Oxidant stress in rat liver after lipopolysaccharide administration: effect of inducible nitric-oxide synthase inhibition. J Pharmacol Exp Ther. 2000;293:968–72. [PubMed] [Google Scholar]
- 61.Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–62. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- 62.Sumanont Y, Murakami Y, Tohda M, Vajragupta O, Matsumoto K, Watanabe H. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol Pharm Bull. 2004;27:170–3. doi: 10.1248/bpb.27.170. [DOI] [PubMed] [Google Scholar]
- 63.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–18. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 64.Chiao CW, Lee SS, Wu CC, Su MJ. Thaliporphine increases survival rate and attenuates multiple organ injury in LPS-induced endotoxaemia. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:34–43. doi: 10.1007/s00210-004-1014-6. [DOI] [PubMed] [Google Scholar]
- 65.Silva AT, Bayston KF, Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis. 1990;162:421–7. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- 66.Inoue K, Takano H, Shimada A, et al. Cytoprotection by interleukin-6 against liver injury induced by lipopolysaccharide. Int J Mol Med. 2005;15:221–4. [PubMed] [Google Scholar]
- 67.Choda Y, Morimoto Y, Miyaso H, et al. Failure of the gut barrier system enhances liver injury in rats: protection of hepatocytes by gut-derived hepatocyte growth factor. Eur J Gastroenterol Hepatol. 2004;16:1017–25. doi: 10.1097/00042737-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 68.Guler R, Olleros ML, Vesin D, et al. Inhibition of inducible nitric oxide synthase protects against liver injury induced by mycobacterial infection and endotoxins. J Hepatol. 2004;41:773–81. doi: 10.1016/j.jhep.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 69.Izeboud CA, Hoebe KH, Grootendorst AF, et al. Endotoxin-induced liver damage in rats is minimized by beta 2-adrenoceptor stimulation. Inflamm Res. 2004;53:93–9. doi: 10.1007/s00011-003-1228-y. [DOI] [PubMed] [Google Scholar]
- 70.Nagai H, Matsumaru K, Feng G, Kaplowitz N. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36:55–64. doi: 10.1053/jhep.2002.33995. [DOI] [PubMed] [Google Scholar]
- 71.Okuyama H, Nakamura H, Shimahara Y, et al. Overexpression of thioredoxin prevents acute hepatitis caused by thioacetamide or lipopolysaccharide in mice. Hepatology. 2003;37:1015–25. doi: 10.1053/jhep.2003.50203. [DOI] [PubMed] [Google Scholar]
- 72.Gaddipati JP, Sundar SV, Calemine J, Seth P, Sidhu GS, Maheshwari RK. Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock. 2003;19:150–6. doi: 10.1097/00024382-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Yao QH, Wang DQ, Cui CC, et al. Curcumin ameliorates left ventricular function in rabbits with pressure overload: inhibition of the remodeling of the left ventricular collagen network associated with suppression of myocardial tumor necrosis factor-alpha and matrix metalloproteinase-2 expression. Biol Pharm Bull. 2004;27:198–202. doi: 10.1248/bpb.27.198. [DOI] [PubMed] [Google Scholar]
- 74.Hewett JA, Schultze AE, VanCise S, Roth RA. Neutrophil depletion protects against liver injury from bacterial endotoxin. Lab Invest. 1992;66:347–61. [PubMed] [Google Scholar]
- 75.Madan B, Ghosh B. Diferuloylmethane inhibits neutrophil infiltration and improves survival of mice in high-dose endotoxin shock. Shock. 2003;19:91–6. doi: 10.1097/00024382-200301000-00017. [DOI] [PubMed] [Google Scholar]