Abstract
Immunosuppression induced by the human immunodeficiency virus (HIV-1) increases the risk of death. We measured the influence of immunological and virological factors and the type of highly active anti-retroviral therapy (HAART) on this risk. Adaptive (lymphocyte) and innate (natural killer) immune correlates and maximum HIV viral loads were assessed for association with mortality using univariate and multivariate analyses. The protective effect of HAART regimens, containing protease inhibitors (PI) and/or non-nucleoside reverse transcriptase inhibitors (NNRTI) on mortality were also examined in a prospectively recorded cohort of 9621 HIV-infected individuals. From this entire cohort, 5873 HIV infected individuals (61%) have been followed-up in the HAART era and of these 499 (8·5%) have died. In multivariate analyses, CD4 counts below the 50th centile and CD8 and CD19 counts below the 25th centile were significantly associated with mortality, as was increased age (P < 0·001). Innate immune subset levels had no effect on mortality. A maximum HIV viral load greater than the 75th centile was also associated independently with mortality (P < 0·035). Exposure to either a PI or an NNRTI-containing HAART regimen, or both together, was protective against death compared with no anti-retrovirals (P < 0·001). Effective HAART-induced maintenance of the adaptive immune system (CD4, CD8 and CD19 counts) protects from HIV-related mortality.
Keywords: AIDS, death, HAART, HIV, immune, mortality
Introduction
In those for whom it is available, highly active anti-retroviral therapy (HAART), consisting of at least three drugs, has decreased the morbidity and mortality associated with HIV, mainly by reducing the incidence of opportunistic infections such as Pneumocystis carinii pneumonia [1–5]. The protective effect of HAART has also been shown to result in a decreased incidence of HIV-associated cancers, including an increased time to disease progression [6,7]. These improvements have largely been explained as related to an improvement in immune function, by virtue of reconstitution of the CD4 repertoire [8–10]. Consistent with these immunological data, large studies in both the pre-HAART and HAART eras have demonstrated that the baseline or nadir CD4 count is associated strongly with probability of progression to AIDS or death [11–13]. Data concerning the effects of cytotoxic T cell (CD8), B cell (CD19) and natural killer (CD16/56) counts in this setting are lacking.
There are many options for anti-retroviral therapy for infection with HIV-1, the cause of AIDS. Current standards of care include using two nucleoside reverse-transcriptase inhibitors (NRTIs; nucleoside analogues) plus a third agent that is either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor [14,15]. Regimens should ideally be well tolerated, provide durable and potent viral suppression and preserve future treatment options [16]. In multivariate analyses, we have demonstrated previously that both PI and NNRTI-containing regimens are equally protective against AIDS-defining illnesses such as Kaposi's sarcoma [17] and non-Hodgkin's lymphoma [18]. In addition, we have shown that decreases in adaptive immune parameters (T and B cell counts) predispose individuals to these diseases and increases confer protection [19]. Innate markers (natural killer counts) appear to have a less significant role in established disease [20].
We therefore wished to establish whether adaptive and innate immune parameters may protect or predispose HIV-1 infected individuals to death. In addition, we examined whether NNRTI- or PI-based HAART conferred increased protection against all causes of HIV-related mortality.
Methods
The Chelsea and Westminster HIV cohort is one of the largest single-centre cohorts in Europe and we prospectively collect routine data on the individuals who attend. HIV positive patients are seen at regular intervals for clinical assessment, trial follow-up and immunological assessments. All HIV patients who have attended the Chelsea and Westminster since routine prospective data collection commenced in 1983 were identified, and we have defined HAART as therapy consisting of at least three anti-retroviral drugs in accordance with published guidelines (dual nucleoside analogues alone are not considered HAART, although this is considered a ‘backbone’ of therapy) [15]. This study focuses on a cohort who have continued to be followed-up since the HAART era commenced, which we have defined as 1 January 1996, when HAART became routinely available at our institution and many others. All individuals who ceased follow-up before this date were excluded; appropriate ethical approval was obtained.
