Abstract
Summary
During inflammation, activated neutrophils, monocytes and macrophages produce and release myeloperoxidase (MPO). MPO converts hydrogen peroxide to hypochlorous acid, a highly reactive and oxidizing agent. Proteins subjected to hypochlorous acid become chlorinated. We analysed how chlorination of the cartilage antigen collagen type II (CII) affects its immunogenic and arthritogenic properties by studying immune responses to chlorinated CII in comparison to immune responses to CII and by studying the development of arthritis in rats immunized with CII–Cl. CII–Cl immunization of LEW.1AV1 rats caused a 100% incidence of arthritis with a mean maximum score of 9·2 (maximal score possible 16). The same dose of non-chlorinated CII did not induce arthritis at all. Rats immunized with CII–Cl developed high anti-CII–Cl IgG titres and also developed IgG antibodies recognizing the non-chlorinated form of CII. Analysis of cytokine mRNA expression in lymph nodes 10 days after immunzation revealed an increased expression of interferon (IFN)-γ mRNA and interleukin (IL)-1β mRNA in CII–Cl-immunized rats compared to CII-immunized rats. Thus, chlorination of CII increased its immunogenicity as well as its arthritogenicity. As neutrophils, monocytes and macrophages are abundant cells in arthritic joints of patients with rheumatoid arthritis, chlorination might be a mechanism by which immunoreactivity to CII is induced and by which chronic joint inflammation is supported.
Keywords: arthritis, chlorination, collagen type II (CII), hypochlorous acid
Introduction
When an organism is attacked by pathogens or is injured in any other way, so that body trauma occurs, the organism defends itself by mounting an inflammatory reaction. Contributing to the inflammatory reaction are both cells and molecules of the immune system. In the early inflammatory response the innate arm of the immune system is activated, whereas if the trauma persists the adaptive immune system becomes activated. Neutrophils and monocytes are among the first cells to enter the trauma site, where they engage in phagocytosis, production of proinflammatory mediators and release of bactericidal substances.
Activated neutrophils are highly reactive cells with the propensity to release matrix-degrading enzymes as well as free radicals. Neutrophils also produce myeloperoxidase (MPO), an enzyme which converts hydrogen peroxide to hypochlorous acid (HOCl). HOCl is a strong oxidant that rapidly chlorinates surrounding proteins. Such MPO-catalysed chlorination of proteins is a part of our innate, anti-bacterial defence but can also occur with endogenous proteins. MPO is synthesized by and released from activated neutrophils, and to a lesser extent from monocytes. In addition, MPO-uptake by macrophages has been demonstrated to occur [1]. The formation of HOCl and subsequent chlorination of proteins can thus occur both intracellularly and extracellularly. HOCl reacts with sulphydryl groups on proteins; tryptophane and methionine can be chlorinated by HOCl in concentrations as low as 10 µM. Chlorination of proteins increases their susceptibility to proteolytic cleavage and enhanced immunogenicity for chlorinated antigens have been demonstrated in in vitro T cell assays [2].
Rheumatoid arthritis (RA) is a chronic, destructive, inflammatory joint disease with unknown aetiology. An autoimmune component in the disease pathogenesis is implicated by the presence of a diverse set of autoantibodies. Some of these are detected frequently in RA patients, including antibodies directed to the Fc-part of Ig molecules (rheumatoid factor) and those reactive with citrullinated proteins [3]. Others can be recorded in subgroups of patients, such as anti-collagen II (CII) antibodies [4,5], anti-human cartilage glycoprotein-39 (gp-39) [6] antibodies and antibodies against stress protein endoplasmatic reticulum chaperone BiP [7]. Joint inflammation in RA, as well as in experimental arthritis models, is characterized by proliferation of synoviocytes and infiltration of the synovial tissue with macrophages, neutrophils, T cells and B cells. In arthritic synovial fluid the dominant cell type is neutrophil, with more than 90% of all cells belonging to this cell type. Neutrophils have been reported previously to contribute to cartilage degradation in RA by their production of collagenolytic enzymes [8–10].
