Abstract
Infection with the cestode Echinococcus multilocularis causes human alveolar echinococcosis (AE), a life-threatening disease affecting primarily the liver. Despite the severity of AE, clinical symptoms often develop only many years after infection, which suggests that E. multilocularis has developed mechanisms which depress anti-parasite immune response, thus favouring immune evasion. In this study we examined the production of cytokines, chemokines and the expression of CD molecules on peripheral blood mononuclear cells (PBMC) from AE patients and healthy controls in response to E. multilocularis metacestode culture supernatant, viable E. multilocularis vesicles and E. multilocularis vesicle fluid antigen in vitro. After 48 h of co-culture, E. multilocularis metacestode culture supernatant and E. multilocularis vesicles depressed the release of the proinflammatory cytokine interleukin (IL)-12 by PBMC. This effect was dose-dependent and a suppression of tumour necrosis factor (TNF)-α and IL-12 was observed even when PBMC were activated with lipopolysaccharide (LPS). Comparing proinflammatory cytokine release by AE patients and controls showed that the release of IL-12 and TNF-α was reduced in AE patients, which was accompanied by an increased number of CD4+ CD25+ cells and a reduced release of the Th2 type chemokine CCL17 (thymus and activation regulated chemokine, TARC), suggesting an anti-inflammatory response to E. multilocularis metacestode in AE patients. Instead the production of interferon (IFN)-γ and the expression of CD28 on CD4+ T cells were increased in PBMC from AE patients when compared to controls. This was accompanied by a higher release of the Th2-type chemokine CCL22 (macrophage derived chemokine, MDC) supporting that E. multilocularis also generates proinflammatory immune responses. These results indicate that E. multilocularis antigens modulated both regulatory and inflammatory Th1 and Th2 cytokines and chemokines. Such a mixed profile might be required for limiting parasite growth but also for reducing periparasitic tissue and organ damage in the host.
Keywords: alveolar echinococcosis, cytokine and chemokine responses, Echinococcus multilocularis
Introduction
In infected humans, proliferating metacestodes of Echinococcus multilocularis progressively infiltrate infested tissues and organs, mainly liver, and cause alveolar echinococcosis (AE). Infection is often undetected for many years of parasite persistence, and often found only incidentally by imaging diagnostic techniques [1]. Epidemiological and clinical data, e.g. the high prevalence of E. multilocularis in foxes in endemic areas but the very low incidence of AE in the human population, suggest that exposure to E. multilocularis does not progress to clinical disease in all cases because many subjects present abortive and spontaneously healed lesions after infection [2–5]. The progressive parasite growth in human host tissues appears not to cause fulminant and exacerbated inflammation and immediate organ damage, but often a ‘latent’, non-apparent disease. This supports that E. multilocularis metacestodes have developed mechanisms which depress anti-parasite responses favouring immune evasion and, moreover, metacestodes may restrict or modulate inflammatory responses which could cause tissue damage and pathology to the human host. Cellular effector mechanisms are considered to be the key defence against metacestode growth and dissemination [6]. Peripheral blood mononuclear cells (PBMC) from AE patients generate substantial amounts of Th1 and Th2 cytokines and chemokines when activated with E. multilocularis metacestode antigens or viable E. multilocularis vesicles. While interleukin (IL)-5 was found to be the predominant cytokine produced by activated PBMC in AE patients [7], the levels of tumour necrosis factor (TNF)-α, IL-10, IL-12, IL-13 and sIL-2R in sera correlated with the actual state of clinical disease [8–11]. Therefore, regulatory and inflammatory immune mediators, notably cytokines and chemokines, may contribute to tissue and organ damage and disease progression in AE patients [12–14]. However, metacestodes and their secreted products will also depress proinflammatory cytokine and proliferative responses over time [12,15]. In the present study we analysed the anti-inflammatory properties of E. multilocularis metacestodes on the cellular production of proinflammatory cytokines and Th2-type chemokines in AE patients and healthy controls. Furthermore, the potential of E. multilocularis metacestodes to promote CD4+ CD25+ differentiation and the capacity of secretory products of E. multilocularis to modulate inflammatory cytokine generation, also after lipopolysaccharide (LPS)-induced activation, were examined. We found that proliferating E. multilocularis selectively stimulated Th2-type chemokine release, depressed proinflammatory cytokines, also in the presence of LPS, and viable vesicles of E. multilocularis promoted CD4+ CD25+ T cell differentiation in AE patients. While such parasite–host interplay may only limit metacestode growth and dissemination to some extent, it may favour parasite growth without generating inflammation and immediate organ damage to the host.
Materials and methods
Study participants
The study population consisted of a total of 28 AE patients admitted to the University Hospitals of Ulm and 55 healthy controls received from the University Hospitals of Tübingen. The AE patients and infection-free controls came from south-western Germany (Baden-Württemberg and Bayern). The Echinococcosis Centre and University Clinics of Ulm and the Clinics of Tübingen are situated in an endemic area for E. multilocularis, both towns being 70 km apart. The mean age of AE patients was 57·1 years, ranging from 18 to 79 years. Seventeen AE patients were female, 11 male. In the AE patient group were 10 cured patients, 12 patients had stable infections and six patients had progressive AE. The control group for fluorescein activated cell sorter (FACS) analysis and cytokine/chemokine determination consisted of 34 healthy controls (16 female, 18 male) with an mean age of 40·4 years (23–63 years). Diagnosis of AE was achieved by positive imaging, serology and histology, and most patients were re-examined regularly during follow-up. All patients gave written consent to participate in this study and approval for the investigation was obtained from the ethical board of the University Clinics of Ulm (Ethikkommission Antrag no. 71/2004). All patients except five were treated with benzimidazole (albendazole or mebendazole).