Person-days of follow-up were converted to person-years at risk (PYAR). PYAR was estimated from entry into the cohort to either the end of the study period or the development of death. In order to keep the coefficient of the PYAR constant, this was log-transformed and used as the offset in the Poisson regression. These data were analysed using the genmod procedure in sas version 8·0 with loge link and Poisson error distributions. This fits generalized linear models, allowing time-dependent measures of probability; all P-values presented are two-sided. The nadir immunological cell counts used were the lowest ever recorded during follow-up until the time of death. If a patient had died, then the lowest ever cell counts prior to death diagnosis were used, otherwise lowest ever observed at the time the data were censored.
Median and interquartile ranges were used to create categorical data. A separate category was created for all variables with missing data. This ensured that no degrees of freedom were lost when building multivariate models. Log-linear models with single variables were used initially to estimate rate ratio of death, intrinsically corrected for the length of time diagnosed with HIV. All variables found to approach significance (P < 0·2) in the univariate log-linear model were then used to build a multivariate model, which allowed the risk of a particular prognostic variable to be assessed while controlling for the others in the model. The final multivariate model presented was tested for its distributional assumptions using Cox Snell residual plots and adjusted for possible confounding or residual effects.
Total lymphocyte and subset analysis was performed using whole blood stained with murine anti-human monoclonal antibodies to CD4 (T helper cells), CD8 (a cytotoxic T cell marker), CD19 (B cells) and CD16/56 (natural killer cells) (TetraOne, Beckman Coulter®, High Wycombe, UK) and were evaluated on an Epics XL-MCL (Beckman Coulter®) multi-parametric four-colour flow cytometer. Plasma viral loads (Quantiplex HIV RNA 3·0, Chiron, Halstead, UK) have been recorded since 1998 with a lower limit of detection of 50 copies/ml.
Results
Univariate analysis
Patients and demographic data
Clinical information on a total of 9621 HIV-1 seropositive patients has been collected prospectively since 1983 and of these, 5873 patients have been observed in the HAART era, comprising 61% of the total cohort. In the HAART era, a total of 499 individuals have died. The male : female ratio was approximately 7 : 1 and females had a significantly higher mortality rate, data confounded by an unequal ratio between genders (Table 1). There were also significant differences between ethnic origin groups although, once again, this demographic information has a large imbalance in numbers between groups. Patient age at entry to the cohort was, however, found to be significant and for each year's increase in age, the likelihood of mortality increased by 3% (P < 0·001).
Table 1.
Univariate log linear regression model showing rate ratio of mortality rate. We selected all human immunodeficiency virus (HIV) cases who have been followed-up and are part of the Chelsea and Westminster cohort during the highly active anti-retroviral therapy (HAART) era, defined as since 1 January 1996. Incidence during the HAART era according to demographic and immunological information. All cell counts were measured in cells/mm3.
