Abstract
Transcription of the nrdDG operon, which encodes the class III nucleotide reductase, which is only active under anaerobic conditions, was strongly induced after a shift to anaerobiosis. The induction was completely dependent on the transcriptional activator FNR and was independent of the ArcA-ArcB two-component response regulator system. The nrdD transcript start site was mapped to a position immediately downstream of two FNR binding sites. Transcription of the other two nucleotide reductase operons, nrdAB and nrdEF, did not respond to oxygen conditions in a wild-type background, but nrdAB expression was increased in the fnr mutant under anaerobic conditions.
The Escherichia coli genome contains three operons (nrdAB, nrdHIEF, and nrdDG) encoding ribonucleotide reductases, which supply the deoxynucleoside triphosphate (dNTP) substrates for DNA replication. The nrdAB operon codes for the primary aerobic reductase, a class Ia tyrosine-cysteine radical enzyme that reduces NDP substrates by using thioredoxin and glutaredoxin (15). The nrdEF genes encode an auxiliary weakly expressed class Ib enzyme (15) that, in addition to glutaredoxin, utilizes the nrdH gene product as an electron transporter (14). The nrdD gene encodes a class III reductase containing an oxygen-sensitive glycyl radical (15), similar to that of pyruvate formate lyase. The NrdD reductase is activated by the nrdG-encoded activase under anaerobic growth conditions (22) and is irreversibly inactivated by oxygen. The nrdAB-encoded reductase is essential under aerobic growth conditions, while the nrdD gene is essential under strict anaerobic growth conditions (9) in which the FeIII tyrosyl radical of the class Ia enzyme is inactive.
The nrdAB operon is transcribed from two promoters (7), and transcription is activated by Fis (4) and IciA (12) and probably also by DnaA, because mutations in the two DnaA binding sites in the promoter region reduce expression (4). The operon is cell cycle regulated (21), and expression is stimulated by inhibition of the nrdAB-encoded nucleotide reductase by hydroxyurea (9, 10) as well as by inactivation of the genes encoding thioredoxin and glutaredoxin (18). Like nrdAB, the nrdHIEF operon is induced by hydroxyurea (13) and in strains lacking thioredoxin and glutaredoxin (17). In addition, nrdHIEF is induced by oxidative stress (17). Very little is known about regulation of expression of the nrdDG operon except that the level of enzyme activity is increased under anaerobic growth conditions (9). Here we have constructed single-copy transcriptional lacZ fusions of the three nrd operons and investigated their expression as a function of aeration and in strains carrying mutations in the two main transcriptional regulators for aerobic/anaerobic shifts, FNR and ArcA. FNR activates transcription of a number of genes coding for enzymes required under anaerobic growth conditions and represses transcription of some genes for aerobic functions (11, 20). Under anaerobic conditions, ArcA, the response regulator of the two-component ArcA-ArcB system, represses many genes for aerobic functions and activates transcription of some other genes (11).
Construction of transcriptional lacZ fusions to the nrd promoters.
The promoter regions of the three nrd operons (Fig. 1) were amplified by PCR and cloned in front of the lacZ gene of the promoter cloning vector pTAC3953 (6). The fusions were subsequently integrated into the λ attachment site as described previously (2) and transferred to strain LJ24 by P1 transduction to obtain the strains BOS7 to BOS9 illustrated in Table 1, and these strains were used to construct isogenic derivatives carrying the fnr1 and arcA1 mutations.
FIG. 1.