Isolation of peripheral blood mononuclear cells (PBMC)
Whole blood samples of donor blood from AE patients as well as controls were processed at the blood transfusion centre at University Clinics of Ulm. PBMC from AE patients and healthy endemic controls were isolated by Ficoll-Paque (Pharmacia, Freiburg, Germany) density gradient centrifugation of heparinized venous peripheral blood. Cell culture experiments were conducted as described previously [12]. Briefly, PBMC were adjusted to 1 × 107/ml in complete medium: RPMI-1640 containing 25 mM HEPES, 2 mM l-glutamine (Gibco, Eching, Germany), 10% heat-inactivated fetal calf serum (FCS) (Biochrom KG, Berlin, Germany), 100 U/ml penicillin, 100 µg/ml streptomycin and 0·25 µg/ml amphotericin B (Sigma, St Louis, MO, USA). PBMC (1 × 107) were plated in duplicate in 48-well flat-bottomed tissue culture plates (Costar 3548, New York, USA) and co-cultured with either 60 µg/ml EmVF (E. multilocularis vesicle fluid antigen), 5 µg/ml Escherichia coli LPS (026:B6; Sigma), 5 µg/ml phytohaemagglutinin (PHA) (Sigma), EmMed (E. multilocularis metacestode culture supernatant) or 5–10 EmVes (viable E. multilocularis metacestode vesicles), both constituting 20% of the final cell culture volume and incubated for 24–96 h at 37°C, saturated humidity and 5% CO2. Cells from AE patients were cultured in corresponding concentrations. To analyse the effect of EmMed and EmVes on LPS-induced cytokine release by PBMC, cells were adjusted to 2 × 106/ml in complete medium (as above) and preincubated for 2 h in the presence of EmMed and EmVes before stimulation with 100 ng/ml E. coli LPS (026:B6; Sigma).
In vitro culture of E. multilocularis metacestodes
In vitro culture of E. multilocularis metacestodes was carried out as described previously [10,14]. Briefly, metacestode tissues were removed aseptically from the peritoneal cavity of infected jirds (Meriones unguiculatus) and cut into tissue blocks. These were cultivated in complete medium (as above) at 37°C, saturated humidity and 5% CO2. Several weeks later metacestodes started to proliferate and produce daughter vesicles. Twice a week the culture medium was renewed and E. multilocularis metacestode culture supernatant (EmMed) and vesicles (EmVes) were collected and used immediately for in vitro stimulation of PBMC or stored below − 20 ° C for further use.
Preparation of E. multilocularis vesicle fluid antigen (EmVF)
Cryopreserved E. multilocularis vesicles were homogenized and sonicated (30% intensity, pulse 1 s for 8 min) on ice. The vesicle homogenate was centrifuged at 5000 g for 30 min at 4°C. The supernatant was sterile-filtrated (0·22 µm). The protein concentration was determined by the bicinchoninic acid (BCA) protein determination kit (Pierce, Rockford, IL, USA).
Determination of cytokine and chemokine production by PBMC
Cell-free culture supernatants were collected after 24, 48, 72 and 96 h and stored below − 20 ° C until further use. Cytokine and chemokine secretion by stimulated PBMC was quantified by sandwich enzyme-linked immunosorbent assay (ELISA) using monoclonal and polyclonal antibodies for TNF-α, IL-12 + p40, IFN-γ (Biosource, Ratingen, Germany), macrophage-derived chemokine (MDC) and thymus and activation regulated chemokine (TARC) (R&D Systems, Minneapolis, MN, USA) as recommended by the manufacturers and as described previously [12].
FACS analysis
Freshly isolated PBMC were cultured at 37°C, with saturated humidity and 5% CO2 in a concentration of 1 × 107 cells/ml in complete medium (as above) in the presence of either 60 µg/ml EmVF, 5 µg/ml E. coli LPS (026:B6) or EmVes constituting 20% of the final cell culture volume. After 24 h vesicles were carefully removed and the remaining supernatant and cells were collected and centrifuged at 400 g, 4°C for 5 min. Cells were stained for 30 min at a concentration of 4 × 105 cells/well in 96-well flat-bottomed plates (Costar, NY, USA). Staining was conducted with phycoerythrin (PE)-conjugated mouse IgG anti-human CD4 (BD PharMingen, San Diego, USA), PE-conjugated mouse IgG anti-human CD8 (BD PharMingen, San Diego, USA), fluorescein isothiocyanate (FITC)-conjugated mouse IgG anti-human CD25 (BD PharMingen, San Diego, CA, USA) and FITC-conjugated mouse IgG anti-human CD28 (BD PharMingen). All staining was compared against the relevant isotype controls (IgG1 for FITC and IgG2α for PE). Following two washing steps with FACSflow (400 g, 4°C, 5 min) cells were resuspended with phosphate-buffered saline (PBS) containing 0·2% formaldehyde and stored overnight at 4°C. For FACS analysis a FACSCalibur running CellQuest version 3·3 was used.
