Abstract
Autoantibodies against C1q have been described in many immune-complex diseases including hypocomplementaemic urticarial vasculitis and systemic lupus erythematosus (SLE). No study has focused on the role of anti-C1q antibodies in hepatitis C virus (HCV) infection. The aim of this study was (i) to evaluate the prevalence of anti-C1q antibodies in HCV infection; and (ii) to analyse the association of anti-C1q antibodies with clinical and biological features of HCV–mixed cryoglobulinaemia (MC) vasculitis. We searched for anti-C1q antibodies using an enzyme-linked immunosorbent assay (ELISA) test in 111 HCV patients (75 had cryoglobulin and 23 systemic vasculitis), 60 SLE patients and 109 blood donors. Anti-C1q antibodies were detected in 26% of HCV patients compared to 10% of healthy donors (P < 0·01), and 38% in patients with SLE. Although there was a higher prevalence of anti-C1q antibodies among HCV patients with type III cryoglobulin (50%, P < 0·01), the overall prevalence of anti-C1q antibodies was similar in HCV patients being cryoglobulin-positive or cryoglobulin-negative (26% versus 25%, P = 0·98). A significant association was found between anti-C1q antibodies and low C4 fraction of complement (P < 0·05). No association was found between anti-C1q antibodies and HCV genotype, severity of liver disease or with specific clinical signs of HCV–MC vasculitis. This study shows an increased prevalence of anti-C1q antibodies in HCV-infected patients. Anti-C1q antibodies were associated with low C4 levels. No association was found between anti-C1q antibodies and HCV–MC vasculitis, nor between anti-C1q antibodies and cryoglobulinaemia.
Keywords: anti-C1q antibodies, autoantibodies, hepatitis C, mixed cryoglobulinaemia, vasculitis
Introduction
Hepatitis C virus (HCV) infection is a major cause of liver disease but is also associated with a spectrum of extrahepatic manifestations, mainly mixed cryoglobulinaemia (MC). MC may be asymptomatic or lead to clinical manifestations ranging from a MC syndrome (purpura, arthralgia, asthenia) to a more serious vasculitis with neurological and/or renal involvement [1]. HCV–MC is a systemic vasculitis characterized by the proliferation of B cell clones producing pathogenic IgM with rheumatoid factor (RF) activity. Although MC are found in 30–50% of patients with chronic hepatitis C, only 10–15% of them will develop symptomatic MC [2].
Autoantibodies against a variety of self-antigens can be detected in the sera of patients with HCV infection. C1q is the first component of the classical pathway of complement activation and its main function is to clear immune complexes from tissues and self-antigens generated during apoptosis [3]. Anti-C1q antibodies are associated strongly with immune complex diseases, most prominently with hypocomplementaemic urticarial vasculitis syndrome, systemic lupus erythematosus (SLE), diffuse proliferative lupus nephritis and severe rheumatoid arthritis [4].
The pathogenesis of HCV–MC vasculitis is complex and is likely to involve many mechanisms. The Arthus phenomenon and its equivalents are considered useful experimental models for immune complex (IC) vasculitis and MC. Effective IC clearance is achieved via the mononuclear phagocytic system and the classical pathway of the complement system. Defects in one of the mechanisms of IC clearance may give rise to immune complex disease. To date, there are no data regarding the prevalence of anti-C1q in patients with HCV chronic infection and their potential association with HCV–MC vasculitis.
The aim of this study was (i) to evaluate the prevalence of anti-C1q antibodies in HCV infection; and (ii) to analyse the association of anti-C1q antibodies with clinical and biological features of HCV-related systemic vasculitis.
Patients and methods
Study population
The study population included 111 patients with HCV chronic infection, all positive for anti-HCV antibodies and serum HCV–RNA [60 (54%) female, mean age 61 ± 12 years)], 75 of whom had detectable cryoglobulin [type II (n = 61), type III (n = 14)] and 24 systemic vasculitis [mean age 66 ± 10 years, clinical manifestations included: purpura (n = 16), peripheral neuropathy (n = 13), arthralgia (n = 12) and glomerulonephritis (n = 2)]; 60 patients with SLE (mean age 48 ± 15 years) (fulfilling at least four of 11 American College of Rheumatology criteria for SLE diagnosis [5]; and 109 blood donors (mean age 52 ± 11 years) (Table 1). Collection of samples occurred after ethical committee approval and appropriate patient consent. All plasma samples were aliquoted and kept at − 80 ° C until further analysis.
