Abstract
Summary
The protozoan parasite Trypanosoma cruzi circulates in the blood as trypomastigotes and invades a variety of cells to multiply intracellularly as amastigotes. The acute phase triggers an immune response that restricts the proliferation of the parasite. However, parasites are able to persist in different tissues causing the pathology of Chagas’ disease. Natural killer (NK) cells play an important role in innate resistance to a variety of pathogens. In the present study we demonstrate that NK cells trigger trypanocidal mechanisms in infected L929 cells that are critically dependent on inducible nitric oxide (NO) synthase (iNOS) induction which is, to a major degree, triggered by interferon (IFN)-γ provided by NK cells. This work provides a more detailed analysis of how NK cells as a part of the innate immune system participate in the control of parasites that reside intracellularly in fibroblast-like L929 cells.
Keywords: iNOS, NK cells, Trypanosoma cruzi
Introduction
Trypanosoma cruzi is the aetiological agent of Chagas’ disease. Approximately 18 million people in Latin America are infected with the protozoan parasite, and an additional 100 million, i.e. 25% of the population of Latin America, are at risk of acquiring Chagas’ disease (WHO, 2004; http://www.who.org).
The acute phase of infection is characterized by high parasitaemia in the blood, followed by the chronic phase that is associated with pathology of Chagas’ disease: a life-long persistence of parasites in various tissues but mainly in the heart and muscle tissue [1], leading to the death of 50 000 people annually [1,2].
After invasion of infectious metacyclic trypomastigotes in mammalian host, T. cruzi infects a variety of cell types such as macrophages [3] and fibroblasts [4]. The latter cell types are numerous in the extracellular matrix of the skin [5], and thus presumably one of the first cells infected. Whereas T. cruzi-infected macrophages and cardiomyocytes are strongly affected in terms of degradation of the cytoskeleton and induction of apoptosis [6], fibroblasts remain refractory to apoptosis [7], which is accompanied by a slight change in gene expression [8]. This indicates that the parasite is disguised in these cells.
In contrast to the inconspicuous infection of fibroblasts, macrophages release interleukin (IL)-12 immediately after infection, which is known to induce a Th1 type of immune response and act directly on natural killer (NK) cells that are prompted to produce interferon (IFN)- γ [9–11]. NK cells are described to be the source of early IFN-γ during T. cruzi infection, and thus depletion of NK cells leads to a strong decrease of IFN-γ production and to an enhanced parasitaemia [12,13]. Protective immunity against intracellular parasites such as Leishmania spp. [14,15], Toxoplasma gondii [16] and T. cruzi [17,18] has been shown to depend on the production of nitric oxide (NO) after induction of inducible NO synthase (iNOS, NOS2).
In the present study, we demonstrate that T. cruzi-infected fibroblasts activate NK cells by contact-independent mechanisms. Activated NK cells subsequently initiate trypanocidal effector mechanisms in infected L929 cells that are triggered mainly by IFN-γ, but also other soluble mediators, and thus contribute to the control of intracellular parasites.
Materials and methods
Parasites and mice
T. cruzi Tehuantepec epimastigotes were stably transfected with 5 µg of a β-galactosidase expression plasmid (pBS-CL-Neo-LacZ, provided by F. S. Buckner), as described previously [19]. Transfected parasites were maintained in liquid culture in the presence of G418 (Gibco, Invitrogene, Karlsruhe, Germany; 150 µg/ml). For in vitro infection of cells parasites were cultured using the murine L929 fibroblast cell line or human 86-HG-39 myoblastoma cells by serial passage of the supernatant of infected cells to uninfected cells. CBA/j and SCID mice were obtained from Charles River Laboratories (Sulzfeld, Germany). C57BL/6 IFN-α/β-R–/– mice were obtained from MPI for Infection Biology (Berlin, Germany).
Treatment of spleen cells
Spleen cells from CBA/j mice were cultured with 20 U/ml IL-2, and in some experiments additionally with 0·2 mg/ml poly I:C (Sigma, Taufkirchen, Germany) overnight. For neutralization of IFN-γ, 30 µg/ml of the monoclonal antibody (clone XMG 1·2, BNI), IFN-α 10-µg/ml of monoclonal antibody (clone RMMA-1, RDI Research Diagnostics), IFN-β 40 units of polyclonal anti-serum (RDI, Concord, MA, USA) and 10 µg/ml for IL-12 monoclonal antibody (clone C17·8, Becton Dickinson, Heidelberg, Germany) were added to spleen cells. Blocking iNOS was achieved by application of 5 mm aminoguanidin to the culture.