Statistical analysis
To analyse differences in concentrations of cytokines and chemokines between AE patients and controls, mean values over time (24, 48, 72 and 96 h) were determined. Significant differences between groups were determined after logarithmic transformation to stabilize the variance of data [log (pg/ml + 0·5)] for t-test. The level of significance was adjusted according to Bonferroni–Holm (25 tests; α= 0·002).
To illustrate the influence of EmMed and EmVes on spontaneous or LPS-induced cytokine release, cytokine levels were expressed as mean in pg/ml or ng/ml ± s.e.m. Dunnett’s test was used for statistical analysis, and the variance of data was stabilized by logarithmic transformation [log (pg/ml + 0·5)]. The level of significance was adjusted according to Bonferroni–Holm (16 tests; α= 0·0031).
For FACS analysis mean values of gated cells are given as percentage ± s.e.m. and differences between AE patients and controls were evaluated by t-test. For this analysis data were stabilized by converting percentage values to arcsin values [arcsin (square root of values in percentage/100)].
Results
Cytokine and chemokine production by AE patients and controls
PBMC from healthy controls and AE patients were stimulated in vitro with E. multilocularis antigen EmMed, EmVes, EmVF or the mitogen PHA and cellular production of the Th1 cytokines IL-12, TNF-α and IFN-γ, as well as the Th2 chemokines MDC and TARC (Table 1), were quantified by ELISA. Results from AE patients with cured, stable or progressive disease were grouped, as they showed no significant differences in cytokine and chemokine release after stimulation with E. multilocularis antigens or PHA/E. coli LPS, except for TNF-α in the presence of PHA. Furthermore, in those AE patients from our study who were receiving benzimidazole treatment, cytokine and chemokine production did not differ from those AE patients not being treated.
Table 1.
In vitro production of Th1 cytokines interleukin (IL)-12, tumour necrosis factor (TNF)-α and interferon (IFN)-γ as well as Th2 chemokines macrophage derived chemokine (MDC) and thymus and activation regulated chemokine (TARC) by peripheral blood mononuclear cells (PBMC) from alveolar echinococcosis patients (EP) and infection-free controls (BS) is shown in pg/ml as means and ranges. Spontaneous (medium) cytokine/chemokine production or in response to Echinococcus multilocularis metacestode culture supernatant (EmMed), vesicles (EmVes), vesicle fluid antigen (EmVF), photohaemagglutinin (PHA) and Escherichia coli lipopolysaccharide (LPS) (used only for TARC), respectively, are shown. The number of AE patients tested for cytokine and chemokine production as well as their number according to their state of infection (cured/stable/progressive) is indicated for each experiment. Concentrations of cytokines/chemokines released into the cell culture supernatant were quantified after 24, 48, 72 and 96 h by enzyme-linked immunosorbent assay (ELISA) and means over time are given. Concentration of MDC was analysed after 96 h. Differences in cytokine/chemokine production between alveolar echinococcosis patients and controls were evaluated as log values [log (pg/ml + 0·5)] by t-test and the level of significance adjusted by Bonferroni–Holm (* P < 0·05, Bonferroni-Holm: 25 tests, ** α = 0·002).
| EP (cured/stable/progressive) | Medium | EmMed | EmVes | EmVF | PHA/E. coli LPS | |
|---|---|---|---|---|---|---|
| IL-12 | BS(n = 9) | 376(0–2974) | 385(0–3013)* | 510(0–3915)** | 382 (n = 8)(0–2394)* | 365 (n = 8)(22–2179) |
| AE (n = 12) (6/3/3) | 28(0–133) | 14(0–87) | 10(0–49) | 24(0–123) | 105(0–368) | |
| TNF-α | BS(n = 22) | 2958(56–23039)* | 3534(94–16871)* | 6396 (n = 21)(215–24129)** | 2222(117–14370)* | 16453 (n = 6)(428–37182) |
| AE (n = 23) (8/10/5) | 648(0–3110) | 564(0–3189) | 1425(0–10013) | 1182(0–8563) | 6987 (n = 12)(875–25096) | |
| IFN-γ | BS(n = 19) | 27(0–101) | 29(0–102)* | 46(0–169) | 107(0–881)* | 3170 (n = 4)(250–10451) |
| AE (n = 17) (6/7/4) | 132(0–467) | 259(0–1620) | 252(0–1487) | 542(0–2073) | 380 (n = 6)(121–658) | |
| MDC | BS(n = 8) | 2011(207–6322)* | 2295(48–8897)** | 279(0–1572)** | 4089(0–11855)* | 3700(0–9247)* |
| AE (n = 12) (6/3/3) | 4390(1552–6622) | 6700(2717–11441) | 3542(0–11272) | 8255(1855–15713) | 8494(4841–11847) | |
| TARC | BS(n = 14) | 396(35–3509)** | 159(32–425)* | 34(12–64) | 316(30–1187) | 74(16–198) |
| AE (n = 11) (3/6/2) | 32(0–87) | 71(4–172) | 34(0–100) | 123(4–549) | 119(0–356) |
IL-12
Most AE patients showed a diminished spontaneous release of IL-12 when compared with healthy controls. Addition of antigens from E. multilocularis enhanced this effect, i.e. significantly reduced IL-12 production by AE patients compared to healthy controls in presence of EmVes (EmVes P = 0·001; EmMed P = 0·01; EmVF P = 0·02). Activation of PBMC with the mitogen PHA resulted in a similar IL-12 production by PBMC from AE patients and controls (P = 0·10).