Structure of nrd-lacZ fusions. (A) The promoter regions of the nrdAB, nrdDG, and nrdHIEF operons were amplified from chromosomal DNA of strain LJ24 with the primers Nrd5 and Nrd6 (AAGACGGCGTATTTAATCGC and CGAGATACTGATAATCCGGC, respectively), NrdD-F and NrdD-B (TTATGTCGACCACTGGCAACAAGGAATGCAC and TAAGATCTTTCCGCTGCTTTAGCTGCAC, respectively), and NrdE-1 and NrdE-2 (TTCTGTCGACGGATCATATGTTTAACCGACC and AGGTTAGATCTGAAGATACGGCGCGATG, respectively). The nrdA fragment was digested with TaqI and cloned into the promoter cloning vector pTAC3953 (6) digested with BstB1, while the nrdD and nrdH promoter fragments were digested with SalI and BglII and inserted into pTAC3953 digested with the same enzymes. The lacZ fusions from the three plasmids were subsequently transferred into the λ attachment site as described in reference 2 to obtain strains BOS7, BOS8, and BOS9. The correct promoter lacZ fusion in these strains was verified by sequencing. Restriction sites used in the construction are indicated above the bars showing the relevant segments of the plasmids. Light gray arrows show the position and direction of genes. Gray rectangles represent DnaA boxes with up to one mismatch to the consensus sequence TTATNCACA, while a white rectangle represents the Fis binding site (4), and black rectangles represent FNR binding sites. Black triangles represent promoters. The positions of the promoters for nrdA and nrdH are according to references 23 and 13, respectively, whereas the position of the nrdD promoter is according to our data from Fig. 4. (B) Sequence of the nrdD promoter region. The −35 and −10 regions of the promoter sequence are indicated with lines below the sequence, and the transcript start site, determined from Fig. 4, is indicated by an asterisk below the sequence. The FNR binding sites are boxed. Bases fitting the consensus sequence ANANTTGATNNANATCAAT (20) are indicated in boldface, and those not fitting are in lowercase.
TABLE 1.
E. coli K-12 strains used in this study
| Strain | Genotypea | Source or construction |
|---|---|---|
| LJ24 | thi-1 leu-6 lacY1 lacI-ZΔ(Mlu) glnV44 tonA21 rpsL rfbD1 rpoS396(Am) | 3, 19 |
| TC3266 | fnr1 zci::Tn10 | 6 |
| LB97 | arcA1 zjj::Tn10 | 6 |
| BOS7 | attB::pndrA′-lacZ | This workb |
| BOS8 | attB::pndrD′-lacZ | This workb |
| BOS9 | attB::pndrH′-lacZ | This workb |
| BOS10 | arcA1 zjj::Tn10 attB::pndrA′-lacZ | P1(BOS7) × LB97 |
| BOS11 | arcA1 zjj::Tn10 attB::pndrD′-lacZ | P1(BOS8) × LB97 |
| BOS13 | fnr1 zci::Tn10 attB::pndrA′-lacZ | P1(BOS7) × TC3266 |
| BOS14 | fnr1 zci::Tn10 attB::pndrD′-lacZ | P1(BOS8) × TC3266 |
Genetic symbols are according to reference 5. The genotypes of TC3266 to BOS14 are otherwise like LJ24.
The lacZ fusions from the three plasmids in Fig. 1 were inserted into the λ attachment site as described previously (2) and transferred by P1 transduction to strain LJ24 by selection for the kanamycin resistance gene linked to the fusions.
Expression of the nrd operons under aerobic and anaerobic conditions.
The wild-type strains carrying the three nrd-lacZ fusions were grown with full aeration, and part of the cultures were then shifted to anaerobic growth conditions as described in reference 6. Cell mass (optical density at 450 nm [OD450]) and β-galactosidase activity were measured throughout the experiment. As expected, expression of the pnrdA′-lacZ fusion was very high, and that of the pnrdH′-lacZ fusion was very low (Table 2). Expression of both fusions was unaffected by the shift to anaerobiosis (Table 2). During steady-state anaerobic growth, expression of the pnrdA′-lacZ and pnrdH′-lacZ fusions was reduced to 50% of that observed immediately after the shift (data not shown). In contrast, the level of nrdD expression was low during exponential aerobic growth and increased 20-fold upon a shift to anaerobic growth conditions (Fig. 2 and Table 2). The pnrdD′-lacZ fusion was also strongly induced in the aerobic culture during the deceleration phase of growth (Fig. 2A), reaching approximately 50% of the anaerobic value (Table 2), while entry into the stationary phase had no effect on expression from the nrdAB or nrdHIEF promoters (Table 2).
TABLE 2.