Experimental arthritis can be induced in rodents either by provoking an inflammatory response, as is performed when using Freund's complete or incomplete adjuvants (adjuvant arthritis and oil-induced arthritis, respectively) or by inducing an autoimmune response to cartilage antigens in conjuction with an inflammatory response (collagen-induced arthritis, gp-39-induced arthritis and COMP-induced arthritis) [6,11,12]. Which mode of induction results in arthritis is dependent on the genetic background of the animal. Hence, one can hypothesize that in certain individuals inflammatory triggering is sufficient to cause arthritis development, whereas in other individuals an autoimmune reaction must develop for arthritis to occur. We therefore considered it interesting to investigate whether chlorination of autoantigens mediated by activated neutrophils, as part of an inflammatory reaction, can induce breakage of self-tolerance and thereby induction of autoimmunity. In a previous study we demonstrated that immunization with chlorinated rat serum albumin (RSA) breaks the immunological tolerance to this systemic autoantigen in rats. Rats immunized with native, unmodified RSA mounted a weak proliferative T cell response to RSA but no detectable antibody response. In contrast, rats immunized with chlorinated rat serum albumin (RSA–Cl) developed a proliferative T cell response as well as a humoral response to RSA–Cl. The humoral response cross-reacted with unmodified RSA, thus indicating that chlorination of an autoantigen by HOCl can induce an autoimmune response [13].
The presence of neutrophils in the arthritic joints of RA patients, together with the report that RA patients have elevated serum levels of MPO, suggests that protein chlorination might occur during RA [14] and could be a link between arthritic inflammatory reactions and the initiation of autoimmune antibody responses. Hence, in order to explore the potential role of chlorination in arthritis we set out to investigate the immune responses to chlorinated CII versus CII and their arthritogenicity. We induced arthritis in the rat strain LEW1.AV1. These rats were selected based on their intermediate sensitivity to arthritis induction. This allowed us to study the increased arthritogenicity caused by the modification of CII, which would not have been possible to investigate if we had chosen a more sensitive rat strain to arthritis, i.e. DA rats.
Our results demonstrate that chlorination of CII increases the immunogenic and arthritogenic properties of this protein and that this increase was mediated in part by a stronger interleukin (IL)-1β and interferon (IFN)-γ induction in lymph nodes upon immunization.
Materials and methods
Rats
LEW.1AV.1 rats were bred and kept at the animal department, Karolinska Institutet, Stockholm, Sweden. They were free from pathogens as determined by a health-monitoring programme by the National Veterinary Institute, Uppsala, Sweden. Animals were kept in a 12-h light/dark cycle, housed in polystyrene cages containing wood shavings with free access to food and water. Female rats, 8–14 weeks old at the start of the experiment, were used throughout the study. All procedures involving the animals were performed according to the guidelines provided by the central board for animal experiments at the Swedish Department of Agriculture. The Ethical Board for Animal Experiments in Stockholm North approved the experiments.
Antigens
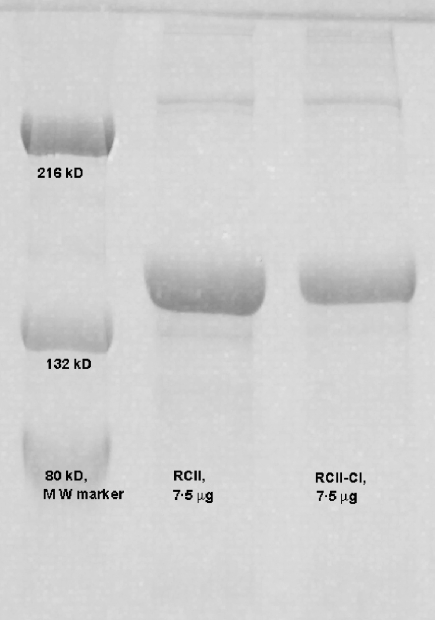
Rat collagen type II (RCII) extracted from a rat chondrosarcoma [15,16] was dissolved in 0·01 m acetic acid, at a concentration of 1·5 mg/ml. Chlorination of RCII was performed through the direct addition of HOCl (cat. no. 23,930-5; Sigma Aldrich, St Louis, MO, USA) 1·5 µmol HOCl/mg protein at room temperature. The concentration of active HOCl in stock solutions of NaOCl was calculated by measuring absorbance at 290 nm and by using the extension coefficient ɛ = 350 nm/M−1 cm−1. The chlorination reaction was terminated after 1 h by the addition of Na2S2O3: (cat. no. S-6672, Sigma Aldrich) in molar ratio 2 : 1 (Na2S2O3: HOCl). RCII used for control experiments was treated in a similar manner, except for the addition of HOCl. Successful chlorination was verified by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis (7·5%) (Fig. 1).