TNF-α
Stimulation with EmVes and PHA increased in AE patients and controls TNF-α production, while EmMed and EmVF had no effect on TNF-α release. Production of the proinflammatory cytokine was constantly lower in AE patients compared to controls, i.e. cells from AE patients spontaneously released less TNF-α (P = 0·003) as well as after antigen stimulation with EmMed (P = 0·003), EmVes (P = 0·0005) and EmVF (P = 0·04). AE patients with acute infections (n = 3) produced higher amounts of TNF-α after PHA stimulation (16123 pg/ml, range 8124–25096 pg/ml) compared to AE patients with abortive infection (3942 pg/ml, range 875–6510 pg/ml; n = 6; P = 0·03).
IFN-γ
Spontaneous IFN-γ secretion did not differ significantly between AE patients and controls, but PBMC from AE patients tended to have a higher secretion of IFN-γ than controls. Stimulation with EmMed (P = 0·01) and EmVF (P = 0·01) enforced this effect clearly; however, PHA augmented production of IFN-γ by PBMC from controls, while stimulation with EmVF and PHA increased IFN-γ in AE patients.
MDC
Macrophage derived chemokine (MDC, CCL22) was released in higher amounts by PBMC from AE patients than by those from controls. This was observed in unstimulated cell cultures (P = 0·006) and also following activation with EmMed (P < 0·002), EmVes (P < 0·002), EmVF (P = 0·02) or PHA (P < 0·02). Interestingly, PBMC from AE patients released more MDC in the presence of EmMed, EmVF or the mitogen PHA compared to their spontaneous production. In contrast, these antigens did not trigger PBMC from healthy controls to produce MDC, while EmVes even depressed MDC release when compared to unstimulated control cultures.
TARC
The spontaneous release of TARC (CCL17) by PBMC was reduced significantly in AE patients when compared to controls (P = 0·0001). This depression was preserved after stimulation with EmVes (P = 0·009), and also the combination with EmVF or E. coli LPS did not change TARC production. Although the TARC production was reduced in AE patients compared to controls, EmMed and EmVF stimulated TARC release by PBMC in AE patients, whereas EmVes reduced TARC release by PBMC from controls.
FACS analysis of CD4+ CD25+ and CD28+ T cells
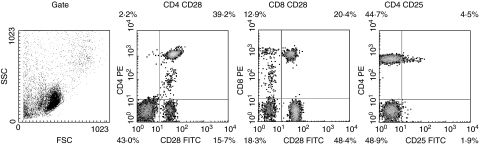
FACS analysis was performed using PBMC stimulated with EmVes, EmVF or E. coli LPS. The unspecific binding of isotype controls (IgG1 and IgG2α) was always less than 1% (data not shown). The spontaneous expression of CD4+ CD25+ was elevated in AE patients’ PBMC compared to controls, and the contingent of CD4+ CD25+ cells increased three- to fivefold in AE patients’ PBMC after stimulation with EmVes or E. coli LPS compared to healthy controls (Table 2). Neither activation with E. multilocularis antigens nor E. coli LPS could change the expression of CD4+ CD25+ cells in both groups (Fig. 1).
Table 2.
Fluorescein activated cell sorter (FACS) analysis of peripheral blood mononuclear cells (PBMC) isolated from alveolar echinococcosis patients (AE) and healthy controls (BS) after 24 h of in vitro cultivation with viable Echinococcus multilocularis vesicles (EmVes), vesicle fluid antigen (EmVF), Escherichia coli lipopolysaccharide (LPS) or without stimulation (medium). Mean values of gated cells as percentage ± s.e.m. from n= 7 AE patients or n= 8 BS are shown. Cell surface CD marker expression was compared between patients and controls by t-test and significant differences between groups (* < 0·05) are shown. For t-test analysis the data were stabilized by converting percentage values to arcsin values [arcsin(square root of values in percentage/100)].
| Medium | EmVes | EmVF | E. coli LPS | |||||
|---|---|---|---|---|---|---|---|---|
| BS | AE | BS | AE | BS | AE | BS | AE | |
| CD4+ CD28+ | 27·2 ± 6·5 | 46·5 ± 5·5 | 34·4 ± 5·5 | 53·9 ± 4·7 * | 32·4 ± 6·0 | 53·2 ± 4·5 * | 27·6 ± 6·8 | 46·2 ± 9·9 |
| CD8+ CD28+ | 14·6 ± 3·3 | 15·4 ± 3·2 | 14·6 ± 3·2 | 16·5 ± 3·1 | 15·7 ± 4·4 | 15·4 ± 3·0 | 10·5 ± 3·6 | 16·7 ± 3·3 |
| CD4+ CD25+ | 3·7 ± 1·4 | 7·7 ± 1·8 | 3·5 ± 1·4 | 26·1 ± 9·3 * | 6·0 ± 2·9 | 10·0 ± 2·5 | 4·9 ± 1·6 | 29·2 ± 8·1 * |
Fig. 1.