Oxygen and growth-phase regulation of transcription of the nrd operons
| Strain | Promoter-lacZ fusion | Regulatory genotype | β-Galactosidase sp act (U · ml−1 · OD450−1)a
|
|||
|---|---|---|---|---|---|---|
| Exponential growth phase
|
Stationary phase
|
|||||
| Aerobic | Anaerobic | Aerobic | Anaerobic | |||
| BOS8 | nrdD | Wild type | 1.5 | 30 | 16 | 50 |
| BOS11 | nrdD | arcA1 | 1.5 | 30 | 20 | 50 |
| BOS14 | nrdD | fnr1 | 1.1 | 1 | 4 | 4 |
| BOS7 | nrdA | Wild type | 370 | 350 | 320 | 200 |
| BOS10 | nrdA | arcA1 | 380 | 360 | 330 | 220 |
| BOS13 | nrdA | fnr1 | 380 | 320 | 330 | 800 |
| BOS9 | nrdH | Wild type | 0.2 | 0.2 | 0.2 | 0.2 |
The strains were grown and β-galactosidase activity was determined as described in the legend to Fig. 2. The values for aerobic exponential growth are the average of specific β-galactosidase activity in at least six samples taken from the cultures at a cell mass represented by an OD450 of between 0.1 and 0.8. The values for anaerobic exponential growth are the slopes from differential plots of β-galactosidase versus OD450 (see Fig. 3 for an example), where the slopes are based on at least six points at OD450s of between 0.1 and 0.8. The specific β-galactosidase activity values for the stationary phase are the average of the two to three samples taken after cessation of growth. All values are the average of at least two experiments, which all showed less than 25% variation between experiments.
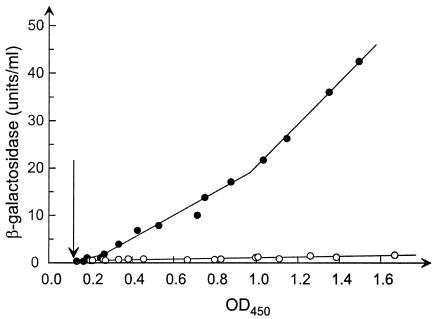
FIG. 2.
pndrD′-lacZ expression under aerobic (A) and anaerobic (B) growth conditions. Strains BOS8 (fnr+) and BOS14 (fnr1) were grown with good aeration at 37°C in AB minimal medium (8) supplemented with 1% Casamino Acids (Difco), 0.2% glucose, and 1 μg of thiamine per ml. At the time corresponding to t = 0 min, part of the cultures were shifted to anaerobic growth conditions obtained as previously described (6), while the other part of the cultures remained under aerobic conditions. Samples were taken for determination of cell mass and β-galactosidase activity as described previously (6). Solid symbols indicate BOS8 (fnr+), and open symbols indicate BOS14 (fnr1). Squares indicate OD450, and triangles indicate β-galactosidase specific activity (units per milliliter × OD450).
Effect of fnr and arcA on anaerobic and growth-phase induction of nrdD.
Introduction of the arcA1 mutation had no effect on expression of the nrdD operon, while introduction of the fnr1 mutation completely eliminated the anaerobic induction of the ndrD promoter (Fig. 2B). The differential rate of synthesis of the pnrdD′-lacZ fusion increases very shortly after the shift to anaerobiosis in the wild type (Fig. 3). Thus, the kinetics of induction of the nrdDG operon are very similar to those observed for other anaerobically induced genes, both the FNR-regulated pfl operon (19) and ArcA-regulated genes (1, 6).
FIG. 3.
Differential plot of pndrD′-lacZ expression during a shift to anaerobiosis. The strains BOS8 (fnr+) and BOS14 (fnr1) were grown as described in the legend to Fig. 2 and shifted to anaerobic growth conditions at the OD indicated by the arrow. Solid circles represent BOS8 (fnr+), and open symbols represent BOS14 (fnr1).