Infection system
Cells (1 × 104 L929) were cultured in a 24-well cell culture plate (Greiner, Frickenhausen, Germany) to a single cell monolayer, before the cells were infected with 1 × 105 lacZ-transfected T. cruzi for 3 days. Cells were washed to remove extracellular T. cruzi. Then 3 × 106 spleen cells were added directly or separated in transwell inserts (Nunc, Roskilde, Denmark) for an additional 2 days and the parasitic burden was measured using chlorophenol red-beta D galactopyranoside (CPRG) (Roche, Mannheim, Germany) staining as substrate for β-Gal. Cytokine and nitric oxide (NO) production was quantified by enzyme-linked immunosorbent assay (ELISA) and Griess assay, respectively.
Reverse transcription–polymerase chain reaction (RT–PCR) of type I interferons
Cells (1 × 106 L929) were lysed in 1 ml TriReagent (Molecular Research Center, Cincinnati, OH, USA) and after supplementation of 100 µl bromo-chloropropane (BCP) (Sigma, Taufkirchen, Germany) cell lysate was centrifuged for 30 min at 14 000 g. RNA was pelleted with isopropanol and washed with ethanol. The pellet was dried and resuspended in Aqua dest. For reverse transcription, 2 µg RNA filled with diethyl pyrocarbonate (DEPC)-water up to 11 µl was incubated with 4 µl oligo(dT)15-primer, 4 µl 5 × buffer, 2 µl dithiothreitol (DTT) (0·1 M), 2 µl dideoxy nucleotide triphosphate (dNTP) (10 mM) for 5 min at 65°C and afterwards for 5 min on ice. Subsequently 1 µl Super-Script™ II (Gibco, Eggenstein, Germany) was added and incubated for 1 h at 37°C. Expression of type I interferon cDNA was exemplarily analysed for IFN-α and IFN-β, using primers for IFN-α: sense 5′-TGTCTGATGCAGCAGGTGG-3′ and anti-sense 5′-AAGACAGGGCTCTCCAGAC-3′ and for IFN-β: sense 5′-CCATCCAAGAGATGCTCCAG-3′ and anti-sense 5′-GTGGAGAGCAGTTGAGGACA-3′. As control the expression of the housekeeping gene β-actin with primer sense 5′-GTCGTACCACAGGCATTGTGATGG-3′ and anti-sense 5′-GCAATGCC TGGGTACATGGTG G-3′ was analysed. Polymerase chain reaction (PCR) of type I interferon was performed in 35 cycles with 1 min 94°C denaturation, 1 min 62°C annealing and 1 min 72°C elongation with PCR cycler (Hybaid OmniGene, Middlesex, UK). DNA amplification was controlled in 1% TBE agarose gels.
NK depletion
Spleen cells from CBA mice were washed in PBS supplemented with 1% fetal calf serum (FCS) and stained on ice with fluorescein isothiocyanate (FITC)-labelled anti-mouse CD49b/pan NK cell (DX5) monoclonal antibody (Becton Dickinson). The cells were then washed in PBS with 1% bovine serum albumin (BSA) and 2 mm ethylenediamine tetraacetic acid (EDTA), centrifuged and resuspended in 80 µl PBS. Twenty µl Macs anti-FITC-MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) was added and incubated on ice for 15 min. Cells were resuspended with PBS to a final volume of 500 µl and applied on an equilibrated LS+ separation column (Miltenyi Biotec). Cells were eluted by washing the column twice with 500 µl PBS/1% BSA and resuspended in medium. NK-deficient spleen cell suspension and isolated NK cells were added to the infected fibroblasts separately.
Immunoblot
Infected and uninfected fibroblasts (1 × 106) were lysed with 1% Triton lysis buffer. After centrifugation supernatants were supplemented with glycerin/sodium dodecyl sulphate (SDS) buffer and applied on a gradient SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gel (8–18%). After electrophoresis the gel was blotted using the semidry technique on Hybond-ECL nitrocellulose (Amersham Bioscience, Freiburg, Germany). Nitrocellulose was then blocked in 4% BSA/PBS and washed twice with PBS supplemented with 0·1% Tween. The blot was incubated with 1 µg/ml rabbit anti-mouse iNOS/NOS II (Upstate Biotechnology) for 1 h at room temperature followed by horseradish peroxidase (HRP)-labelled goat anti-rabbit antibody (Dako, Glostrup, Denmark) for 1 h at room temperature. After washing with PBS/0·1% Tween the blot was developed with ECL Western blotting detection solution and Hyperfilm-ECL (Amersham Bioscience).
Statistical analysis
Results are presented as the mean ± standard deviation. Each experiment was performed independently three to four times. Statistical analysis was generally performed with the unpaired Student’s t-test using prism software (Graph Pad Software, San Diego, CA, USA). The level of significance was set at *P ≤ 0·05 and **P ≤ 0·01.