| Variable | Total no. of HIV patients at risk during the HAART era | No. who died n (%) | Mortality rate¶ per 1000 patient-years | Rate ratio¶ | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Demographic information | ||||||
| Sex | ||||||
| Female | 770 | 54 (7·0) | 17·6 | 1·29 | (1·14–1·47) | < 0·001 |
| Male | 5103 | 445 (8·7) | 13/6 | 1* | ||
| Ethnic origin | ||||||
| Caucasian | 2556 | 329 (12·9) | 39·00 | 1·42 | (1·20–1·70) | < 0·001 |
| Bl African | 353 | 33 (9·4) | 17·82 | 1·09 | (0·95–1·25) | |
| Other | 554 | 87 (15·7) | 8·49 | 0·52 | (0·47–0·58) | |
| Not available | 2410 | 50 (2·1) | 16·39 | 1·00 | ||
| Age at entry (years) | 35·8 (10·6) | 1·03† | (1·029–1·039) | < 0·001 | ||
| Immunological information | ||||||
| Nadir CD4 T helper count | ||||||
| Missing | 205 | 12 (5·9) | 101·17 | 10·49 | (8·12–13·54) | < 0·001 |
| ≤ 14 | 1432 | 257 (18·0 | 18·02 | 1·87 | (1·62–2·16) | |
| 15–143 | 1406 | 164 (11·7) | 14·55 | 1·51 | (1·30–1·75) | |
| 144–289 | 1422 | 45 (3·2) | 9·65 | 0·84 | ||
| > 289 | 1408 | 21 (1·5) | ||||
| Nadir CD16/56 natural killer cells | ||||||
| Missing | 1618 | 76 (4·7) | 23·95 | 2·39 | (2·05–2·77) | < 0·001 |
| < 17 | 1033 | 169 (16·4) | 15·08 | 1·50 | (1·30–1·74) | |
| 17–35 | 1091 | 101 (9·3) | 11·12 | 1·11 | (0·95–1·30) | |
| 36–70 | 1053 | 91 (8·6) | 10·03 | 1·13 | ||
| ≥ 71 | 1078 | 62 (5·8) | ||||
| Nadir CD19 B cell count | ||||||
| Missing | 1614 | 75 (4·7) | 23·78 | 2·92 | (2·48–3·43) | < 0·001 |
| < 39 | 1086 | 244 (22·5) | 18·89 | 2·32 | (1·99–2·70) | |
| 39–76 | 1085 | 90 (8·3) | 10·71 | 1·31 | (1·11–1·56) | |
| 77–130 | 1055 | 49 (4·6) | 7·87 | 0·97 | (0·80–1·16) | |
| ≥ 131 | 1033 | 41 (4·0) | 8·15 | 1 | ||
| Most recent CD8 T cell count | ||||||
| Missing | 1604 | 74 (4·6) | 23·69 | 3·44 | (2·93–4·03) | < 0·001 |
| 595 | 1109 | 269 (24·3) | 22·35 | 3·24 | (2·79–3·77) | |
| 596–845 | 1049 | 62 (5·9) | 8·66 | 1·26 | (1·05–1·50) | |
| 846–1159 | 1052 | 44 (4·2) | 7·00 | 1·02 | (0·84–1·22) | |
| ≥ 1160 | 1059 | 50 (4·7) | 6·89 | 1·00 | ||
| Virological information | ||||||
| Max VL (if dead then maximum VL pre-death) | ||||||
| Missing | 760 | 181 (23·8) | 35·30 | 2·91 | (2·55–3·33) | < 0·001 |
| < 14 860 | 1279 | 54 (4·2) | 10·99 | 0·91 | (0·78–1·05) | |
| 14 860–82 988 | 1278 | 56 (4·4) | 9·78 | 0·81 | (0·70–0·93) | |
| 82 989–86 177 | 1278 | 82 (6·4) | 10·00 | 0·83 | (0·72–0·95) | |
| > 286 177 | 1278 | 126 (9·9) | 12·12 | 1 | ||
| Anti-retroviral information | ||||||
| ARV class exposure | ||||||
| No ARV history | 2010 | 184 (9·2) | 24·3 | 1 | < 0·001 | |
| NA only | 341 | 63 (18·5) | 19·5 | 0·80 | (0·67–0·95) | |
| PI only | 782 | 88 (11·3) | 12·5 | 0·52 | (0·45–0·59) | |
| NNRTI only | 1359 | 56 (4·1) | 8·7 | 0·36 | (0·31–0·41) | |
| PI + NNRTI | 1381 | 108 (7·8) | 8·3 | 0·34 | (0·30–0·38) |
Data for CD4 counts are shown in quartiles and above and below 200 cells/mm3. Person-years at risk (PYAR) was defined as the time from entry to the study as an HIV case until either (i) death occurred; or (ii) if not a dead case then date of death; or (iii) if not a dead case and alive during the study period, then the last ever date recorded. ARV = antiretroviral, NA = nucleoside analogue, PI = protease inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor, VL = viral load in RNA copies/ml. Missing data, the largest confounder in this cohort, are fewer with CD4 counts as these were measured routinely since 1996, when HAART became available. Routine HIV-1 viral load testing and prospective recording was introduced since 1998 and other lymphocyte subsets were recorded thereafter.
refers to the reference category. Person time at risk was used as a rate multiplier in the log linear model with Poisson error distribution. The rate ratio accounts for time to event.