Fig. 1.
Verification of chlorination by denaturing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis. Samples of non-chlorinated CII and chlorinated CII were run on a denaturing SDS-PAGE together with a molecular weight marker. Chlorinated collagen type II (CII) (lane 3) were detected as an increase in high molecular weight bands, compared to non-chlorinated CII (lane 2).
Immunizations
RCII and chlorinated RCII (RCII–Cl), dissolved in 0·01 M HAc at a concentration of 1·5 mg/ml were emulsified with Freund’s incomplete adjuvant (Difco Laboratories, Detroit, MI, USA) at a volume ratio of 1 : 1. Rats were immunized intradermally at the base of the tail with 300 µl emulsion; hence each rat received 225 µg antigen. All immunization procedures were performed under anaesthesia.
Arthritis evaluation
Arthritis development was followed by visual inspection of paws from day 10 post-immunization (p.i.) until the end of the experiment (i.e. day 34 p.i.). Arthritis severity was evaluated using a scoring system presented previously [17]. In brief, each paw is graded individually from 0 to 4 and a cumulative score calculated with a maximum of 16 points for each rat. One point corresponds to one group of swollen joints, two points equals two groups of swollen joints, three points equals three swollen types of joints and four points are given when the entire paw is swollen.
Collection of sera
Blood samples were collected by tail bleeding at two time-points during the study, day 10 p.i. and day 34 p.i. Sera were prepared by centrifugation for 20 min at 500 g in 4°C and aliquots were stored frozen in −18°C until analysed.
Detection of anti-RCII/RCII–Cl IgG by enzyme-linked immunosorbent assay (ELISA)
ELISA plates (Maxisorp plates, Nunc, Roskilde, Denmark) were coated with 100 µl/well of 10 µg/ml RCII or RCII–Cl diluted in phosphate-buffered saline (PBS) at 4°C overnight. Excess protein was removed by repeated washing in PBS/0·05% Tween (cat. no. 8·22184·0500 Merck, Schuchardt, Hohenbrunn, Germany). Sera samples were diluted in PBS/0·05% Tween and the following dilutions were added to the plates in duplicate: 1 : 100, 1 : 500, 1 : 1000 and 1 : 5000. Subsequently the plates were incubated at room temperature for 2 h, washed three times with PBS/0·05% Tween and a secondary antibody, alkaline phosphatase-conjugated affinity pure goat anti-rat IgG (H + 1) (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA) was added to each well. The plates were incubated at room temperature for 2 h and washed as above. A colour reaction was developed by addition of phosphatase substrate (cat. no. 104–105, Sigma, Stockholm, Sweden) and absorbance measured at 405 nm in a microplate reader (Molecular Devices Emax precision). All volumes were 50 µl/well.
Absorbance values of all samples were recalculated with respect to a reference sample added to all plates and background values subtracted. As reference sample, a serum sample from a rat immunized with RCII–Cl obtained at day 17 p.i. was used. The reference sample was from a previous study.
