Abstract
Systemic lupus erythematosus (SLE) is characterized by a deviation of the immune system that involves T cell-dependent autoantibody production. The aim of this study was to investigate the role of co-stimulatory markers on T cells in this disease. Twenty-eight patients with SLE as defined by the American College of Rheumatology (ACR) criteria and 11 healthy controls were included into the study. Eleven patients had biopsy-proven lupus nephritis while 17 patients had no clinical evidence of lupus nephritis. Clinical disease activity was assessed according to the systemic lupus erythematosus disease index (SLEDAI). CD4+ T cell populations in the peripheral blood were analysed for the expression of co-stimulatory markers CD45RO, CD70, CD80, CD86, CD137, CD137L, CD134, CD152, CD154 and ICOS. SLE patients showed an increased frequency of peripheral CD4+ T cells expressing high levels of CD80, CD86 and CD134 compared to healthy controls (7·1 ± 1·5% versus 1·7 ± 0·9%; P < 0·005; 2·3 ± 0·4% versus 1·0 ± 0·2%; P = 0·008, 20·2 ± 2·0% versus 10·6 ± 1·9%; P < 0·005, respectively). Significantly higher levels of CD80 on CD4+ T cells were detected in SLE patients with lupus nephritis compared to patients without nephritis (11·9 ± 3·3% versus 4·0 ± 0·7%; P < 0·005). There was an increased presence of CD134+ CD4+ cells in SLE patients with lupus nephritis (27·5 ± 4·0%versus 15·5 ± 1·3%; P < 0·005). CD80 and CD134 expression was significantly correlated with SLEDAI (r = 0·42, P = 0·03; r = 0·56, P < 0·005). Co-stimulatory molecules on CD4+ T cells are associated with renal disease and disease activity in patients with systemic lupus erythematosus.
Keywords: CD134, costimulation, lupus nephritis, systemic lupus erythematosus, T cell
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disorder that is thought to involve disturbances in both innate and adaptive immune mechanisms, including complex interactions between T lymphocytes, B lymphocytes and other antigen-presenting cells (APCs). T cell activation requires not only the interaction between the T cell receptor and MHC/antigen complexes, but also co-stimulation provided by molecules expressed on APCs. In addition to the well-characterized T cell co-stimulatory molecules CD28 and CD152 which bind to CD80 and CD86 on APCs, several members of the tumour necrosis factor (TNF) superfamily, especially CD134 (OX40), CD137 (4–1BB) and CD154, have been shown to induce co-stimulatory signals upon binding to their cognate receptors [1,2]. CD134 (OX-40) and CD137 (4-1BB) are structurally distinct from CD28 and ICOS, and expressed on activated T cells. Foell et al. [3] could show suppressing effects of anti-CD137 treatment in terms of IgG production, immune complex formation in kidney and exacerbation of renal disease in mice. The relevance of co-stimulatory molecules for immune-mediated disease was investigated in several murine models resembling human SLE. Blockade of the CD28–CD80/CD86 interaction and the CD154/CD40 pathway resulted in amelioration of disease in these mouse models [4–6]. High levels of co-stimulatory molecules, especially CD80 and CD86 on B cells, were also found in human SLE. The expression of these markers correlated with disease activity as assessed by the SLEDAI score [7]. Surprisingly, CD80 and CD86, that are usually found on APCs, were also found on T cells of patients with SLE. However, the meaning of this finding for disease development and activity remained unclear in this study [8]. Furthermore, expression of CD134L has been shown to be up-regulated in proliferative lupus nephritis, suggesting a role for the CD134–CD134L pathway in its pathogenesis [9]. A co-stimulatory antagonist against CD154 has also been examined as a possible therapeutic approach in human SLE but this was not fruitful, as short-term administration of the anti-CD154 was correlated with life-threatening prothrombotic activity despite initial encouraging data in the serology and renal function of the patients [10,11].
However, literature about the clinical importance of novel markers such as CD134, CD137 and CD137Ligand, in particular, is rare. The main goal of our study was to elucidate the clinical importance of these markers in SLE against the background of in vitro and animal studies.
Furthermore, it was the aim of this study to look for a correlation of co-stimulatory molecules on T cells with disease activity and renal involvement in patients with SLE in order to identify possible new therapeutic targets for SLE.