Representative individual fluorescein activated cell sorter (FACS) analysis of peripheral blood mononuclear cells (PBMC) isolated from an alveolar echinococcosis patient after 24 h of in vitro cultivation. The plots show side scatter/forward scatter (FSC/SSC) gated lymphocytes as well as CD4+ CD28+, CD8+ CD28+ and CD4+ CD25+ cells.
Stimulation of PBMC with E. multilocularis antigens (EmMed and EmVF) increased activated CD4+ CD28+ cells in AE patients’ PBMC and in controls, while E. coli LPS did not (Table 2). AE patients’ PBMC expressed spontaneously markedly more CD4+ CD28+ than control PBMC, as well as after antigen (P < 0·05) or mitogen stimulation. In contrast, the number of CD8+ CD28+ T cells was similar in both groups and antigen or mitogen stimulations showed no effect on CD8+ CD28+ expression.
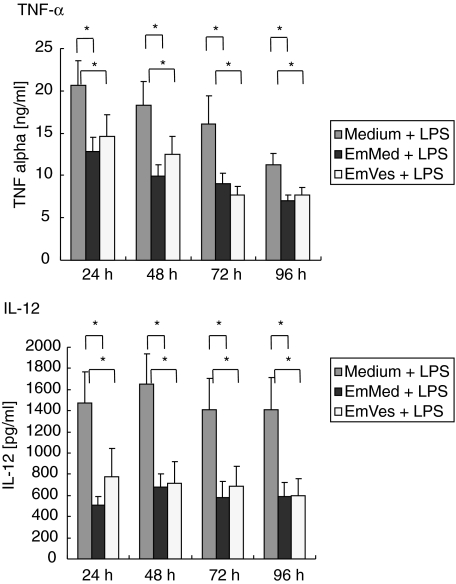
Reduction of LPS-induced IL-12 and TNF-α by EmMed and EmVes
The kinetics of IL-12 and TNF-α production by PBMC after LPS stimulation was determined in the presence of EmMed or EmVes. EmMed and EmVes were able to reduce the LPS-induced cytokine release of IL-12 and TNF-α when compared to cell cultures stimulated only with LPS (Fig. 2). This reduction was observable after 24 h and lasted until 96 h of incubation.
Fig. 2.
Kinetics of tumour necrosis factor (TNF)-α (ng/ml + s.e.m.) and interleukin (IL)-12 (pg/ml + s.e.m.) production in vitro in the presence of Escherichia coli lipopolysaccharide (LPS) by peripheral blood mononuclear cells from healthy donors is shown. Cells were preactivated with 100 ng/ml E. coli LPS and spontaneous cytokine production (medium + LPS, n= 15 and n= 13) or in response to Echinococcus multilocularis metacestode culture supernatant (EmMed + LPS, n= 11) as well as viable vesicles (EmVes + LPS, n= 8) were quantified by enzyme-linked immunosorbent assay (ELISA) after 24, 48, 72 and 96 h of incubation. Dunnett’s test was used for statistic analysis, and the data were stabilized by logarithmic transformation [log (pg/ml + 0·5)]. The level of significance was adjusted according to Bonferroni–Holm (16 tests; *α = 0·0031).
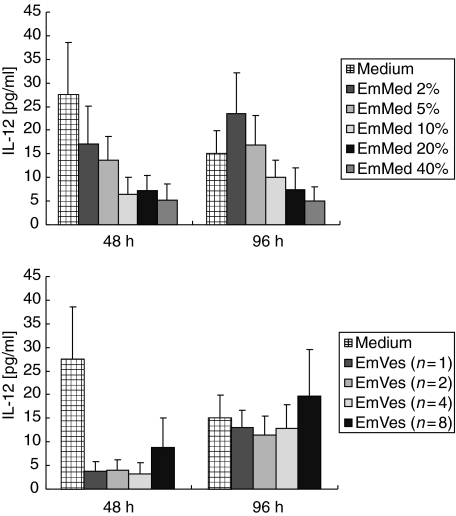
Reduction of spontaneously released IL-12 by EmMed and EmVes in a dose-dependent manner
To analyse the effect of EmMed and EmVes on the spontaneous production of IL-12 from PBMC, different concentrations of EmMed (2–40%) or increasing numbers of vesicles (1–8) were added to PBMC cell cultures. EmMed reduced the spontaneous IL-12 production in a dose-dependent manner after 48 and 96 h of incubation. A single vesicle of E. multilocularis (diameter 2–3 mm) was sufficient to reduce the spontaneous IL-12 production below the detection limit after 48 h (Fig. 3).
Fig. 3.
In vitro production of interleukin (IL)-12 (pg/ml + s.e.m.) by peripheral blood mononuclear cells from healthy donors (n = 6). Spontaneous IL-12 release (medium) or in response to different concentrations of Echinococcus multilocularis metacestode culture supernatant (EmMed 2–40%) as well as increasing numbers of viable vesicles (EmVes 1–8) are shown.