Determination of cytokine mRNA in lymph node cells
Draining lymph nodes from immunized rats were dissected at day 7 p.i. and lymph node cells were washed, counted and kept at − 70°C until RNA preparation. RNA was extracted from 5 × 106 cells using a total RNA extraction kit (Qiagen, Hilden, Germany) and synthesis of cDNA was performed as described previously [18]. The Taqman™ method was used for quantitative polymerase chain reaction (PCR) analysis of cDNA (Perkin Elmer, Norwalk, CT, USA) according to the manufacturer's instructions. Primers and probes for IL-1β and IFN-γ were designed using the software PrimerExpress™ (Perkin Elmer) [19].
Statistical analysis
All data were evaluated using the Mann–Whitney U-test for independent groups. P-values lower than 0·05 were considered statistically significant.
Results
Chlorination of rat collagen II
RCII was chlorinated by HOCl addition and modification was verified by reducing SDS-PAGE electrophoresis (Fig. 1). Chlorination of CII was detected as an increase in high molecular weight bands, which are due probably to chlorination of tyrosine and lysine residues in the collagen molecule. It has been reported previously that chlorination of lysine residues in low density lipoproteins (LDL) resulted in increased aggregation of this protein [20] and has similarly increased aggregation of ovalbumin (OVA), reported as a consequence of chlorination [21]. Thus, our results indicate a similar chlorination of RCII as reported previously for LDL and OVA.
Arthritis development
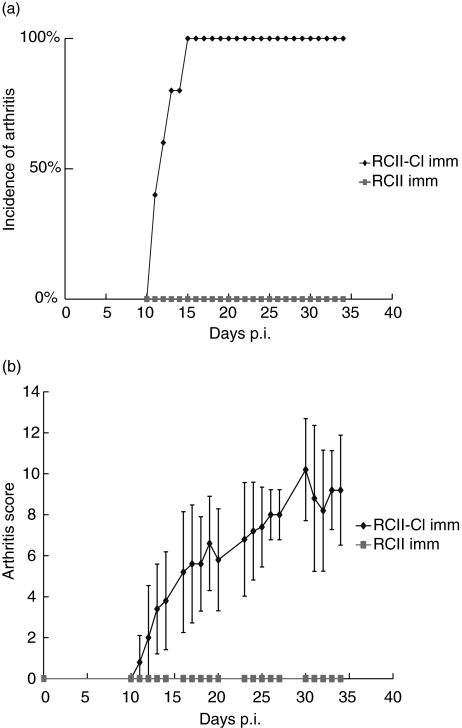
LEW1.AV.1 rats were immunized with RCII or with RCII–Cl. In order to facilitate detection of increased arthritogenicity of CII–Cl, a suboptimal dose of antigen was utilized so that immunization with unmodified CII did not result in arthritis development. The same suboptimal dose of chlorinated CII resulted in arthritis development with an incidence reaching 100% by day 15 p.i., thus indicating that chlorination increased the arthritogenicity of CII. By day 15 p.i. an average score of 5·2 was reached, which subsequently increased to a maximum of 9·2 by day 34 p.i. (i.e. the end of the experiment). Standard deviations in arthritic animals varied between 1·3 (day 11 p.i.) and 3·6 (day 31 p.i.). (Fig. 2a,b).
Fig. 2.
Incidence and severity of arthritis induced by collagen type II (CII)–Cl. (a) In rats immunized with CII–Cl (n = 5), clinical arthritis started to develop by day 11 post-immunization (p.i.) and had reached a 100% incidence by day 15 p.i. In contrast, rats immunized with non-chlorinated CII (n = 5) did not develop arthritis during the time period of the experiment. (b) Arthritis severity in rats immunized with CII–Cl had reached an average of 5·2 by day 15 p.i. and continued to rise until the end of the experiment when the average score was 9·2. P-values lower than 0·05 were obtained from day 11 p.i. to the end of the experiment by Mann–Whitney nonparametric test. The shown experiment is one of two experiments performed both giving similar results.