Patients and methods
Twenty-eight patients (24 female, four male, mean age 43 ± 15 years, range 20–71) fulfilling at least four of the American College of Rheumatology (ACR) revised criteria for the diagnosis of SLE, 11 age-matched healthy controls (eight female, three male, mean age 41 ± 16 years, range 27–71) and 10 patients with rheumatoid arthritis (10 female, mean age 63·6 ± 3·7 years, range 52–85) who fulfilled the ACR criteria for rheumatoid arthritis (RA) were included into the study. The mean disease duration was 11 ± 8 years (range 4–28). Eleven patients had biopsy-proven lupus nephritis [World Health Organization (WHO) class II: two patients, class III: one patient, class IV: six patients, class V: two patients] while 17 patients had no clinical evidence of lupus nephritis (absence of proteinuria and/or glomerular haematuria). Clinical disease activity at the time of the study was assessed according to the systemic lupus erythematosus disease index (SLEDAI). Furthermore, a SLEDAI–N was created, which excludes the four renal parameters of the SLEDAI (urinary casts, haematuria, proteinuria and pyuria) to have a parameter of disease activity independent of renal involvement. Organ involvement and SLEDAI of individual patients is shown in Table 1. Twenty-three patients were treated with prednisone (15 ± 4 mg/day). In addition, patients were without (n = 12) or receiving a constant dose of immunomodulating drugs [n = 16, azathioprin (n = 4), leflunomide (n = 1), mycophenolate mofetil (n = 5), cyclosporin A (n = 2), hydroxychloroquinsulphate (n = 3) and a combination of azathioprin and hydroxychloroquinsulphate (n = 1)]. The study protocol was approved by the institutional review board. All patients gave informed consent for the participation in this study.
Table 1.
Anamnestic clinical features of the patients [leukopenia (LEU), thrombocytopenia (THR), arthritis (ART), skin involvement (SKI) and glomerulonephritis classification (GN class)]. The current disease activity at the time of measurement was assessed by systemic lupus erythematosus disease index (SLEDAI).
| Sex | Age | SLEDAI | LEU | THR | ART | SKI | ZNS | GN class |
|---|---|---|---|---|---|---|---|---|
| Female | 30 | 22 | – | – | + | + | + | V |
| Female | 42 | 6 | + | + | + | + | – | II |
| Female | 38 | 14 | – | – | – | + | – | IV |
| Female | 26 | 8 | + | – | + | + | – | III |
| Female | 26 | 6 | + | + | + | + | IV | |
| Female | 32 | 20 | + | – | + | + | – | V |
| Female | 50 | 24 | – | – | + | – | – | IV |
| Male | 21 | 16 | + | + | – | + | – | IV |
| Female | 49 | 10 | – | – | + | – | – | IV |
| Male | 38 | 16 | – | – | + | + | – | II |
| Female | 20 | 8 | – | – | + | + | – | IV |
| Female | 49 | 20 | – | – | + | + | + | – |
| Female | 54 | 2 | – | – | – | + | + | – |
| Female | 66 | 18 | – | – | – | + | + | – |
| Female | 44 | 6 | – | – | + | – | – | – |
| Female | 35 | 0 | – | + | + | – | – | – |
| Male | 41 | 4 | – | – | + | – | – | – |
| Female | 69 | 4 | – | – | + | + | – | – |
| Female | 26 | 8 | – | – | + | + | – | – |
| Female | 71 | 14 | + | – | + | + | – | – |
| Male | 56 | 4 | – | – | + | – | – | – |
| Female | 37 | 10 | – | + | – | + | – | – |
| Female | 33 | 8 | – | – | + | + | – | – |
| Female | 68 | 16 | – | – | + | + | – | – |
| Female | 35 | 0 | – | – | + | + | – | – |
| Female | 39 | 10 | – | – | + | – | – | – |
| Female | 63 | 8 | – | – | + | + | – | – |
| Female | 38 | 4 | – | – | + | – | – | – |
Flow cytometry
Expression of co-stimulatory molecules was measured on T cells. Phycoerythrin (PE) or fluorescein isothiocyanate (FITC) and peridin chlorophyll protein (PerCP)-labelled antibodies in phosphate-buffered saline (PBS; containing 2% bovine serum albumine and 0,1% sodium azide) were used for double-colour surface staining: CD45RO (mouse IgG2, FITC), CD70 (mouse IgG3, PE), CD80 (mouse IgG1, PE), CD86 (mouse IgG1, PE), CD134 (mouse IgG1, PE), CD137 (mouse IgG1, PE), CD137L. (mouse IgG1, PE), CD152 (mouse IgG2, PE), CD154 (mouse IgG1, PE), ICOS (mouse IgG1, PE), CD3 (PerCP), CD4 (PerCP), CD8 (PerCP), CD14 (PerCP). As well as MultiTest CD3/CD16+ 56/CD45/CD19, MultiTest CD3/CD8/CD45/CD4 (Becton Dickinson, Mountain View, CA, USA) were used for four-colour surface staining. Appropriate isotype controls were used (Becton Dickinson). Briefly, peripheral blood was stained with labelled monoclonal antibodies (mAbs) for 20 min at room temperature. The cell suspension was incubated with lysing reagent for 15 min in the dark and prepared as indicated. Analysis was performed with a fluorescence activated cell sorter (FACS)Calibur flow cytometer (Becton Dickinson).