In the absence of the FNR protein, the aerobic exponential expression of nrdD was slightly reduced (Table 2) and the stationary-phase induction was virtually absent, indicating that the induction during the deceleration phase of growth is caused by oxygen limitation. A slight residual stationary-phase induction was, however, observed in the fnr mutant. This induction occurred at a much later stage (higher cell density) under both aerobic (Fig. 2A) and anaerobic (Fig. 2B) growth conditions. In the wild type, there was also an approximately twofold stationary-phase induction under anaerobic conditions (Table 2). This stationary-phase induction is probably not due to RpoS, the stationary-phase sigma factor, because the promoter does not contain any of the RpoS-dependent promoter signatures (Fig. 1B) and the induction occurs very late in the growth cycle relative to the time at which maximal RpoS levels are reached in this strain under these growth conditions (T. Atlung, unpublished data).
Localization of the nrdD promoter.
Analysis of the sequence between the treC and nrdD genes revealed two good homologies to FNR binding sites (20) located upstream of two putative −10 promoter sequences (Fig. 1B). To determine which of these is actually the promoter, we mapped the transcript start site by primer extension using the nrdDG2 primer, complementary to sequences 160 residues downstream of FNR site 1. We detected only one transcript start site in RNA from anaerobically grown wild-type cells, while no specific primer extension products were detected with RNA from the fnr mutant (Fig. 4). The same result was obtained with a primer located closer to the promoter (data not shown). Thus the nrdDG operon is transcribed from a single promoter that has two FNR sites: one centered at position −65 relative to the transcript start site and the second located on top of the −35 sequence of the promoter. It is therefore very likely that transcription from the nrdD promoter is stimulated directly by FNR upon its activation by a shift to anaerobiosis.
FIG. 4.
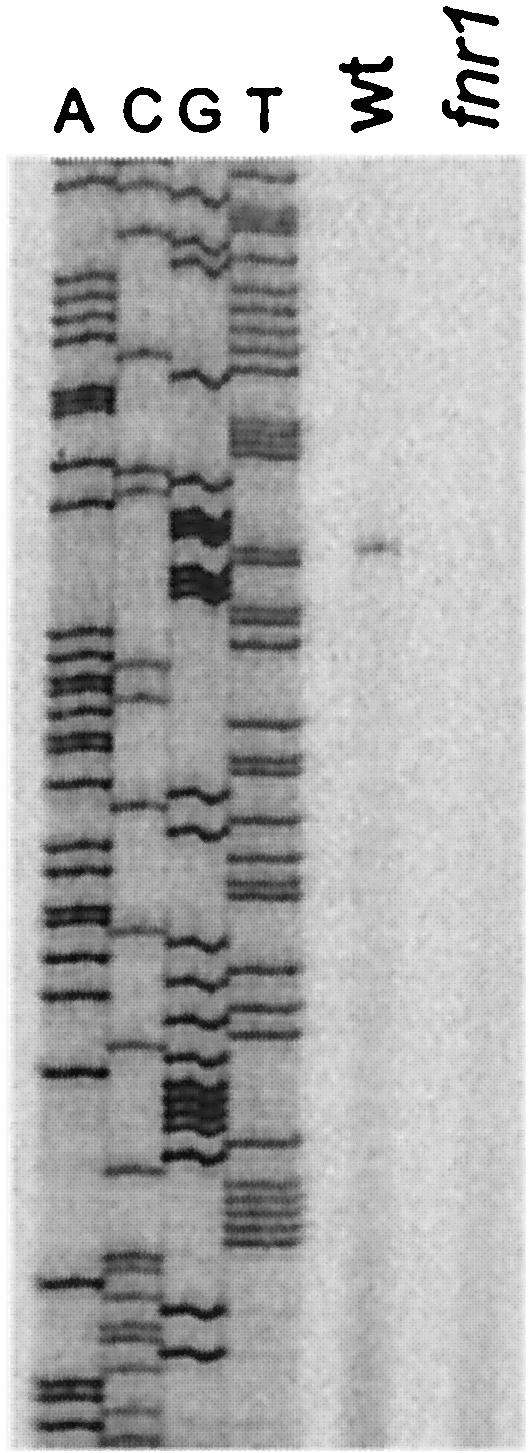
Primer extension analysis of the nrdD transcript start site. The wild-type (wt) strain BOS8 (fnr+) and mutant strain BOS14 (fnr1) were grown under anaerobic conditions as described in the legend to Fig. 2, and RNA was prepared with the MasterPure RNA purification kit from EPICENTRE. Primer extension analysis (16) was carried out with 5 μg of RNA and the 33P end-labeled primer nrdDG2 (GGTTATCCACAGAAATTGGGAAAGG). The primer extension products were analyzed by electrophoresis on an 8% acrylamide gel containing 8 M urea, alongside sequencing reactions performed with the same end-labeled primer and the nrdD promoter PCR fragment as a template.