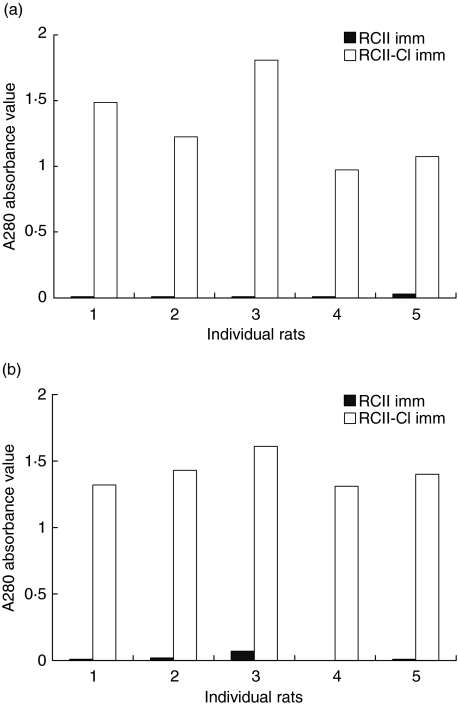
Antibodies induced by RCII–Cl immunization cross-reacts with RCII
Rats immunized with RCII–Cl developed high titres of IgG antibodies directed against RCII–Cl. The antibody response was detectable at day 10 p.i. (data not shown), as well as day 34 p.i. We could detect antibodies to the chlorinated form of CII, cross-reacting with the non-chlorinated form, at both of these time-points in animals immunized with chlorinated CII. Interestingly, two of five animals that were immunized with RCII also developed antibodies that cross-reacted with the chlorinated form of RCII; however, we did not detect similar cross-reactivity at day 34 p.i., when the B cell selection had proceeded further (Fig. 3).
Fig. 3.
Antibody responses in individual rats immunized with rat collagen type II (RCII) and RCII–Cl. (a) Total IgG antibody response to RCII in rats immunized with RCII and RCII–Cl at 34 days post-immunization (p.i.). Rats immunized with RCII–Cl had a stronger response to RCII compared to rats immunized with RCII. (b) Total IgG antibody response to RCII–Cl in rats immunized with RCII and RCII–Cl, at day 34 p.i. No differences in IgG titres were observed in response to the two antigens in each individual animal.
Induction of IL-1β and IFN-γ in immunized animals
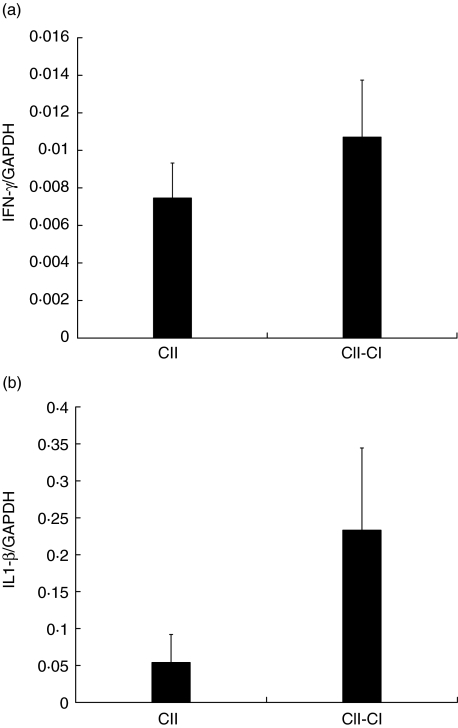
Real-time PCR was conducted to analyse the cytokine response in the two experimental groups. mRNA levels of type 1 cytokine IFN-γ and proinflammatory cytokine IL-1β were investigated at day 7 p.i., an optimal time-point for this type of analysis based on previous experiments. mRNA levels of IFN-γ and IL-1β were found to be elevated in the arthritic CII–Cl-immunized animals compared to the non-arthritic CII–immunized animals. The major difference in cytokine ratios was detected with IL-1β, which was increased almost fourfold in CII–Cl-immunized rats compared to CII-immunized rats. (Fig. 4a,b)
Fig. 4.