Increase in mortality rate in years.
Immune parameters
In the univariate analysis (Table 1), we observed the expected results that lower T cell lymphocyte counts (both CD4 and CD8), B cell lymphocyte counts (CD19, adaptive immune markers), the CD16/56 natural killer counts (innate markers) and higher maximum HIV-1 viral loads significantly predisposed HIV-infected individuals to death (P < 0·001 in all cases). These data were consistent in that the lowest lymphocyte subset counts, in all cases, were associated with the highest mortality rate.
Anti-retroviral treatment
Receiving any anti-retroviral was significantly more protective against the development of death than not receiving therapy (Table 1). This included receiving nucleoside analogues alone, which are not considered HAART. There were no statistically significant differences between anti-retroviral classes; NNRTI-based therapy only (rate ratio 0·36, 95% CI 0·31–0·41) conferred an almost identical degree of protection to the combination of PI and NNRTI-based therapy together (RR 0·34, 95% CI 0·30–0·38). The rate ratio for PI-based therapy was significantly higher than NNRTI-based regimens and the combination of an NNRTI and PI together (RR 0·52, 95% CI 0·45–0·59).
Multivariate analysis
The multivariate analysis (Table 2) demonstrated the following: (i) age at entry to cohort remained significantly associated with mortality, ages > 39 having an increase in rate per year of increase in age (RR 1·03, 95% CI 1·02–1·03, P < 0·001); (ii) being female is associated with an increased mortality (RR 1·19, 95% CI 1·02–1·38, P = 0·028); (iii) T lymphocyte CD4 counts of <144 cells/mm3 (P = 0·001) and CD8 counts of less than 595 cells/mm3 (P = 0·001) were associated with an increased risk of death; (iv) nadir B cell CD19 counts < 39 cells/mm3 were associated with an increased risk of death (RR 1·41, 95% CI 1·18–1·67); and (v) decreased maximal HIV-1 viral loads, below the 75th centile of measured viraemia, was associated with protection from death (RR 0·85, 95% CI 0·73–0·98). When the parameters that approached significance were entered in a multivariate analysis, we found that low natural killer counts were not associated with death.
Table 2.
Multivariate log-linear regression model showing independent predictors of mortality rate during the highly active anti-retroviral therapy (HAART) era. As for the univariate analysis, nucleoside analogue (NA) alone-based therapy is not considered HAART, even though these patients received this treatment in the HAART era.
| Variable | Rate ratio¶ | 95% CI | Wald statistics P-value |
|---|---|---|---|
| Demographic information | |||
| Sex | |||
| Female | 1·19 | (1·02–1·38) | 0·028 |
| Male | 1 | ||
| Age at entry to cohort | |||
| > 39 years | 1·03* | (1·02–1·03) | < 0·001 |
| Immunological information | |||
| Nadir CD4 count (if dead then nadir prior to death) | |||
| Missing | 3·64 | (2·66–4·98) | < 0·001 |
| ≤ 14 | 1·93 | (1·62–2·30) | < 0·001 |
| 15–143 | 1·78 | (1·49–2·11) | < 0·001 |
| 144–289 | 1·14 | (0·95–1·36) | 0·162 |
| > 289 | 1 | ||
| Nadir CD19 count | |||
| Missing | 0·93 | (0·32–2·67) | 0·886 |
| < 39 | 1·37 | (1·15–1·63) | < 0·001 |
| 39–76 | 1·06 | (0·88–1·27) | 0·553 |
| 77–130 | 0·87 | (0·72–1·05) | 0·142 |
| > 131 | 1 | ||
| Most recent CD8 (if dead then CD8 pre-death) | |||
| Missing | 2·35 | (0·81–6·80) | 0·114 |
| 595 | 1·92 | (1·63–2·26) | < 0·001 |
| 596–845 | 1·10 | (0·92–1·32) | 0·303 |