Table 1.
Patients data and laboratory parameters*.
| Group | Patients (n) | Age (years) (mean ± SD) | AntiC1q prevalence (%, n) | AntiC1q levels (IU/ml) |
|---|---|---|---|---|
| Healthy Donors | 109 | 52 ± 11 | 10% (11/109) | 60 ± 30 |
| HCV-infected patients | 111 | 61 ± 12 | 26% (29/111) | 83 ± 40 |
| MC positive | 75 | 62 ± 11 | 26% (20/75) | 86 ± 44 |
| Type II MC | 61 | 63 ± 14 | 21% (13/61) | 65 ± 17 |
| Type III MC | 14 | 60 ± 10 | 50% (7/14) | 107 ± 53 |
| Low C4 level | 28 | 64 ± 12 | 43% (12/28) | 137 ± 45 |
| MC negative | 36 | 58 ± 14 | 25% (9/36) | 77 ± 49 |
| Systemic vasculitis | 24 | 66 ± 10 | 21% (5/24) | 75 ± 33 |
| SLE | 60 | 48 ± 15 | 38% (23/60) | 295 ± 71 |
MC, mixed cryoglobulin; SLE, systemic lupus erythematosus; SD, standard deviation; IU/ml, international unit per milliliter.
Enzyme-linked immunosorbent assay (ELISA) for anti-C1q autoantibodies
Anti-C1q antibodies were determined using the method described by Siegert [6], as modified by Trendelenburg [7]. Briefly, ELISA wells (MaxisorpNunc Immuno plates, Roskilde, Denmark) were coated overnight with 1 µg/well of C1q (Calbiochem, La Jolla, CA, USA) in sodium hydrogen carbonate buffer, pH 9·6, at room temperature. After washing plates, 100 µl of the plasma diluted 1 : 25 in phosphate-buffered saline (PBS) 0·05% Tween containing 1% fetal calf serum (FCS) (PBSTwFCS) and 1 M NaCl were incubated for 1 h at 37°C. Bound IgG was detected using biotinylated mouse monoclonal anti-human IgG (1 : 10000) (Southern Biotechnology Associates, Bioreba AG, Reinach, Switzerland) diluted in PBSTwFCS and 1 M NaCl, and revealed with streptavidin–horseradish peroxidase (Jackson ImmunoResearch, Cambridge, UK). The C1q solid-phase assay (Calbiochem, La Jolla, CA, USA) has a purity of more than 95%.
Non-organ-specific (NOSA) antibody testing
Immunological factors included anti-nuclear antibodies (ANA), anti-liver kidney microsomes antibodies (LKM1), anti-smooth muscle antibodies (SMA), C3 and C4 fractions of complement, cryoglobulin and rheumatoid factor. Indirect immunofluorescence performed on HEp-2 cells was used for anti-nuclear antibody detection (BMD, Paris, France), with a positive result defined as > 1/80. Cytochrome CYP2D6 (liver–kidney microsomal type 1) autoantibodies were determined by radio ligand assay. Anti-smooth muscle cells were detected by indirect immunofluorescence using an unfixed 4 mm cryostat sections of rat liver, stomach and kidney.
Cryoglobulins were searched using a previously described technique [2], whereby they were isolated from the patient sera, purified and then characterized by immunoblotting at 37°C. Following the system of Brouet et al. [8], all positive patients had either type II or type III mixed cryoglobulins characterized, respectively, by the presence of a monoclonal or polyclonal rheumatoid factor component.