Analysis of interferon (IFN)-γ and interkeukin (IL)-1β mRNA expression in draining lymph nodes 7 days post-immunization (p.i.). Levels of mRNA are expressed as ratios of GADPH mRNA levels. (a) IFN-γ mRNA was expressed to significantly higher levels in collagen type II (CII)–Cl-immunized rats compared to CII-immunized rats. P-value 0·047 calculated by Mann–Whitney test for nonparametric samples. (b) IL-1β mRNA levels were fourfold higher in rats immunized with CII–Cl compared with rats immunized with CII. A P- value of 0·009 was obtained as calculated by Mann–Whitney test for nonparametric samples. The analysis was performed on five rats in each group and repeated twice.
Discussion
In a previous study have we been able to demonstrate that chlorination of an autoantigen (RSA) can break immunological tolerance to the autoantigen in question. We then formulated a hypothesis that chlorination, as an inflammation-mediated event, might be a mechanism by which autoimmunity can be induced and thereby add to the chronicity of an inflammatory reaction. With this study, we thus set out to test our hypothesis further in an experimental model, where we could study the effects of chlorination of a disease-inducing antigen on specific immunity. As our working model, we chose collagen-induced arthritis, which is induced in rats by immunization with CII. We were able to demonstrate an increased arthritogenicity of CII which had been chlorinated in vitro by means of incubation with hypochlorous acid. When utilized for immunization of rats CII–Cl induced arthritis, whereas non-chlorinated CII did not. Furthermore, the CII–Cl-immunized rats developed antibodies recognizing both the chlorinated and non-chlorinated form of CII, i.e. a true autoimmune response had been provoked. Analysis of the arthritis-associated cytokines IL-1β and IFN-γ as mRNA expression in lymph nodes after immunization revealed up-regulated expression in CII–Cl-immunized rats compared to CII-immunized rats.
Neutrophils, as the major producers of hypochlorous acid, are the dominant cell type in the synovial fluid of RA patients. Interestingly, neutrophils in the synovial fluid (SF) of RA patients have lower levels of MPO compared to blood-derived neutrophils from the same patients. This indicates that neutrophils in SF have secreted their MPO excellulary in the arthritic joint [14], which might lead to the production of hypochlorous acid extracellulary and thereby chlorination of joint antigens. Destruction of CII by hypochlorite and by collagenases leading to cartilage breakdown has been suggested as a principal mechanism for joint destruction in RA [22].
Our results demonstrate clearly that chlorination of collagen type II induced increased arthritogenicity. This might be caused by at least two different mechanisms: first, by an increased immunogenicity of CII–Cl, as mirrored by the stronger antibody-inducing capacity. At day 10 p.i., four of five rats immunized with CII–Cl had developed antibodies against CII–Cl whereas only two of five rats immunized with CII had detectable antibody levels. This could be caused by an increased presentation of CII by antigen-presenting cells due to chlorination. It has been demonstrated previously that chlorinated antigens can bypass the need of proteolysis and can be presented by MHC II without previous endocytic processing of the antigen [23]. By this mechanism they can be recognized directly by antigen-specific T cells without intracellular processing. Chlorination might also create T cell neoepitopes not subjected to tolerance control, thus inducing a T cell response which can provide help to autoreactive B cells.
Secondly, our analysis of IFN-γ and IL-1β mRNA expression in LN after immunization with CII or CII–Cl demonstrate a higher production of mRNA for these cytokines in the CII–Cl immunized rats. As we focused on dissecting the disease-inducing mechanisms, we opted to study the cytokine mRNA expression in lymph nodes only 7 days p.i. and chose not to study cytokine expression in paws of rats with established disease. Thus, it is possible that chlorinated proteins are experienced as danger signals by the innate immune system and thereby give rise to a stronger inflammatory response that will affect the development of CII-specific arthritogenic T and B cells.