Enzyme-linked immunosorbent assay (ELISA) for soluble CD134L in the serum
Concentrations of soluble CD134L (CD134Ligand) were measured in duplicate wells in serum of patients with SLE and healthy controls using Quantikine® human OX40 Ligand kit (R&D Systems, Wiesbaden, Germany), as recommended by the manufacturer. CD134L concentrations were expressed in pg/ml according to a standard curve.
Confocal laser scanning microscopy
Peripheral blood mononuclear cells were isolated from peripheral blood by centrifugation over Ficoll-Hypaque (Pharmacia Biotech, Buckinghamshire, UK). Lymphocytes were washed twice in PBS (Biosource, Rockville, MD, USA) and fixed in 1% paraformaldehyde (w/v) in PBS (pH 7·3) for 15 min. Samples were washed in PBS and blocked with 1% bovine serum albumin (BSA) in PBS to reduce unspecific antibody binding. Samples were stained for 45 min with mAb to ceramide (Alexis Biochemicals, San Diego, CA, USA), Samples were washed three times in PBS and stained for 45 min with cyanine 3·18 (Cy5)-coupled F(ab′)2 fragments of donkey anti-mouse IgM antibodies. Samples were washed three times in PBS and stained for 45 min with PE-CD134 (Becton Dickinson) and then again washed three times and stained with Cy3-coupled F(ab′) fragments of sheep anti-mouse IgG antibodies (all from Jackson ImmunoResearch, West Grove, PA, USA). Thereafter, cells were again washed three times in PBS and stained for 45 min with FITC-CD3 (Becton Dickinson). Cells were again washed and 10 µl pipetted on glass cover slips. The cover slips were embedded in Moviol and viewed with a Leica TCS NT scanning confocal microscope (Munich, Germany). Clustering was defined as one or several intense spots of fluorescence on the cell surface [12]. In each experiment, the presence or absence of clustering in samples of at least 200 cells was scored by two independent observers. The results are given as percentage of cells showing a cluster.
Statistics
All values are expressed as mean ± s.e.m. Significance for the differences between groups was determined using the Mann–Whitney U-test. Spearman’s rank correlation was applied for detecting correlation between different study parameters.
Results
Lymphocyte subsets
Lymphocyte subset distribution in the peripheral blood of SLE patients with lupus nephritis, SLE patients without lupus nephritis and healthy controls is shown in Table 2. SLE patients had a lower number of circulating lymphocytes compared to healthy controls. The absolute numbers of circulating CD3+ cells (851 ± 484 and 945 ± 546 versus 1511 ± 391 cells/µl; P = 0·003 and P = 0·02) and CD19+ cells (101 ± 91 and 136 ± 113 versus 305 ± 129 cells/µl; P = 0·003 and P = 0·005) in SLE patients with lupus nephritis and SLE patients without lupus nephritis were significantly lower compared to healthy blood donors. SLE patients with and without lupus nephritis had significantly decreased numbers of CD4+ T cells (432 ± 361 and 584 ± 367 versus 970 ± 262 cells/µl; P = 0·004 and P = 0·01) compared with healthy donors, while the numbers of circulating CD8+ T cells (396 ± 182 and 338 ± 228 versus 488 ± 188) were similar in all groups. Furthermore, numbers of circulating natural killer (NK) cells were significantly higher in healthy donors compared to SLE patients (267 ± 98 cells/µl versus 108 ± 64 cells/µl and versus 156 ± 108 cells/µl; P = 0·0008 and P = 0·03). There were no significant differences in the absolute number of all circulating lymphocyte subsets between SLE patients with and without lupus nephritis.