| 846–1159 | 0·98 | (0·81–1·18) | 0·833 |
| > 1160 | 1 | ||
| Virological information | |||
| Max VL (if dead then maximum VL pre-death) | |||
| Missing | 1·31 | (1·10–1·56) | 0·002 |
| < 14860 | 0·84 | (0·71–0·99) | 0·034 |
| 14860–82988 | 0·82 | (0·70–0·96) | 0·014 |
| 82989–286177 | 0·85 | (0·73–0·98) | 0·030 |
| > 286177 | 1 | ||
| Anti-retroviral information | |||
| ARV class exposure (if dead then exposure pre-death otherwise at the time of censoring) | |||
| NA only | 0·68 | (0·56–0·82) | < 0·001 |
| PI only | 0·57 | (0·48–0·66) | < 0·001 |
| NNRTI only | 0·45 | (0·39–0·53) | < 0·001 |
| PI + NNRTI | 0·40 | (0·34–0·46) | < 0·001 |
| No ARV hist | 1 |
Adjusted for ethnic origin.
Increase in incidence of mortality per year increase in age.
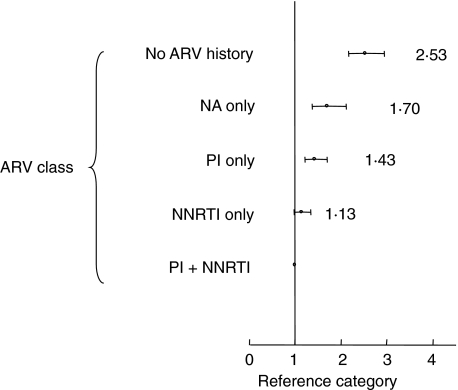
Importantly, NNRTI-based HAART and NNRTI and PI-based combinations were significantly more protective than nucleoside analogues alone (Fig. 1, using the NNRTI and PI combination as a reference category). PI-based regimens were more protective than no anti-retroviral therapy (P < 0·001). Due probably to missing data, the major (time-dependent) confounder in this cohort, PI-based HAART regimens were not significantly more protective than nucleoside analogues alone, although there are significant controlled data for PI-based regimens being better than two nucleoside analogues [21,22]. Interestingly, nucleoside alone-based regimens, such as the combination of stavudine and didanosine (which we have studied without use of a PI or NNRTI in a small randomized trial [23]) were more protective than no anti-retrovirals (RR 0·68, 95% CI 0·56–0·82, P < 0·001; Table 2).
Fig. 1.
Multivariate log-linear regression model showing rate ratio of mortality according to anti-retroviral therapy, using the combination of protease inhibitor (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) as the reference category. All values are point estimates; error bars 95% confidence intervals. ARV, anti-retroviral therapy; NA, nucleoside analogue; Adjusted for gender, age, viral load, nadir CD4, CD19 count and recent CD8 count.
The data from CD4, CD8 and CD19 counts in quartiles demonstrated statistical significance for the lowest counts, which led to an increased risk of death. The rate ratios here also provide significant evidence of a time-dependent effect, those individuals with lower nadir CD4 counts (RR = 1·93, 95% CI 1·62–2·3) dying more rapidly than those with increased nadir CD4 counts (RR = 1·0), results supported by database cohort studies [24].
Discussion
As we embark well into the third decade of the HIV pandemic, AIDS-related mortality remains a major focus of world attention, despite well over 40 million individuals living with HIV [4,25,26]. Anti-retroviral therapy for HIV infection decreases viraemia and increases CD4 cell counts [21,27] and most trials focus on these end-points, which are imperfect surrogates for clinical progression or death. In many studies, follow-up is restricted to less than 1 year and current treatments usually require life-long therapy [24,28]. While this is not a prospective randomized study, we show that HAART protects from death, and the data presented suggest that this occurs by increasing immune subset counts and decreasing viraemia. While HAART ‘maintains’ immunocompetence, this may occur in conjunction with viral suppression itself having a direct beneficial effect on adaptive and innate parameters.