Discussion
Severe alveolar echinococcosis (AE) is associated with a general immune suppression [13,16], while Th2-type responses will favour parasite growth and disease progression, and dominant Th1-type cytokines and vigorous lymphocyte proliferation are linked with protection and abortive AE lesions [17,18]. Furthermore, E. multilocularis-derived molecules could inhibit and modulate cellular immune responses − not only by polarizing cytokine profiles [12], suppressing proliferation of blood cells [15] and the accessory cell function and the capacity of macrophages to present antigens [19], but also by impeding the generation of CD4+ memory cells [20]. In the present study, we showed that E. multilocularis metacestode antigens promoted the expression of CD25+ on CD4+ T cells, i.e. suggesting a differentiation into regulatory T cells (Table 2). As CD25 is inducible on both Th1- as well as Th2-type helper cells, and is also present on other effector cell populations, e.g. activated B cells, dendritic cells and monocytes, further detailed studies should include CD25 high or low levels of expression together with forkhead box P3 (FoxP3) and intracellular cytokine profiling to support these results. The baseline expression of the IL-2 receptor CD25 on CD4+ cells was elevated on PBMC from AE patients (Table 2) and stimulation with E. multilocularis vesicles further increased CD25 expression on T cells. Previously, CD25+ CD11b– cells, identified morphologically as macrophages, were observed in the periparasitic cellular lining around E. multilocularis metacestodes [21]. Such cells were described as monocytes recruited to the intestine with an acquired profound inflammatory anergy, i.e. they will not produce inflammatory cytokines [22]; these observations add to our understanding of how E. multilocularis metacestodes may evade host immune surveillance. However, a probable explanation for a greater frequency of CD25 is more ‘activated’ CD4 cells − as observed for CD28. The higher numbers of activated CD4+ CD28+ T cells in AE patients in this study (Table 2) suggested that activated CD4+ cells may be present around and within the periparasitic granulomas caused by E. multilocularis metacestodes. CD28 is a co-stimulator promoting T cell proliferation, cytokine production, T cell effector functions and antibody production. In addition, peripheral blood cells from AE patients secreted the CC chemokines MDC/CCL22 and TARC/CCL17 in response to E. multilocularis metacestode antigens –both attract monocytes, lymphocytes, eosinophils and basophils to numerous organs, including the liver, lung and dermal tissues [23]. MDC and TARC are implicated in chronic inflammatory skin disease and systemic sclerosis [24,25]; they were found elevated in helminth infection with Paragonimus westermani [26] and Onchocerca volvulus [27], and systemic neutralization of TARC diminished granuloma formation in mice challenged with S. mansoni eggs [28].
The extent to which MDC or TARC may contribute to metacestode growth or healing of AE lesions remains unknown. Our observations suggest that reduced levels of TARC, released by PBMC from AE patients after stimulation with E. multilocularis metacestode antigens (Table 1), may prevent the pathological features of lesions induced by infection with E. multilocularis. In contrast, the elevated MDC production by PBMC from AE patients (Table 1) may recruit effector cells into periparasitic tissues, thus supporting the development of efficient granulomas which restrain metacestode growth and dissemination and favour the outcome of infection. Clinical observations in AE patients and data from mass screenings have identified abortive cases where AE was cured spontaneously [3,4,8] and it remains to be determined how these chemokines contribute to clearance or parasite persistence.
As protection and abortive AE lesions were linked with dominant Th1-type cytokine responses [6,29,30], the elevated IFN-γ production by PBMC in AE patients following stimulation with metacestode culture supernatant or vesicle fluid antigen (Table 1) may slow progression of lesions and help to stop larval development [18]. Similarly, Shi et al. [9] have shown elevated IFN-γ concentrations in sera from AE patients irrespective of their stage of disease, and suggested that IFN-γ may have to act in synergy with other factors to slow parasite growth or to achieve curative effects.
The constantly diminished TNF-α release by PBMC in response to E. multilocularis antigens in patients from this study may indicate that metacestode vesicles will actively modulate the host immune responses, which may facilitate parasite survival. As necrosis and fibrosis are the main pathological features of echinococcosis, the cellular immune response, especially TNF-α synthesis, was considered responsible for clinical complications [21]. The AE patients studied here were with cured, stable or progressive disease and their PBMC secreted similar amounts of TNF-α in response to E. multilocularis antigens or PHA/E. coli LPS, except that the PHA-induced TNF-α release was stronger in cases with a progressive AE compared to those with a cured AE. An augmented production of TNF-α was seen in AE patients with severe tissue damage [9], while no TNF-α mRNA expression was detected in abortive lesions [21]. Therefore, the depressed TNF-α release, in response to E. multilocularis antigens, by cells from patients appears as an immune adaptation to prevent inflammatory host damage.
Furthermore, the Th1 type cytokine IL-12 was reduced in AE patients compared to controls (Table 1) and this has been observed previously in sera from AE patients at distinct states of infection [10]. Suppression of the cellular IL-12 production was mediated by E. multilocularis metacestode culture supernatant in a dose-dependent manner (Fig. 3), suggesting that excretory/secretory products released by E. multilocularis will mediate this effect. In addition, viable E. multilocularis vesicles depress IL-12 release by PBMC below the detection limit after 48 h of co-culture, and a single vesicle sufficed to mediate this effect, but after 96 h of co-culture these suppressive effects vanished. Therefore, E. multilocularis metacestode culture supernatant as well as viable vesicles diminished inflammatory cytokine releases of IL-12 and TNF-α after LPS stimulation after 24–96 h of co-culture (Fig. 2). This shows that E. multilocularis metacestodes have the potential to depress systemic inflammatory responses also induced by non-parasite-derived molecules. Parasite antigens with the capacity to inhibit TNF-α and other cytokines are present in a wide range of helminth parasites; ES-62, a phosphorylcholin-containing glycoprotein from Acanthocheilonema viteae, inhibits TNF-α, IL-6 and IFN-γ release [31]. Soluble egg antigens from Schistosoma mansoni [32], nematode cystatins [33] and the carbohydrate-rich molecules released into the parasite culture medium and contained in vesicles of E. multilocularis [15] have suppressive effects on mitogen [concanavalin A(ConA)] or crude parasite extract-induced cellular proliferation. Parasite extracts, and also entire parasites, have been studied intensively for their anti-inflammatory [32–34], anti-allergic [35,36] or anti-autoimmunological [31,37–39] potential, but E. multilocularis has received little attention, and therefore the robust anti-inflammatory capacity of metacestode-derived molecules merits further study.