Results
Decreased burden of intracellular T. cruzi after co-incubation with spleen cells
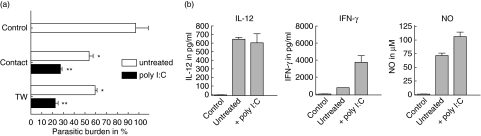
To study the ability of immune cells to affect the number of intracellular T. cruzi in fibroblasts, monolayers of L929 cells were infected in vitro with lacZ-transfected T. cruzi and subsequently spleen cells from CBA/j mice were added. Staining of cells with 4′,6-diamidino-2-phenylindole (DAPI), a dye for nuclear fluorescent labelling, revealed that approximately 80% of the cells were infected after 3 days using a multiplicity of infection (MOI) of one parasite per cell (data not shown). Addition of spleen cells from CBA/j mice for 2 days reduced the parasitic load in L929 cells significantly (Fig. 1a). Spleen cells that were pre-activated with poly I:C, which is known to induce IFN-γ by NK cells, displayed an increased activity in comparison to naive spleen cells. As expected, pre-activated spleen cells produce more IFN-γ and an increased amount of nitric oxide could be found in poly I:C-stimulated cultures (Fig. 1b). TNF-α production was not detectable in this in vitro culture system (data not shown). Trypanocidal activity of naive as well as poly I:C pre-activated spleen cells were independent from cell-to-cell contact, as separation of spleen cells from infected L929 cells by a transwell insert did not abolish the trypanocidal effect (Fig. 1a). These findings suggest not only that the reduction of intracellular parasites is triggered by spleen cell-derived soluble factors, but also that spleen cells were activated by soluble factors that were produced by infected L929 cells.
Fig. 1.
Induction of trypanocidal activity in L929 cells after incubation with CBA/j spleen cells. A monolayer of L929 cells was infected with 1 × 105Trypanosoma cruzi and after 3 days 3 × 106 spleen cells from CBA/j mice that were either untreated or stimulated with 0·2 mg/ml poly I:C were added. After an additional 2 days the number of intracellular lacZ-transfected T. cruzi was measured by staining with chlorophenol red-beta D galactopyranoside (CPRG) (a). The number of intracellular parasites was similar when L929 cells were in direct contact with spleen cells (contact) or when they were separated by a transwell system (TW). The supernatant of this mixed cell culture system was analysed for interleukin (IL)-12, interferon (IFN)-γ and nitric oxide (NO) (b). Graphs indicate cytokine production after direct (contact) incubation.
Depletion of NK cells abrogates the activity against intracellular T. cruzi
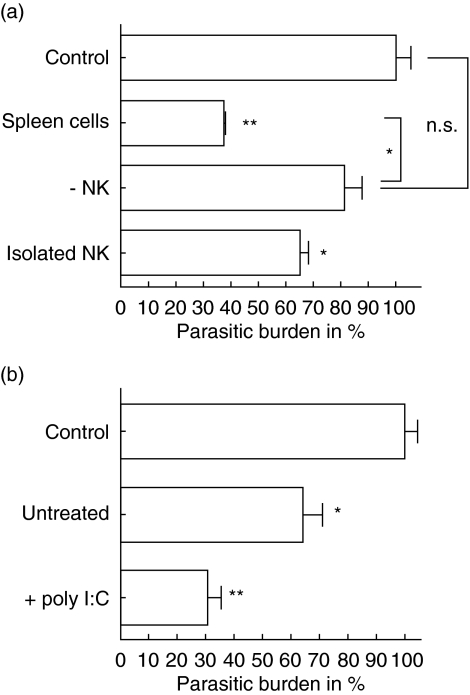
Due to the short incubation time of naive spleen cells with infected fibroblasts, an involvement of specific T cells in this trypanocidal effect is unlikely. However, it is well accepted that NK cells produce IFN-γ very quickly after the infection of spleen cell cultures with T. cruzi. To investigate if NK cells contribute to the reduction of intracellular parasites in L929 cells, NK cells were depleted from spleen cell cultures of CBA/j mice and the remaining cells were incubated with the infected fibroblasts. The depletion of NK cells led to a strong decrease of trypanocidal activity against T. cruzi-infected L929 cells (Fig. 2a). In addition, we found that purified NK cells exhibit trypanocidal activity, although they were not found to be as active as naive spleen cells. The central role of NK cells was corroborated by experiments using spleen cells from SCID mice. Although these mice lack T and B cells their trypanocidal activity was as high as those from wild-type CBA/j mice and could be increased further by stimulation with poly I:C (Fig. 2b).
Fig. 2.