In a recent study by Marcinkiewicz et al., in which they studied the effects of CII-chlorination in murine collagen-induced arthritis, results directly opposite to ours were presented. In their study, chlorinated CII was less immunogenic and arthritogenic than non-chlorinated CII. In addition, both forms of CII could be used to vaccinate animals against subsequent arthritis induction, which was interpreted as modification of arthritogenic B and T cell epitopes, while the regulatory B and T cell epitopes remained intact. A possible explanation for these different results could be the extent to which CII was chlorinated. We have chlorinated CII by incubating the protein with 2·2 mM HOCl, whereas Marcinkiewicz et al. [24] used 3 mM. It has been demonstrated previously that chlorination of CII leads ultimately to fragmentation of the protein, with the formation of low molecular fragments correlating to the concentration of HOCl used. Different degrees of chlorination also affect the secondary structure of the CII molecule to different degrees. The latter effect might explain the intact tolerogenic capacity of CII–Cl despite the loss of arthritogenicity. It has long been known that heat-denatured CII is not arthritogenic, while it can still have tolerogenic properties [25]. We calibrated our model so that native collagen type II did not induce arthritis, thus facilitating the detection of increased arthritogenic effects of chlorination. This was not performed in the mouse model of arthritis used by Marcinkiewicz.
However, in areas where neutrophils are attached to the cartilage surface, so-called frustrated phagocytosis [26], it is possible that the HOCl concentrations are much higher, reaching the concentration we utilized in vitro.
In conclusion, this study demonstrates that chlorination, a modification occurring during inflammation, is of importance for both immunogenicity and arthritogenicity of collagen II. As a subgroup of RA patients have T and B cell responses to CII and as CII reactivity can either induce or worsen experimental arthritis, we suggest that chlorination of CII might be a mechanism contributing to the development of chronic joint inflammation. In future studies, it would be of interest both investigate the presence of B and T cell reactivity to CII–Cl in RA patients as well as the presence of CII–Cl in joints. Furthermore, evaluation of whether blocking of chlorination in experimental models could ameliorate arthritis would also be of interest.
Acknowledgments
This work was supported by grants to H. Erlandsson Harris by the Swedish Science Council, the Swedish Association against Rheumatism, the Af Ugglas foundation, the Börje Dahlin Foundation and the Åke Wiberg Foundation.
References
- 1.Shepherd VL, Hoidal JR. Clearance of neutrophil-derived myeloperoxidase by the macrophage mannose receptor. Am J Respir Cell Mol Biol. 1990;2:335–40. doi: 10.1165/ajrcmb/2.4.335. [DOI] [PubMed] [Google Scholar]
- 2.Marcinkiewicz J, Chain BM, Olszowska E, Olszowski S, Zgliszczynski JM. Enhancement of immunogenic properties of ovalbumin as a result of its chlorination. Int J Biochem. 1991;23:1393–5. doi: 10.1016/0020-711x(91)90280-z. [DOI] [PubMed] [Google Scholar]
- 3.Schellekens GA, Visser H, de Jong BA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Clague RB, Morgan K, Reynolds I, Williams HJ. The prevalence of serum IgG antibodies to type II collagen in American patients with rheumatoid arthritis. Br J Rheumatol. 1994;33:336–8. doi: 10.1093/rheumatology/33.4.336. [DOI] [PubMed] [Google Scholar]
- 5.Morgan K, Clague RB, Collins I, Ayad S, Phinn SD, Holt PJ. Incidence of antibodies to native and denatured cartilage collagens (types II, IX, and XI) and to type I collagen in rheumatoid arthritis. Ann Rheum Dis. 1987;46:902–7. doi: 10.1136/ard.46.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verheijden GF, Rijnders AW, Bos E, et al. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997;40:1115–25. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]