In summary, proliferating metacestodes of E. multilocularis were found to depress proinflammatory cytokine responses even when PBMC were preactivated with LPS; they promoted CD4+ CD25+ T cell differentiation in AE patients and stimulated selectively Th2 type chemokine release. Such an immune modulatory capacity of E. multilocularis metacestodes may favour parasite growth and dissemination, limit adverse inflammatory and clinical effects to the host and may contribute to the often unnoticed progression of alveolar echinococcosis.
Acknowledgments
This work was supported by the fortüne-programme of the University Hospital of Tübingen (grants no. 1246-0-0 and 1041-0-0). M. P. Hübner was supported by the Deutsche Gesellschaft für Pneumologie, the National Genome Research Network (NGFN) programme of the BMBF (grant no. 01GSO403). For statistical consultations, data analyses and help in manuscript preparation we thank Professor Dr K. Dietz from the Department of Medical Biometry, University of Tübingen.
References
- 1.Gottstein B, Hemphill A. Immunopathology of echinococcosis. Chem Immunol. 1997;66:177–208. doi: 10.1159/000058670. [DOI] [PubMed] [Google Scholar]
- 2.Gottstein B, Saucy F, Deplazes P, et al. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg Infect Dis. 2001;7:408–12. doi: 10.3201/eid0703.010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresson-Hadni S, Laplante JJ, Lenys D, et al. Seroepidemiologic screening of Echinococcus multilocularis infection in a European area endemic for alveolar echinococcosis. Am J Trop Med Hyg. 1994;51:837–46. doi: 10.4269/ajtmh.1994.51.837. [DOI] [PubMed] [Google Scholar]
- 4.Rausch RL, Wilson JF, Schantz PM, McMahon BJ. Spontaneous death of Echinococcus multilocularis: cases diagnosed serologically (by Em2 ELISA) and clinical significance. Am J Trop Med Hyg. 1987;36:576–85. doi: 10.4269/ajtmh.1987.36.576. [DOI] [PubMed] [Google Scholar]
- 5.Gottstein B, Felleisen R. Protective immune mechanisms against the metacestode of Echinococcus multilocularis. Parasit Today. 1995;11:320–6. doi: 10.1016/0169-4758(95)80184-7. [DOI] [PubMed] [Google Scholar]
- 6.Vuitton DA. The ambiguous role of immunity in echinococcosis. Protection of the host or of the parasite? Acta Trop. 2003;85:119–32. doi: 10.1016/s0001-706x(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 7.Sturm D, Menzel J, Gottstein B, Kern P. Interleukin-5 is the predominant cytokine produced by peripheral blood mononuclear cells in alveolar echinococcosis. Infect Immun. 1995;63:1688–97. doi: 10.1128/iai.63.5.1688-1697.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godot V, Harraga S, Beurton I, et al. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. I. Comparison of patients with progressive and abortive lesions. Clin Exp Immunol. 2000;121:484–90. doi: 10.1046/j.1365-2249.2000.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi DZ, Li FR, Bartholomot B, Vuitton DA, Craig PS. Serum sIL-2R, TNFα and IFN-γ in alveolar echinococcosis. World J Gastroenterol. 2004;10:3674–6. doi: 10.3748/wjg.v10.i24.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreweck CM, Soboslay PT, Schulz-Key H, Gottstein B, Kern P. Cytokine and chemokine secretion by human peripheral blood cells in response to viable Echinococcus multilocularis metacestode vesicles. Parasite Immunol. 1999;21:433–8. doi: 10.1046/j.1365-3024.1999.00243.x. [DOI] [PubMed] [Google Scholar]
- 11.Kilwinski J, Jenne L, Jellen-Ritter A, Radloff P, Flick W, Kern P. T lymphocyte cytokine profile at a single cell level in alveolar echinococcosis. Cytokine. 1998;11:373–81. doi: 10.1006/cyto.1998.0432. [DOI] [PubMed] [Google Scholar]
- 12.Eger A, Kirch A, Manfras B, Kern P, Schulz-Key H, Soboslay PT. Pro-inflammatory (IL-1α, IL-18) cytokines and IL-8 chemokine release by PBMC in response to Echinococcus multilocularis metacestode vesicles. Parasite Immunol. 2003;25:103–5. doi: 10.1046/j.1365-3024.2003.00601.x. [DOI] [PubMed] [Google Scholar]
- 13.Vuitton DA. Echinococcosis and allergy. Clin Rev Allergy Immunol. 2004;26:93–104. doi: 10.1007/s12016-004-0004-2. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill A, Stettler M, Walker M, Siles-Lucas M, Fink R, Gottstein B. In vitro culture of Echinococcus multilocularis and Echinococcus vogeli metacestodes: studies on the host–parasite interface. Acta Trop. 2003;85:145–55. doi: 10.1016/s0001-706x(02)00220-6. [DOI] [PubMed] [Google Scholar]