Effect of fnr and arcA on expression of nrdA.
We also tested the effect of mutations in the two transcriptional regulators on expression of the nrdAB promoter, because we had observed a small but reproducible decrease in the levels of β-galactosidase activity in the wild type in the stationary phase under anaerobic growth conditions (Table 2). Expression of nrdA was independent of ArcA (Table 2), but we found a significant increase in the anaerobic stationary-phase expression of nrdA in the fnr mutant. No FNR sites are found close to the nrdA promoter, but there is a site showing reasonable homology for one half-site overlapping the promoter-proximal IciA binding site, and since IciA stimulates transcription (12), FNR could repress transcription by interfering with IciA binding. It has previously been found that nrdAB-encoded nucleotide reductase levels were increased in an nrdD-null mutant under microaerophilic conditions (9). We therefore favor the alternative hypothesis that the low level of NrdD ribonucleotide reductase activity in the fnr mutant causes the increased expression of nrdA by the same unknown induction mechanism as inhibition of NrdAB reductase activity by hydroxyurea, nrdA inactivation by mutation, or the absence of thioredoxin and glutaredoxin.
Conclusions.
Expression from the nrdD promoter exhibits FNR-dependent and ArcA-independent induction by anaerobiosis and by oxygen limitation in the stationary phase. The promoter is most probably directly activated by binding of FNR to the two FNR binding sites found in positions similar to those found for other FNR-regulated promoters. Expression of the nrdA promoter is independent of oxygen availability and growth phase, except for prolonged growth under anaerobic conditions or entry into the stationary phase during anaerobic growth, where expression is reduced in the wild type but increased in the fnr mutant, probably in response to the sum of activity of the nucleotide reductases.
Acknowledgments
We thank Kirsten Olesen for expert technical assistance and Susanne Christensen at the University of Southern Denmark for help with the primer extension.
This work was supported by a grant from the Danish Natural Science Research Council.
REFERENCES
- 1.Atlung, T., and L. Brøndsted. 1994. Role of the transcriptional activator AppY in regulation of the cyx appA operon of Escherichia coli by anaerobiosis, phosphate starvation, and growth phase. J. Bacteriol. 176:5414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlung, T., A. Nielsen, L. J. Rasmussen, L. J. Nellemann, and F. Holm. 1991. A versatile method for integration of genes and gene fusions into the λ attachment site of Escherichia coli. Gene 107:11-17. [DOI] [PubMed] [Google Scholar]
- 3.Atlung, T., H. V. Nielsen, and F. G. Hansen. 2002. Characterisation of the allelic variation in the rpoS gene in 13 K12 and six other nonpathogenic Escherichia coli strains. Mol. Gen. Genomics 266:873-881. [DOI] [PubMed] [Google Scholar]
- 4.Augustin, L. B., B. A. Jacobson, and J. A. Fuchs. 1994. Escherichia coli Fis and DnaA proteins bind specifically to the nrd promoter region and affect expression of an nrd-lac fusion. J. Bacteriol. 176:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann, B. J. 1990. Linkage map of Escherichia coli K-12, edition 8. Microbiol. Rev. 54:130-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brøndsted, L., and T. Atlung. 1994. Anaerobic regulation of the hydrogenase 1 (hya) operon of Escherichia coli. J. Bacteriol. 176:5423-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casado, C., M. Llagostera, and J. Barbe. 1991. Expression of nrdA and nrdB genes of Escherichia coli is decreased under anaerobiosis. FEMS Microbiol. Lett. 83:153-157. [DOI] [PubMed] [Google Scholar]