Table 2.
Lymphocyte subsets in the peripheral blood of SLE patients with and without nephritis and healthy controls.
| Lymphocyte subset | Healthy controls (n = 11) | SLE with N (n = 11) | SLE without N (n = 17) |
|---|---|---|---|
| CD3+ T cells | 1511 ± 391 | 851 ± 484** | 954 ± 546* |
| CD4+ T cells | 970 ± 262 | 432 ± 361** | 584 ± 367* |
| CD8+ T cells | 488 ± 188 | 396 ± 182 | 338 ± 228 |
| CD19+ B cells | 305 ± 129 | 101 ± 91** | 136 ± 113* |
| CD16/56+ NK cells | 267 ± 98 | 108 ± 64** | 156 ± 108* |
Results are given as absolute counts (cells/µl) and are expressed as mean ± s.d. P-values were determined by Mann–Whitney U-test(
P < 0·05;
P < 0005 compared to healthy controls; SLE patients with nephritis compared to SLE patients without nephritis); NK = natural killer cells.
Co-stimulatory molecules on T cells in the peripheral blood of patients with SLE
The percentage of CD45RO-, CD70-, CD80-, CD86-, CD134-, CD137-, CD152-, CD154- and ICOS-expressing lymphocytes was analysed in the peripheral blood of 28 patients with SLE, 10 patients with RA, 11 healthy controls and four control patients with renal insufficiency without immunological cause. There were no differences in expression of the markers investigated between control patients with renal insufficiency without immunological cause and healthy controls (data not shown).
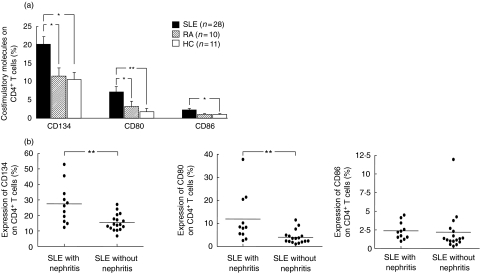
SLE patients showed an increased frequency of peripheral CD4+ T cells expressing high levels of CD80 and CD86 compared to healthy controls (CD80: 7·1 ± 1·5 versus 1·7 ± 0·9%; P < 0·005; CD86: 2·3 ± 0·4 versus 1·0 ± 0·2%; P = 0·008). The percentage of CD134+ CD4+ T cells observed in the SLE patients was significantly higher compared to healthy controls (20·2 ± 2·0 versus 10·6 ± 1·9%; P = 0·001) (Fig. 1a). The absolute numbers of circulating CD86+/CD4+ cells (8·4 ± 5·2, 8·9 ± 5·9 and 8·1 ± 5·5 cells/µl) and CD134+/CD4+ cells (104 ± 89, 88 ± 67 and 101 ± 60 cells/µl) in SLE patients with lupus nephritis, SLE patients without lupus nephritis and healthy blood donors were similar.
Fig. 1.
(a) Expression of co-stimulatory molecules on peripheral CD4+ T cells in patients with systemic lupus erythematosus (SLE) (n = 28), in patients with rheumatoid arthritis (n = 10) and healthy controls (n = 11). (b) Expression of co-stimulatory molecules on peripheral CD4+ T cells in SLE patients with (n = 11) and without nephritis (n = 17). The illustrated data are shown as mean values (± s.e.m.). Significant differences by the Mann–Whitney U-test are indicated: *P < 0·05; **P < 0·005.
There were no significant differences in the expression levels of CD137 and CD152 on CD4+ T cells between SLE patients and healthy controls. There was a tendency for increased CD70 expression in SLE patients compared to RA (6·2 ± 2·3% versus 3·5 ± 0·6%). ICOS and CD154 expression were not different in controls and a subgroup of our SLE patient cohort (n = 6) (1·6 ± 0·6% and 0·9 ± 0·3%versus 2·2 ± 0·6% and 1·7 ± 0·8%). Moreover, there were no differences in the expression levels of CD45RO on CD4+ T cells between SLE patients, 54·4 ± 5·0% versus 52·4 ± 3·2% (RA) versus 58·2 ± 7·0% (controls). However, almost all CD134+, CD80+, CD86+ CD4+ T cells (> 95% each) were also CD45RO+.