- 7.Blass S, Union A, Raymackers J, et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 2001;44:761–71. doi: 10.1002/1529-0131(200104)44:4<761::AID-ANR132>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Gysen P, Malaise M, Gaspar S, Franchimont P. Measurement of proteoglycans, elastase, collagenase and protein in synovial fluid in inflammatory and degenerative arthropathies. Clin Rheumatol. 1985;4:39–50. doi: 10.1007/BF02032316. [DOI] [PubMed] [Google Scholar]
- 9.Hasty KA, Jeffery JJ, Hibbs MS, Welgus HG. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987;262:10048–52. [PubMed] [Google Scholar]
- 10.Fosang AJ, Last K, Maciewicz RA. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J Clin Invest. 1996;98:2292–9. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–68. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlsen S, Hansson AS, Olsson H, Heinegard D, Holmdahl R. Cartilage oligomeric matrix protein (COMP)-induced arthritis in rats. Clin Exp Immunol. 1998;114:477–84. doi: 10.1046/j.1365-2249.1998.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westman E, Harris HE. Alteration of an autoantigen by chlorination, a process occurring during inflammation, can overcome adaptive immune tolerance. Scand J Immunol. 2004;59:458–63. doi: 10.1111/j.0300-9475.2004.01428.x. [DOI] [PubMed] [Google Scholar]
- 14.Torsteinsdottir I, Hakansson L, Hallgren R, Gudbjornsson B, Arvidson NG, Venge P. Serum lysozyme: a potential marker of monocyte/macrophage activity in rheumatoid arthritis. Rheumatology (Oxf) 1999;38:1249–54. doi: 10.1093/rheumatology/38.12.1249. [DOI] [PubMed] [Google Scholar]
- 15.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase–hydrogen peroxide–chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–32. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith BD, Martin GR, Miller EJ, Dorfman A, Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975;166:181–6. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- 17.Åkerlund K, Harris HE, Tracey KJ, et al. Anti-inflammatory effects of a new tumour necrosis factor-alpha (TNF-alpha) inhibitor (CNI-1493) in collagen-induced arthritis (CIA) in rats. Clin Exp Immunol. 1999;115:32–41. doi: 10.1046/j.1365-2249.1999.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattsson L, Lorentzen JC, Svelander L, et al. Immunization with alum-collagen II complex suppresses the development of collagen-induced arthritis in rats by deviating the immune response. Scand J Immunol. 1997;46:619–24. doi: 10.1046/j.1365-3083.1997.d01-163.x. [DOI] [PubMed] [Google Scholar]
- 19.Mattsson L, Lundberg K, Mussener E, Jansson A, Harris HE, Larsson P, et al. Antigen inhibition of collagen-induced arthritis is associated with up-regulation of IL-4 mRNA and induction of Ox40 on T cells in draining lymph nodes. Clin Exp Immunol. 2003;131:241–7. doi: 10.1046/j.1365-2249.2003.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazell LJ, van den Berg JJ, Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem J. 1994;302:297–304. doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olszowski S, Olszowska E, Stelmaszynska T, Krawczyk A, Marcinkiewicz J, Baczek N. Oxidative modification of ovalbumin. Acta Biochim Pol. 1996;43:661–72. [PubMed] [Google Scholar]
- 22.Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–4. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco-Marin E, Paz-Miguel JE, Lopez-Mato P, Alverez-Dominguez C, Leyva-Cobain F. Oxidation of defined antigens allows protein unfolding and increases both proteolytic processing and exposes peptide epitopes which are recognized by specific T cells. Immunology. 1998;95:314–21. doi: 10.1046/j.1365-2567.1998.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcinkiewicz J, Biedron R, Maresz K, et al. Oxidative modification of type II collagen differentially affects its arthritogenic and tolerogenic capacity in experimental arthritis. Arch Immunol Ther Exp (Warsz) 2004;52:284–91. [PubMed] [Google Scholar]
- 25.Hart BA, Bakker NP, Jonker M, Bontorp RE. Resistance to collagen-induced arthritis in rats and rhesus monkeys after immunization with attenuated type II collagen. Eur J Immunol. 1993;23:1588–94. doi: 10.1002/eji.1830230729. [DOI] [PubMed] [Google Scholar]
- 26.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]