Natural killer (NK) cells were needed for the induction of trypanocidal activity in infected L929 cells. NK cells were depleted by magnetic beads that were coated with anti-DX-5 antibodies from cultures of naive spleen cells. A monolayer of L929 cells was infected with 1 × 105T. cruzi and after 3 days 3 × 106 NK cell-deficient spleen cells spleen and isolated NK cells were added, respectively. After an additional 2 days the number of intracellular lacZ transfected T. cruzi was measured by staining with chlorophenol red-beta D galactopyranoside (CRBG) (a). In a similar experiment infected L929 cells were incubated for 2 days with 5 × 105 spleen cells from SCID mice that were either untreated or stimulated with poly I:C (b).
Trypanocidal activity is enhanced by IFN-γ and depends critically on nitric oxide
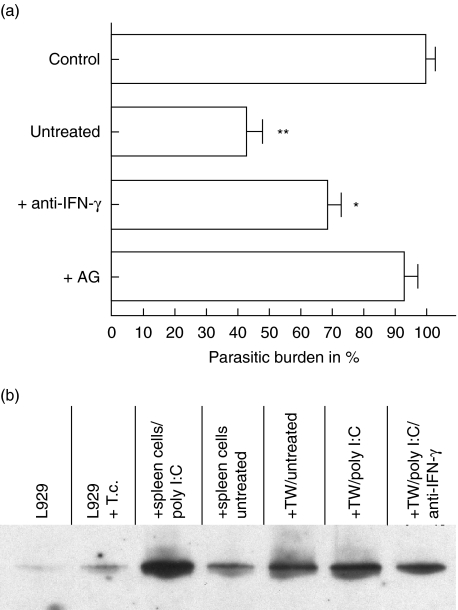
IFN-γ activates macrophages to produce anti-microbial molecules that kill intracellular pathogens. The incubation of spleen cells with infected fibroblasts also led to an IFN-γ secretion and to the production of nitric oxide. To control if this pathway is critical for the trypanocidal activity of T. cruzi-infected fibroblasts, iNOS was blocked with aminoguanidin and IFN-γ was neutralized by monoclonal antibodies. Naive spleen cells minimized the parasitic burden significantly. Treatment with aminoguanidin inhibited the synthesis of nitric oxide and abrogated the trypanocidal activity against intracellular parasites. Whereas after iNOS blockade almost no differences were found in the parasitic load in comparison to the control, the neutralization of IFN-γ caused a lower but statistically significant inhibition of trypanocidal activity (Fig. 3a). Neutralization of IFN-γ correlates with a decreased production of nitric oxide (data not shown), which indicates that the trypanocidal activity of infected fibroblasts depends critically on nitric oxide. However, in the absence of IFN-γ other soluble factors might also contribute to the induction of iNOS. In order to test if NO production by infected L929 cells was due to an increased iNOS expression we performed immunoblot analysis (Fig. 3b). In vitro infection of L929 cells with T. cruzi causes only a weak induction of iNOS. In contrast, in the presence of spleen cells iNOS was found to be significantly up-regulated. This was also found when spleen cells were separated by a transwell insert from infected fibroblasts. This demonstrates further that iNOS induction was contact-independent, and in addition exclude that iNOS was expressed only in macrophages present in spleen cells, which are known for their strong iNOS expression after stimulation with IFN-γ. It is interesting to note that neutralization of IFN-γ from spleen cells led to a decreased expression of iNOS in fibroblasts, although the expression remains higher than in infected L929 cells in the absence of spleen cells. These findings are further evidence that not only IFN-γ is able to induce iNOS.
Fig. 3.
Inhibition of nitric oxide abrogates trypanocidal activity. 3 × 106 naive spleen cells from CBA/j mice were incubated with infected L929 cells either in the presence of 30 µg/ml anti-interferon (IFN)-γ neutralizing antibodies or with 5 mm aminoguanidin, which blocks inducible NO synthase (iNOS) activity (a). Induction of iNOS in infected fibroblasts after incubation with spleen cells (b) 1 × 106 L929 cells were lysed in Triton X-100 containing lysis buffer and Western blotting was performed after gradient (8–18%) sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). The samples were a mixture of L929 cells and 3 × 106 spleen cells (+ spleen cells, untreated or poly I:C stimulated) or L929 cells were analysed separately from spleen cells (TW = transwell) to exclude contamination with iNOS-expressing macrophages.