The amount of CD86+ CD8+ T cells in the peripheral blood of SLE patients was significantly higher compared to healthy controls (2·8 ± 0·4 versus 1·0 ± 0·3%; P < 0·05), as were the levels of circulating CD80+ CD8+ T cells (1·2 ± 0·5 versus 0·2 ± 0·1%; P < 0·005). There were no significant differences in the expression levels of CD134, CD137 and CD152 on CD8+ T cells between SLE patients and healthy controls.
Expression of co-stimulatory molecules on T-cells in the course of disease and in dependence of the immunosuppressive therapy
The expression of CD80, CD86 and CD134 was stable in six SLE patients with a stable course of disease (CD80: 12·1 ± 6·3% versus 11·4% ± 5·5%, CD86: 2·8 ± 0·7% versus 2·7 ± 0·9% and CD134: 23·6 ± 6·6% versus 22·5 ± 6·7%). The time-point of the second measurement (visit 2) varied from 3 to 10 months (Fig. 2).
Fig. 2.
Expression of CD80, CD86 and CD134 on CD4+ T cells in six systemic lupus erythematosus (SLE) patients in the course of time is illustrated. The horizontal bars represent the mean percentage of CD134/CD4+ T cells.
The 12 patients not taking additional immunosuppressants (prednisone + versus prednisone alone) showed no significant changes in the expression levels of CD80 (5·79 ± 1·3% versus 8·9 ± 3·2%), CD86 (2·31 ± 0·7% versus 2·21 ± 0·5%) and CD134 (19·3 ± 1·8% versus 21·4 ± 4·4%) compared to the 16 patients taking additional immunosuppressants.
Expression of CD80+ and CD134+ on CD4+ T cells with and without renal disease
Eleven of 28 patients had biopsy-proven lupus nephritis. Significantly higher levels of CD80 on CD4+ T cells were detected in the peripheral blood of SLE patients with lupus nephritis compared to patients without renal involvement (11·9 ± 3·4 versus 4·0 ± 0·8%; P < 0·005). Furthermore, there was an increased presence of CD134+ CD4+ cells in the peripheral blood of SLE patients with lupus nephritis (27·5 ± 4·0 versus 15·5 ± 1·3%; P < 0·005) (Fig. 1b). There was no correlation between renal function expressed as serum creatinine and expression of the markers on the CD4+ T cells. There were no significant differences between patients with WHO class IV and with other WHO classes of nephritis concerning the expression of CD134 and CD80 on CD4+ T cells (CD134: 27·6 ± 13·2 versus 27·4 ± 13·9%; P = 1; CD80: 14·5 ± 12·9 versus 8·8 ± 7·0%; P = 0·4). There were no significant differences between the mean percentages of CD86, CD137 and CD152 expression levels on CD4+ cells in SLE patients with and without lupus nephritis. In contrast to the CD4+ T cell subsets there were no significant differences between SLE patients with and without lupus nephritis in the expression of co-stimulatory molecules on CD8+ T cells (Fig. 1b).
Disease activity and expression of co-stimulatory molecules
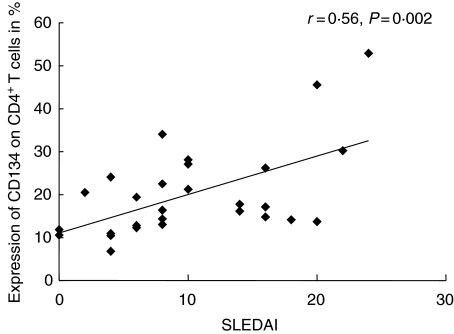
Disease activity as assessed by SLEDAI was correlated with the percentage of co-stimulatory molecules on CD4+ T cells: CD134 expression (r = 0·56, P < 0·005) as well as CD80+ expression (r = 0·42, P = 0·03) correlated significantly with the SLEDAI (Fig. 3). CD134 expression on CD4+ T cells also correlated significantly with the SLEDAI–N (r = 0·5, P = 0·007), whereas CD86 expression did not correlate with the SLEDAI–N (r = 0·1, P = 0·5). No correlation was found between disease activity and CD137+ or CD152+ expression on CD4+ T cells or expression levels of co-stimulatory markers on CD8+ T cells.
Fig. 3.
Correlation between the percentage of CD134 expressing CD4+ T cells and disease activity as measured by systemic lupus erythematosus disease index score analysed for all samples taken (n = 28).