- 8.Clark, D. J., and O. Maaløe. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99-112. [Google Scholar]
- 9.Garriga, X., R. Eliasson, E. Torrents, A. Jordan, J. Barbe, I. Gibert, and P. Reichard. 1996. nrdD and nrdG genes are essential for strict anaerobic growth of Escherichia coli. Biochem. Biophys. Res. Commun. 229:189-192. [DOI] [PubMed] [Google Scholar]
- 10.Gibert, I., S. Calero, and J. Barbe. 1990. Measurement of in vivo expression of nrdA and nrdB genes of Escherichia coli by using lacZ gene fusions. Mol. Gen. Genet. 220:400-408. [DOI] [PubMed] [Google Scholar]
- 11.Gunsalus, R. P., and S. J. Park. 1994. Aerobic-anaerobic gene regulation in Escherichia coli—control by the ArcAB and Fnr regulons. Res. Microbiol. 145:437-450. [DOI] [PubMed] [Google Scholar]
- 12.Han, J. S., H. S. Kwon, J. B. Yim, and D. S. Hwang. 1998. Effect of IciA protein on the expression of the nrd gene encoding ribonucleoside diphosphate reductase in E. coli. Mol. Gen. Genet. 259:610-614. [DOI] [PubMed] [Google Scholar]
- 13.Jordan, A., E. Aragall, I. Gibert, and J. Barbe. 1996. Promoter identification and expression analysis of Salmonella typhimurium and Escherichia coli nrdEF operons encoding one of two class I ribonucleotide reductases present in both bacteria. Mol. Microbiol. 19:777-790. [DOI] [PubMed] [Google Scholar]
- 14.Jordan, A., F. Aslund, E. Pontis, P. Reichard, and A. Holmgren. 1997. Characterization of Escherichia coli NrdH—a glutaredoxin-like protein with a thioredoxin-like activity profile. J. Biol. Chem. 272:18044-18050. [DOI] [PubMed] [Google Scholar]
- 15.Jordan, A., and P. Reichard. 1998. Ribonucleotide reductases. Annu. Rev. Biochem. 67:71-98. [DOI] [PubMed] [Google Scholar]
- 16.Melefors, Ö., and A. von Gabain. 1988. Site-specific endonucleolytic cleavages and the regulation of stability of E. coli ompA mRNA. Cell 52:893-901. [DOI] [PubMed] [Google Scholar]
- 17.Monje-Casas, F., J. Jurado, M. J. Prieto-Alamo, A. Holmgren, and C. Pueyo. 2001. Expression analysis of the nrdHIEF operon from Escherichia coli—conditions that trigger the transcript level in vivo. J. Biol. Chem. 276:18031-18037. [DOI] [PubMed] [Google Scholar]
- 18.Prieto-Alamo, M. J., J. Jurado, R. Gallardo-Madueno, F. Monje-Casas, A. Holmgren, and C. Pueyo. 2000. Transcriptional regulation of glutaredoxin and thioredoxin pathways and related enzymes in response to oxidative stress. J. Biol. Chem. 275:13398-13405. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen, L. J., P. L. Møller, and T. Atlung. 1991. Carbon metabolism regulates expression of the pfl (pyruvate formate-lyase) gene in Escherichia coli. J. Bacteriol. 173:6390-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiro, S., and J. R. Guest. 1990. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol. Rev. 75:399-428. [DOI] [PubMed] [Google Scholar]
- 21.Sun, L., and J. A. Fuchs. 1992. Escherichia coli ribonucleotide reductase expression is cell-cycle regulated. Mol. Biol. Cell 3:1095-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamarit, J., E. Mulliez, C. Meier, A. Trautwein, and M. Fontecave. 1999. The anaerobic ribonucleotide reductase from Escherichia coli—the small protein is an activating enzyme containing a [4Fe-4S]2+ center. J. Biol. Chem. 274:31291-31296. [DOI] [PubMed] [Google Scholar]
- 23.Tuggle, C. K., and J. A. Fuchs. 1986. Regulation of the operon encoding ribonucleotide reductase in Escherichia coli—evidence for both positive and negative control. EMBO J. 5:1077-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]