Activation of spleen cells is not dependent on IL-12
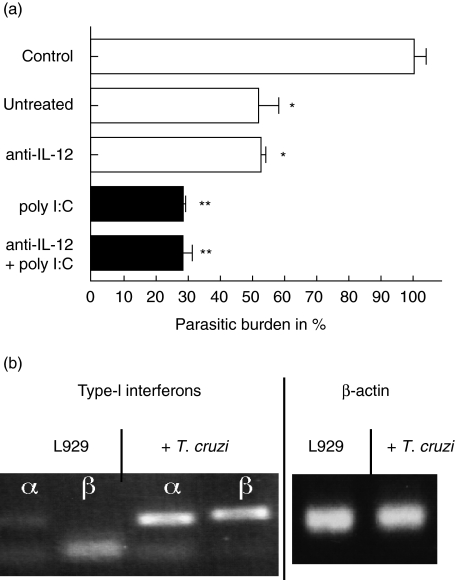
IL-12 is a strong inducer of IFN-γ production. Although infected fibroblasts did not release IL-12 in detectable amounts, IL-12 was produced by spleen cells that were cultured in the presence of infected L929 cells. Accordingly, IL-12 production was accompanied by IFN-γ production of naive spleen cells. To study if IL-12 production by antigen-presenting cells that are present in spleen cells is a prerequisite for the NK cell-induced trypanocidal activity, IL-12 (p70) was blocked by monoclonal antibodies. Interestingly, neither naive nor poly I:C stimulated cells lost their activity when IL-12 was blocked (Fig. 4a).
Fig. 4.
Activation of spleen cells was independent from interleukin (IL-12). 3 × 106 spleen cells from CBA/j mice were either untreated or stimulated with poly I:C and incubated with a monolayer of infected fibroblasts either in the presence or absence of 10 µg/ml neutralizing anti-interleukin (IL)-12 (p70) antibodies (a). Infection of L929 cells by Trypanosoma cruzi is accompanied by an induction of type I interferon RNA (b).
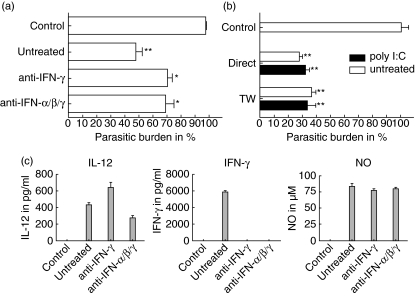
Blockade of type I interferons did not influence the trypanocidal activity
iNOS is still induced in infected L929 cells upon cultivation with spleen cells although IFN-γ was blocked, albeit to a lower extent. Therefore we investigated if type I interferons, namely IFN-α and IFN-β, support iNOS induction in fibroblasts. RT–PCR of infected fibroblasts revealed induction of the most prominent type I interferons, IFN-α and IFN-β (Fig. 4b). Therefore we neutralized type I interferons with a combination of a monoclonal antibody against IFN-β and a polyclonal anti-serum against IFN-α. However, the inhibition of type I interferons had no effect on the reduction of the parasitic load in L929 cells (Fig. 5a). To support these findings, spleen cells from type I receptor-deficient mice were incubated with infected fibroblasts either directly or separated by transwell. In this experiment spleen cells were activated and reduced the burden of intracellular T. cruzi, as described for spleen cells from wild-type mice (Fig. 5b). The activation of trypanocidal activity is accompanied by the production of IL-12, IFN-γ and NO in the supernatant of the culture (Fig. 5c). These data suggest that type I interferons were neither involved in the activation of NK cells by infected L929 cells nor that type I interferon secretion by NK cells is involved in iNOS induction.
Fig. 5.
Type I interferons did not trigger trypanocidal activity in L929 cells. 3 × 106 poly I:C stimulated spleen cells from CBA/j mice were incubated with infected L929 cells either directly or separated by a transwell system (TW). In the latter case the experiment was performed either in the presence or absence of 30 µg/ml anti-interferon (IFN)-γ, 10 µg/ml IFN-α and 40 units IFN-β neutralizing antibodies, respectively (a); 3 × 106 spleen cells from type I receptor deficient mice that were either untreated or poly I:C stimulated were incubated with infected L929 cells directly or separated using a transwell system (b). The supernatant of the TW culture was then analysed for interleukin (IL)-12, IFN-γ and nitric oxide (NO) (c).
Activation of NK cells depends on fibroblast-derived molecules
The search for activating factors for NK cells has led to the question of whether fibroblasts release other cytokines than the ones excluded, or if T. cruzi-derived molecules from extracellular trypomastigotes also present in the culture act as activators for NK cells. To approach this issue we infected human 86-HG-39 cells with T. cruzi and incubated these cells with CBA/j spleen cells in transwells. However, using human cells there was no induction of IFN-γ or IL-12 in the culture detectable (data not shown). This points to fibroblast-derived cytokines as activators for NK cells which could not be defined further at this point.