Clustering of CD134 on CD4+ T cells in confocal microscopy
Confocal microscopy was used to study clustering of CD134 on CD4+ cells in two SLE patients with active renal disease and five SLE patients without active renal disease. The organization of activation molecules into clusters or lipid rafts has been described to be vital to T cell activation pathways [12]. The degree of CD134 clustering was more pronounced in the patients with active renal disease (32% and 35% versus 7%, range 1–15%, of T cells were clustering for CD134). The formation and clustering of CD134 in the membrane of CD3+ lymphocytes is shown in Fig. 4.
Fig. 4.
CD134 clustering was determined by fluorescence microscopy analysing 200 cells/sample. The representative microscopy images show T lymphocytes of patients with active lupus nephritis (a) versus T lymphocytes of patients without active lupus nephritis (b).
Measurement of soluble CD134L in the serum
As the presence of CD134L has been demonstrated within the glomerular capillary wall in proliferative lupus nephritis, we further investigated the CD134/CD134L interaction by measuring soluble CD134L by ELISA [9]. Twenty-seven samples were analysed, including eight from patients with biopsy-proven nephritis. The soluble CD134L concentration in the serum of SLE patients was not significantly different to healthy control subjects (33·9 ± 3·7 versus 27·6 ± 12·6 pg/ml, P > 0·05).
Discussion
The data from this study indicate that the expression of CD80, CD86 and CD134 on peripheral CD4+ T cells is associated with nephritis and increased disease activity in patients with SLE. This suggests that these T cell populations play a pivotal role in the pathogenesis of this disease. While a stimulatory effect of CD134 ligation on T cell function is well characterized, the meaning of CD80 and CD86 expression on T cells is not yet defined. CD134+ CD4 T cells could be involved in the inflammatory process of lupus nephritis by their engagement with CD134L, which is present in glomerular endothelium in almost all cases of proliferative lupus nephritis [9]. Secretion of soluble CD134L by glomerular endothelial cells or APCs with subsequent activation of T cells via CD134 is not likely, as soluble CD134L was not detectable in patients’ sera. Furthermore, interaction between CD134+ lymphocytes and CD134L expressed on endothelial cells has been demonstrated in an in-vitro study using human leukaemic T cells [13].
Numerous abnormalities in T cell function have been described in SLE patients and one common feature appears to be a reduced threshold for T cell activation. Recently, Han et al. described an increased prevalence of activated CD70+ CD4+ cells in SLE in similar amounts as in our study. They pointed out the complementary signalling of CD27/70 to CD40/CD154 in the co-stimulatory cascade. Provision of co-stimulatory signals may be responsible for the heightened sensitivity or prolonged response to TCR activation seen in T lymphocytes from patients with SLE. The data shown in this study show an up-regulation of OX40 on T cells and the microscopic finding that this OX40 receptor tends to appear in a more clustered form on these T cells. This indicates its activity, because oganization of signalling molecules into discrete membrane associated microdomains is vital for the regulation of T lymphocyte activation pathways [14]. A recent publication focused on the cross-linking of 4-1BB, which activates TCR-signalling pathways in CD8+ T lymphocytes [15]. We assume a similar importance for OX40 cross-linking.
There are two hypothetical ways in which CD134 CD4 T cells could mediate lupus nephritis. These cells could provide help to B cells producing anti-dsDNA antibodies and also to other antibodies that may contribute to the kidney lesions [16,17]. Secondly, it is also possible that the CD134+ T cell populations infiltrate glomerular endothelial cells after ligation with CD134L and cause direct damage. The role of CD134 for effector functions has been shown by the observation that treatment with a stimulatory anti-CD134 antibody enhances T cell expansion and differentiation to effector cells in mice. This evidently promoted the secretion of IFN-γ and the upregulation of various interleukin (IL)-receptors with subsequent cytokine-mediated kidney cell damage [18].
The functional role of CD80 and CD86 expression on CD4 T cells observed in our patients appears less clear. Most other reports have focused on the expression of CD80 and CD86 on antigen-presenting cells. Bijl et al. reported a correlation of CD86+ B cells and disease activity as measured by SLEDAI score and autoantibody production [7]. According to our data, Abe et al. have also detected increased levels of CD80 and CD86 on T cells, but did not show an association with disease activity or renal manifestation [8]. It is possible that CD80 and CD86-positive T cells could become independent from co-stimulation of antigen-presenting cells leading to a self-enhancing loop of T cell activation.