The relative efficacies of PI and NNRTI containing HAART regimens in protecting against the development of systemic AIDS-related mortality have not been evaluated previously, although it is now appreciated that both are similarly effective at reducing HIV viraemia and maintaining CD4 counts [29,30]. Large randomized studies show few differences between regimens in terms of these end-points [31]. The multivariate analysis of our single-centre prospective cohort study demonstrates that HAART has clinically significant protective effects against mortality and NNRTI-based regimens as well as PI-based regimens are able to protect against this important end-point. Previously, only PI-based regimens have been shown to protect specifically against AIDS-defining illnesses [21,22]. Here, NNRTI- or PI-based HAART regimens and both in combination appear equally effective at protecting from death, and the combination of two anti-retroviral classes conferred significantly more protection than nucleoside analogues alone (a regimen that is not considered adequate HAART).
We confirmed as independent risk factors for mortality that increased age and low T lymphocyte counts, both CD4 and CD8, led to an increased incidence of death. Low CD19 B cell counts also predisposed individuals to death, reflecting the importance of the humoral immune system (interestingly, B cells were one of the first HIV reservoirs identified [32,33]). HIV leads to numerous perturbations of B cell function through elusive mechanisms. Microarray, phenotypic and functional analyses have shown that a number of interferon-associated genes associated with terminal differentiation were up-regulated in B cells of HIV-infected individuals, leading to decreased survival of these cells. In particular, increased expression of the tumour necrosis factor superfamily receptor CD95 correlated with increased susceptibility to CD95-mediated apoptosis of CD21(low) B cells which, in turn, correlated with plasma viraemia [34]. Other work has demonstrated that the memory B cell autocrine survival factor, nerve growth factor, is decreased in advanced HIV-1 infection, whereas the expression of Fas on B cells was increased [35].
Markers of the innate (CD16/56) immune system did not demonstrate significance when adjustments were made for other factors. Increased age is a risk factor and age-related immune dysfunction is well described [36,37]. Finally, we also showed that higher maximum HIV-1 viraemia was associated with all-cause mortality. In this respect, maximal viraemia greater than the 75th centile was associated significantly (for an HIV-1 viral load of > 286 177 copies/ml, RR 0·85, 95% CI 0·73–0·98, for a viral load of > 14 860 copies/ml, RR 0·82, 95% CI 0·7–0·96) with death. We suggest that not only does improved immune function lead to suppression of viraemia, also that reduced viral loads lead to increased immune reconstitution, both leading to a decrease in all causes of mortality.
These data have particular significance for the developing world where some anti-retrovirals or even classes of anti-retrovirals will be less available than others. Significantly, we show that a wide range of HAART appears protective against HIV-related mortality. This is achieved by maintaining adequate T and B lymphocyte counts, as opposed to natural killer counts which were not significant. Thus, the choice of anti-retrovirals can be considered to be ‘any effective HAART regimen’. Such information is important to patients and their carers, and is significant for an improved understanding of HIV and AIDS.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Katlama C, Johnson AM, et al. Lancet. 2000;356:291–6. doi: 10.1016/s0140-6736(00)02504-6. [DOI] [PubMed] [Google Scholar]
- 3.Lubis N, Baylis D, Short A, et al. Postgrad Med J. 2003;79:164–6. doi: 10.1136/pmj.79.929.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbrook R. N Engl J Med. 2004;351:739. doi: 10.1056/NEJMp048201. [DOI] [PubMed] [Google Scholar]