Discussion
In the mammalian host, T. cruzi cycles between extracellular trypomastigotes, which are involved in dissemination via the blood, and intracellular amastigotes, which are forms of replication. After invasion through the skin macrophages were one of the first cell types that were infected with T. cruzi [3,20]. Nevertheless, other cell types, e.g. fibroblasts that were found in high numbers in the extracellular matrix of the skin, are also highly infected [6]. However, infected fibroblasts exhibit no induction of mRNA expression [8] and apoptosis [7], although activation of infected cells and lymphocytes often results in apoptosis in diverse parasitic infections including T. cruzi [21]. Interestingly, T. cruzi-specific CD8+ T cells from infected mice are able to form tight interactions with T. cruzi-infected fibroblasts which are followed by the death of intracellular parasites [4]. Thus, these data suggest that immune cells are able to recognize infected fibroblasts and trigger trypanocidal mechanisms in these cells.
We found that not only parasite specific T cells but also naive spleen cells were able to induce trypanocidal activity in fibroblasts. This activity could be abrogated completely by inhibition of iNOS with aminoguanidin, which indicates that the trypanocidal effect was mediated by NO. Using immunoblot analysis we found that T. cruzi-infected L929 cells express only marginal amounts of iNOS. In contrast, in the presence of naive spleen cells a strong induction of iNOS expression occurred. This holds also true when spleen cells were separated from fibroblasts using a transwell system. These data suggest a cross-talk between infected fibroblasts and naive spleen cells by soluble mediators that lead to an activation of cells present in spleen cell preparations, which in turn activate L929 cells. The trypanocidal activity of infected L929 cells was dependent on iNOS, induced by soluble molecules that were secreted by spleen cells. In vivo, the importance of nitric oxide as a mediator of trypanocidal activity remains unclear. Whereas it was shown that iNOS-deficient mice were highly susceptible for T. cruzi infection [17,22], a recent report described a similar parasitaemia and parasitic burden in muscle tissue for wild-type and iNOS–/– mice [23], respectively. It has already been shown by Saeftel et al. that inhibition of iNOS activity in vivo increased the susceptibility of infected mice in the very early phase of infection and had no effect when iNOS was blocked 10 days after infection [24]. These data may indicate that the contribution of NO to the control of T. cruzi depends critically on the stage of infection and on the tissue that is involved. An impaired function of iNOS-dependent trypanocidal activity might reflect the role of myoglobin as a nitric oxide scavenger [24], leading to a preferential persistence in heart and skeletal muscle [25,26]. A similar effect was described for Leishmania that persist in fibroblasts due to their lower iNOS activity, whereas parasites in macrophages were highly susceptible to iNOS-mediated killing [27].
Depletion of NK cells in our in vitro infection system abrogates the induction of trypanocidal activity in fibroblasts and was accompanied by a decreased production of NO, which indicates that NK cells were responsible for the observed induction of iNOS. This is in line with our finding that spleen cells from SCID mice (that lack B and T cells) were also able to induce iNOS in fibroblasts in a similar manner to spleen cells from CBA/j mice. In both settings the trypanocidal activity increased when spleen cells were preincubated with poly I:C, which is known to activate NK cells. In addition, highly purified NK cells trigger trypanocidal activity in infected L929 cells, although to a lesser extent, as spleen cells contain the same amount of NK cells. This indicates that other cells present in spleen cells might amplify the activity of NK cells. It has already been shown that depletion of NK cells in vivo causes an increased parasitaemia and in some cases an increased susceptibility [12]. However, we reported recently that depletion of NK cells in vivo had no significant influence on the parasitic load in different tissues during the acute phase as well as in the chronic stage [26]. Further experiments showed that NK cells contribute to the control of extracellular T. cruzi rather than to the control of intracellular amastigotes in the tissue. However, our in vitro data suggest that NK cells are capable of inducing trypanocidal activity in fibroblasts. In vivo the highest parasitic loads were found in heart and skeletal muscle, which might be explained by an impaired activity of iNOS-dependent pathways in theses tissues, as discussed previously.
Using transwell experiments we found that the induction of iNOS in infected L929 cells is contact-independent. As NO production was accompanied by the production of IFN-γ we tested if IFN-γ secretion by NK cells might trigger iNOS expression. Neutralizing IFN-γ by monoclonal antibodies led to a decreased trypanocidal activity in fibroblasts and a marked decrease of iNOS expression. However, although iNOS levels decreased they appeared to be higher, as in control experiments, in the absence of spleen cells. Interestingly, in vitro experiments with T. cruzi infected human fibroblasts revealed that even the addition of recombinant IFN-γ could not affect the parasitic load in these cells [28].