Blocking co-stimulatory molecules, such as the CD28–CD80/CD86 interaction and the CD154/CD40 pathway is an established therapeutic approach to alleviate Ig secretion, autoantibody production and disease activity [11,19,20]. Blocking of CD80 and CD86 could not only decrease B cell activity but could also turn down T cell function. In addition, blocking other pathways such as CD134/CD134L has to be discussed as a possible therapeutic strategy in the future. Furthermore, measurements of these co-stimulatory molecules could be useful for predicting disease activity or recurrence of disease. Other markers, such as ds-DNA titres or complement measurements, are not very reliable tools for this purpose. This issue, however, needs to be investigated in a longitudinal study with a larger patient population.
In conclusion, our data indicate that the expression of co-stimulatory molecules on CD4+ T cells is associated with renal disease and disease activity in patients with systemic lupus erythematosus. Targeting these (i.e. CD134) could be a new therapeutic approach in patients with lupus nephritis.
Acknowledgments
We thank Ute Schmücker for expert technical assistance with flow cytometry. This work was supported by the Werner-Jackstaedt Foundation to S. Patschan.
References
- 1.Watts TH, DeBenedette MA. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–93. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 2.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors. co-stimulation from CD28, inducible co-stimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–13. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 3.Foell J, Strahotin S, O’Neil SP, et al. CD137 co-stimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003;111:1505–18. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang B, Kashgarian MJ, Sharpe AH, et al. Autoantibody responses and pathology regulated by B7-1 and B7-2 co-stimulation in MRL/lpr lupus. J Immunol. 2000;165:3436–43. doi: 10.4049/jimmunol.165.6.3436. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita K, Tesch G, Schwarting A, et al. Costimulation by B7-1 and B7-2 is required for autoimmune disease in MRL-Faslpr mice. J Immunol. 2000;164:6046–56. doi: 10.4049/jimmunol.164.11.6046. [DOI] [PubMed] [Google Scholar]
- 6.Daikh DI, Finck BK, Linsley PS, et al. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 co-stimulation pathways. J Immunol. 1997;159:3104–8. [PubMed] [Google Scholar]
- 7.Bijl M, Horst G, Limburg PC, et al. Expression of co-stimulatory molecules on peripheral blood lymphocytes of patients with systemic lupus erythematosus. Ann Rheum Dis. 2001;60:523–6. doi: 10.1136/ard.60.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe K, Takasaki Y, Ushiyama C, et al. Expression of CD80 and CD86 on peripheral blood T lymphocytes in patients with systemic lupus erythematosus. J Clin Immunol. 1999;19:58–66. doi: 10.1023/a:1020566618980. [DOI] [PubMed] [Google Scholar]
- 9.Aten J, Roos A, Claessen N, et al. Strong and selective glomerular localization of CD134 ligand and TNF receptor-1 in proliferative lupus nephritis. J Am Soc Nephrol. 2000;11:1426–38. doi: 10.1681/ASN.V1181426. [DOI] [PubMed] [Google Scholar]
- 10.Kalunian KC, Davis JC, Jr, Merrill JT, et al. Treatment of systemic lupus erythematosus by inhibition of T cell co-stimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:3251–8. doi: 10.1002/art.10681. [DOI] [PubMed] [Google Scholar]
- 11.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–27. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 12.Grassme H, Jendrossek V, Riehle A, et al. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–30. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 13.Imura A, Hori T, Imada K, et al. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89:2951–8. [PubMed] [Google Scholar]
- 14.Jury EC, Kabouridis PS. T-lymphocyte signalling in systemic lupus erythematosus: a lipid raft perspective. Lupus. 2004;13:413–22. doi: 10.1191/0961203304lu1045rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nam KO, Kang H, Shin SM, et al. Cross-linking of 4-1BB activates TCR-signaling pathways in CD8+ T lymphocytes. J Immunol. 2005;174:1898–905. doi: 10.4049/jimmunol.174.4.1898. [DOI] [PubMed] [Google Scholar]
- 16.Putterman C. New approaches to the renal pathogenicity of anti-DNA antibodies in systemic lupus erythematosus. Autoimmun Rev. 2004;3:7–11. doi: 10.1016/S1568-9972(03)00082-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z, Weinstein E, Tuzova M, et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52:522–30. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 18.Lathrop SK, Huddleston CA, Dullforce PA, et al. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–43. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 19.Grammer AC, Slota R, Fischer R, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154–CD40 interactions. J Clin Invest. 2003;112:1506–20. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toubi E, Shoenfeld Y. The role of CD40–CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37:457–64. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]