- 15.Walker M, Baz A, Dematteis S, et al. Isolation and characterization of a secretory component of Echinococcus multilocularis metacestodes potentially involved in modulating the host–parasite interface. Infect Immun. 2004;72:527–36. doi: 10.1128/IAI.72.1.527-536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emery I, Liance M, Deriaud E. Vuitton DA, Houin R, Leclerc C. Characterization of T-cell immune responses of Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 1996;18:463–72. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 17.Bresson-Hadni S, Vuitton DA, Lenys D, Liance M, Racadot E, Miguet JP. Cellular immune response in Echinococcus multilocularis infection in humans. I. Lymphocyte reactivity to Echinococcus antigens in patients with alveolar echinococcosis. Clin Exp Immunol. 1989;78:61–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Vuitton DA, Bresson-Hadni S, Laroche L, et al. Cellular immune response in Echinococcus multilocularis infection in humans. II. Natural killer cell activity and cell subpopulations in the blood and in the periparasitic granuloma of patients with alveolar echinococcosis. Clin Exp Immunol. 1989;78:67–74. [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon JB. Echinococcosis. Comp Immunol Microbiol Infect Dis. 1997;20:87–94. doi: 10.1016/s0147-9571(96)00019-7. [DOI] [PubMed] [Google Scholar]
- 20.Manfras BJ, Reuter S, Wendland T, Boehm BO, Kern P. Impeded Th1 CD4 memory T cell generation in chronic-persisting liver infection with Echinococcus multilocularis. Int Immunol. 2004;16:43–50. doi: 10.1093/intimm/dxh005. [DOI] [PubMed] [Google Scholar]
- 21.Bresson-Hadni S, Petitjean O, Monnot-Jacquard B, et al. Cellular localisations of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha mRNA in a parasitic granulomatous disease of the liver, alveolar echinococcosis. Eur Cytokine Netw. 1994;5:461–8. [PubMed] [Google Scholar]
- 22.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 24.Fujii H, Shimada Y, Hasegawa M, Takehara K, Sato S. Serum levels of a Th1 chemoattractant IP-10 and Th2 chemoattractants, TARC and MDC, are elevated in patients with systemic sclerosis. J Dermatol Sci. 2004;35:43–51. doi: 10.1016/j.jdermsci.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003;112:1195–202. doi: 10.1016/j.jaci.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 26.Katoh S, Fukushima K, Matsumoto N, et al. Accumulation of CCR4-expressing CD4+ T cells and high concentration of its ligands (TARC and MDC) in bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. Allergy. 2004;58:518–23. doi: 10.1034/j.1398-9995.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 27.Fendt J, Hamm DM, Banla M, et al. Chemokines in onchocerciasis patients after a single dose of ivermectin. Clin Exp Immun. 2005;142:318–26. doi: 10.1111/j.1365-2249.2005.02910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakubzick C, Wen H, Matsukawa A, Keller M, Kunkel SL, Hogaboam CM. Role of CCR4 ligands, CCL17 and CCL22, during Schistosoma mansoni egg-induced pulmonary granuloma formation in mice. Am J Pathol. 2004;165:1211–21. doi: 10.1016/S0002-9440(10)63381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emery I, Leclerc C, Sengphommachanh K, Vuitton DA, Liance M. In vivo treatment with recombinant IL-12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol. 1998;20:81–91. doi: 10.1046/j.1365-3024.1998.00131.x. [DOI] [PubMed] [Google Scholar]
- 30.Amiot F, Vuong P, Defontaines M, Pater C, Dautry F, Liance M. Secondary alveolar echinococcosis in lymphotoxin-alpha and tumor necrosis factor-alpha deficient mice. exacerbation of Echinococcus multilocularis larval growth is associated with cellular changes in the periparasitic granuloma. Parasite Immunol. 1999;21:475–83. doi: 10.1046/j.1365-3024.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 31.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–33. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 32.Kane CM, Cervi L, Sun J, et al. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173:7454–61. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 33.Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect Immun. 2003;71:2422–9. doi: 10.1128/IAI.71.5.2422-2429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harnett W, Harnett MM, Leung BP, Gracie JA, McInnes IB. The anti-inflammatory potential of the filarial nematode secreted product, ES-62. Curr Top Med Chem. 2004;4:553–9. doi: 10.2174/1568026043451212. [DOI] [PubMed] [Google Scholar]
- 35.Mangan NE, Fallon RE, Smith P, Van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346–56. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 36.Pinto LA, Pitrez PM, Fontoura GR, et al. Infection of BALB/c mice with Angiostrongylus costaricensis decreases pulmonary inflammatory response to ovalbumin. Parasite Immunol. 2004;26:151–5. doi: 10.1111/j.0141-9838.2004.00694.x. [DOI] [PubMed] [Google Scholar]
- 37.Sewell D, Qing Z, Reinke E, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 38.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:1117–9. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Zaccone P, Fehervari Z, Jones FM, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]