- 5.Thomas CF, Jr, Limper AH. N Engl J Med. 2004;350:2487–98. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 6.Bower M, Fox P, Fife K, Gill J, Nelson M, Gazzard B. AIDS. 1999;13:2105–11. doi: 10.1097/00002030-199910220-00014. [DOI] [PubMed] [Google Scholar]
- 7.International Collaboration on HIV and Cancer. J Natl Cancer Inst. 2000;92:1823–30. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 8.Douek DC, Brenchley JM, Betts MR, et al. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, Picker LJ, Koup RA. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 10.Jansen CA, De Cuyper IM, Steingrover R, et al. AIDS. 2005;19:1145–54. doi: 10.1097/01.aids.0000176214.17990.94. [DOI] [PubMed] [Google Scholar]
- 11.Mellors JW, Munoz A, Giorgi JV, et al. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet. 2000;355:1131–7. [PubMed] [Google Scholar]
- 13.Egger M, May M, Chene G, et al. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 14.Pozniak A, Gazzard B, Anderson J, et al. HIV Med. 2003;4(Suppl. 1):1–41. [PubMed] [Google Scholar]
- 15.Yeni PG, Hammer SM, Hirsch MS, et al. JAMA. 2004;292:251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 16.Clavel F, Hance AJ. N Engl J Med. 2004;350:1023–35. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 17.Portsmouth S, Stebbing J, Gill J, et al. AIDS. 2003;17:17–22. [Google Scholar]
- 18.Stebbing J, Gazzard B, Mandalia S, et al. J Clin Oncol. 2004;22:2177–83. doi: 10.1200/JCO.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 19.Stebbing J, Gazzard B, Newsom-Davis T, et al. Int J Cancer. 2004;108:473–4. doi: 10.1002/ijc.11601. [DOI] [PubMed] [Google Scholar]
- 20.Stebbing J, Gazzard B, Flore O, et al. AIDS. 2003;17:1998–2000. doi: 10.1097/01.aids.0000088168.01779.01. [DOI] [PubMed] [Google Scholar]
- 21.Hammer SM, Squires KE, Hughes MD, et al. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 22.Portsmouth S, Stebbing J, Gazzard B. Curr Top Med Chem. 2003;3:1458–66. doi: 10.2174/1568026033451808. [DOI] [PubMed] [Google Scholar]
- 23.Stebbing J, Nelson M, Orkin C, et al. J Antimicrob Chemother. 2004;53:501–5. doi: 10.1093/jac/dkh116. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, Hernan MA, Ledergerber B, et al. Lancet. 2005;366:378–84. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 25.HIV/AIDS: doing what's right. Lancet. 2005;365:1003. [Google Scholar]
- 26.Predicting the failure of 3 by 5. Lancet. 2005;365:1597. [PubMed] [Google Scholar]
- 27.Gulick RM, Ribaudo HJ, Shikuma CM, et al. N Engl J Med. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 28.Jordan R, Gold L, Cummins C, Hyde C. BMJ. 2002;324:757. doi: 10.1136/bmj.324.7340.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafer RW, Smeaton LM, Robbins GK, et al. N Engl J Med. 2003;349:2304–15. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez E, Arnaiz JA, Podzamczer D, et al. N Engl J Med. 2003;349:1036–46. doi: 10.1056/NEJMoa021589. [DOI] [PubMed] [Google Scholar]
- 31.Gallant JE, DeJesus E, Arribas JR, et al. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 32.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 33.Stebbing J, Gazzard B, Douek DC. N Engl J Med. 2004;350:1872–80. doi: 10.1056/NEJMra032395. [DOI] [PubMed] [Google Scholar]
- 34.Moir S, Malaspina A, Pickeral OK, et al. J Exp Med. 2004;200:587–99. [PubMed] [Google Scholar]
- 35.Titanji K, Nilsson A, Morch C, et al. Clin Exp Immunol. 2003;132:297–303. doi: 10.1046/j.1365-2249.2003.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalayjian RC, Landay A, Pollard RB, et al. J Infect Dis. 2003;187:1924–33. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 37.Alberti S, Cevenini E, Ostan R, et al. Mech Ageing Dev. 2006;127:560–6. doi: 10.1016/j.mad.2006.01.014. [DOI] [PubMed] [Google Scholar]