Other possible candidates that might trigger iNOS expression are type I interferons, namely IFN-α and β, the most important type I interferons. We found that type I interferons were induced in the infected fibroblasts. Interestingly, type I interferons were also induced in spleen cells that were separated from infected L929 cell by a transwell insert, which might suggest a signal amplification between infected fibroblasts and NK cells. However, neutralizing antibodies against IFN-α and -β did not lead to a further decrease in IFN-γ-independent trypanocidal activity. Although these data suggest that type I interferons were not involved as mediators between NK cells and infected fibroblasts, we cannot exclude that members of the type I interferon family, which were not neutralized by the antibodies used, might account for the remaining activity. In particular, this might be the case for IFN-α, for which a great variety of different members have been described.
We focused our interest not only on the induction of iNOS in fibroblasts by NK cells but were also interested in signals that activate naive spleen cells. Preincubation of spleen cells by poly I:C led to their strong activation and to a subsequent activation of trypanocidal in L929 cells, which were found to be contact-independent. However, infected L929 cells were also able to activate naive spleen cells that were separated by a transwell insert. This was accompanied by IL-12 and IFN-γ production. Depletion of NK cells abrogated IFN-γ production (data not shown) and reduced trypanocidal activity. We assume that IL-12 production by antigen-presenting cells (APC) might trigger IFN-γ production by NK cells. However, blocking IL-12 by antibodies does not prevent the induction of trypanolytic activity. This is in line with the finding that isolated NK cells were also able to induce trypanocidal activity in L929 cells and that in this case no IL-12 could be detected due to the absence of APC. This is in contrast to our recent results showing that the cytotoxic activity of NK cells was dependent on the IL-12, which was provided by APC [26], indicating that cytokine secretion and cytotoxic activity of NK cells is regulated differentially. In conclusion, our experiments concerning type I interferons suggested that these, although induced in infected fibroblasts, had no influence on the activation of spleen cells. Another possibility might be that the activation of naive spleen cells is elicited by Toll-like receptors (TLR), as binding of T. cruzi-derived molecules to TLR-2, which is highly expressed on APC, was reported [29]. T. cruzi-derived molecules that are always present in the cell culture, although the parasites remain intracellular, might bind to TLR 2 leading to the induction of IL-12 and IL-18, which acts synergistically on NK cells [30]. However, infection of the human cell line 86-HG-39 with T. cruzi did not induce cytokine production in murine NK cells. Thus, the possibility of T. cruzi-derived molecules as a source of activation can be excluded.
We have demonstrated in this work that T. cruzi-infected L929 cells secrete soluble molecules that activate NK cells. Although type I interferons were induced, they appeared to be neither critical for NK cell activation nor for the induction of trypanocidal activity in L929 cells. The trypanocidal activity of L929 cells was dependent on endogenous iNOS activity which is, at least to a major part, induced by IFN-γ produced by NK cells. This work provided a more detailed analysis of how as a part of the innate immune system NK cells could participate in the control of parasites and in the regulation of the specific immune response.
Acknowledgments
The kind provision of the β-galactosidase expression plasmid pBS-CL-Neo-LacZ by Frederick S Buckner is gratefully acknowledged.
References
- 1.Tarleton RL, Zhang L. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol Today. 1999;15:94–9. doi: 10.1016/s0169-4758(99)01398-8. [DOI] [PubMed] [Google Scholar]
- 2.Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M. Chagas’ disease. Clin Microbiol Rev. 1992;5:400–19. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golden JM, Tarleton RL. Trypanosoma cruzi: cytokine effects on macrophage trypanocidal activity. Exp Parasitol. 1991;72:391–402. doi: 10.1016/0014-4894(91)90085-b. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn RE, Murnane JE. Trypanosoma cruzi : immune destruction of parasitized mouse fibroblasts in vitro. Exp Parasitol. 1977;41:66–73. doi: 10.1016/0014-4894(77)90130-8. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Fan J, Chen M, Woodley DT. Mechanisms of human skin cell motility. Histol Histopathol. 2004;19:1311–24. doi: 10.14670/HH-19.1311. [DOI] [PubMed] [Google Scholar]
- 6.de Souza EM, Araujo-Jorge TC, Bailly C, et al. Host and parasite apoptosis following Trypanosoma cruzi infection in in vitro and in vivo models. Cell Tissue Res. 2003;314:223–35. doi: 10.1007/s00441-003-0782-5. [DOI] [PubMed] [Google Scholar]
- 7.Clark RK, Kuhn RE. Trypanosoma cruzi does not induce apoptosis in murine fibroblasts. Parasitology. 1999;118:167–75. doi: 10.1017/s0031182098003631. [DOI] [PubMed] [Google Scholar]
- 8.Vaena de Avalos S, Blader IJ, Fisher M, Boothroyd JC, Burleigh BA. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J Biol Chem. 2002;277:639–44. doi: 10.1074/jbc.M109037200. [DOI] [PubMed] [Google Scholar]
- 9.Antunez MI, Cardoni RL. IL-12 and IFN-gamma production, and NK cell activity, in acute and chronic experimental Trypanosoma cruzi infections. Immunol Lett. 2000;71:103–9. doi: 10.1016/s0165-2478(99)00172-8. [DOI] [PubMed] [Google Scholar]
- 10.Frosch S, Kraus S, Fleischer B. Trypanosoma cruzi is a potent inducer of interleukin-12 production in macrophages. Med Microbiol Immunol (Berl) 1996;185:189–93. doi: 10.1007/s004300050030. [DOI] [PubMed] [Google Scholar]
- 11.Meyer Zum Buschenfelde C, Cramer S, Trumpfheller C, Fleischer B, Frosch S. Trypanosoma cruzi induces strong IL-12 and IL-18 gene expression in vivo: correlation with interferon-gamma (IFN-gamma) production. Clin Exp Immunol. 1997;110:378–85. doi: 10.1046/j.1365-2249.1997.4471463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardillo F, Cunha FQ, Tamashiro WM, Russo M, Garcia SB, Mengel J. NK1.1+ cells and T-cell activation in euthymic and thymectomized C57Bl/6 mice during acute Trypanosoma cruzi infection. Scand J Immunol. 2002;55:96–104. doi: 10.1046/j.1365-3083.2002.01034.x. [DOI] [PubMed] [Google Scholar]
- 13.Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–34. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green SJ, Meltzer MS, Hibbs JB, Jr, Nacy CA. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–83. [PubMed] [Google Scholar]
- 15.Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–7. [PubMed] [Google Scholar]
- 16.Khan IA, Schwartzman JD, Matsuura T, Kasper LH. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci USA. 1997;94:13955–60. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holscher C, Kohler G, Muller U, Mossmann H, Schaub GA, Brombacher F. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–15. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723–33. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckner FS, Verlinde CL, La Flamme AC, Van Voorhis WC. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–7. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrico F, Heremans H, Rivera MT, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–32. [PubMed] [Google Scholar]
- 21.Luder CG, Gross U, Lopes MF. Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite–host interactions. Trends Parasitol. 2001;17:480–6. doi: 10.1016/s1471-4922(01)02016-5. [DOI] [PubMed] [Google Scholar]
- 22.Arantes RM, Marche HH, Bahia MT, Cunha FQ, Rossi MA, Silva JS. Interferon-gamma-induced nitric oxide causes intrinsic intestinal denervation in Trypanosoma cruzi-infected mice. Am J Pathol. 2004;164:1361–8. doi: 10.1016/s0002-9440(10)63222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings KL, Tarleton RL. Inducible nitric oxide synthase is not essential for control of Trypanosoma cruzi infection in mice. Infect Immun. 2004;72:4081–9. doi: 10.1128/IAI.72.7.4081-4089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saeftel M, Fleischer B, Hoerauf A. Stage-dependent role of nitric oxide in control of Trypanosoma cruzi infection. Infect Immun. 2001;69:2252–9. doi: 10.1128/IAI.69.4.2252-2259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ascenzi P, Salvati L, Brunori M. Does myoglobin protect Trypanosoma cruzi from the antiparasitic effects of nitric oxide? FEBS Lett. 2001;501:103–5. doi: 10.1016/s0014-5793(01)02637-0. [DOI] [PubMed] [Google Scholar]
- 26.Lieke T, Graefe SE, Klauenberg U, Fleischer B, Jacobs T. NK cells contribute to the control of Trypanosoma cruzi infection by killing free parasites by perforin-independent mechanisms. Infect Immun. 2004;72:6817–25. doi: 10.1128/IAI.72.12.6817-6825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogdan C, Donhauser N, Doring R, Rollinghoff M, Diefenbach A, Rittig MG. Fibroblasts as host cells in latent leishmaniosis. J Exp Med. 2000;191:2121–30. doi: 10.1084/jem.191.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceravolo IP, Chaves AC, Bonjardim CA, Sibley D, Romanha AJ, Gazzinelli RT. Replication of Toxoplasma gondii, but not Trypanosoma cruzi, is regulated in human fibroblasts activated with gamma interferon: requirement of a functional JAK/STAT pathway. Infect Immun. 1999;67:2233–40. doi: 10.1128/iai.67.5.2233-2240.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos MA, Almeida IC, Takeuchi O, et al. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–23. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 30.Graefe SEB, Jacobs T, Gaworski I, Klauenberg U, Steeg C, Fleischer B. Interleukin-12 but not interleukin-18 is required for immunity to Trypanosoma cruzi in mice. Microbes Infect. 2003;5:833–9. doi: 10.1016/s1286-4579(03)00176-x. [DOI] [PubMed] [Google Scholar]