Virological and liver histological analysis
Virological factors included HCV viral load and HCV genotype. Histological features of analysed liver specimen belong to the METAVIR scoring system [9]. All HCV-infected patients had a liver biopsy. Liver biopsies more than 10 mm in length were fixed, paraffin-embedded and stained with at least haematoxylin–eosin safran and Masson’s trichrome or picrosirius red for collagen. For each liver biopsy, stage of fibrosis and grade of activity were established according to the following criteria. Liver biopsy was staged on a scale of 0–4 : 0 = no fibrosis, 1 = portal fibrosis without septa, 2 = few septa, 3 = numerous septa without cirrhosis and 4 = cirrhosis. This feature has been shown to be highly reproducible between pathologists. The grading of activity that evaluates the intensity of necroinflammatory lesions was indicated as follows: A0 = no histological activity, A1 = mild activity, A2 = moderate activity, and A3 = severe activity.
Statistical analysis
All quantitative data are expressed as mean ± standard deviation (s.d.). Univariate analysis used χ2 or Fisher’s exact test for comparisons of qualitative values, or the unpaired Student’s t-test for quantitative values. The nonparametric Mann–Whitney test was used for P-value calculation using GraphPad Prism version 3·0 for Macintosh (GraphPad, San Diego, CA, USA). Significance was assessed at P < 0·05.
Results
The overall prevalence of anti-C1q antibodies was higher in HCV-infected patients compared with blood donors [26% (29/111) versus 10% (11/109), respectively; P < 0·01)] (Table 1). Although there was a higher prevalence of anti-C1q antibodies among HCV patients with type III cryoglobulin (50%, P < 0·01), the overall prevalence of anti-C1q antibodies was similar in HCV patients being cryoglobulin-positive or cryoglobulin-negative (26% versus 25%, P = 0·98). There was a higher prevalence of anti-C1q antibodies among HCV-infected patients with low C4 levels [41% (12/28); P < 0·05)] (Table 1). There was no significant association between the presence of anti-C1q antibodies and age, gender or HCV genotype. HCV viral load did not differ significantly between patients with positive or negative anti-C1q antibodies (5·2 ± 0·5 versus 5·4 ± 0·7 log copies/ml, respectively). A significant association was found between anti-C1q antibodies and low C4 levels (P = 0·03) (Table 2). There was no relation between the severity of liver damage (i.e. cirrhosis) and the presence of anti-C1q antibodies in HCV-infected patients. Prevalence of NOSA in HCV chronically infected patients was distributed as follows: ANA 43% (23/53), SMA 8·5% (3/35) and LKM1 3% (1/33). There was no significant association between anti-C1q antibodies and NOSA (Table 2). We found no significant association between anti-C1q antibodies and the presence of specific clinical signs of HCV-related systemic vasculitis (Table 2).
Table 2.
Comparative analysis of HCV-infected patients with or without anti-C1q autoantibodies (C1q Ab)*.
| C1q Ab-positive (n=29) | C1q Ab-negative (n=82) | P (Chi-square) | |
|---|---|---|---|
| Age > 50 (n, %) | 12 (41) | 37 (45) | 0·2 |
| Female sex (n,%) | 17 (59) | 44 (54) | 0·5 |
| Cirrhosis (n, %) | 8 (27) | 19 (23) | 0·4 |
| ANA (n, %) | 9 (31) | 14 (17) | 0·07 |
| SMA (n,%) | 0 (0) | 3 (4) | 0·3 |
| LKM1 (n,%) | 1 (3) | 0 (0) | 0·08 |
| Cryoglobulin (n, %) | 20 (69) | 55 (67) | 0·2 |
| Rheumatoid Factor (n, %) | 7 (24) | 26 (32) | 0·2 |
| Low C4 level (n,%) | 12 (41) | 16 (19) | 0·03 |
| Low C3 level (n,%) | 4 (14) | 7 (9) | 0·08 |
| Systemic Vasculitis (n, %) | 5 (17) | 19 (23) | 0·4 |
| Peripheral neuropathy (n,%) | 4 (14) | 9 (11) | 0·99 |
| Skin purpura (n,%) | 0 (0) | 16 (19) | 0·003 |
| Arthralgia/arthritis (n,%) | 1 (3) | 11 (13) | 0·08 |
| Glomerulonephritis (n,%) | 0 (0) | 2 (2) | 0·4 |
ANA, anti-nuclear Ab; SMA, anti-smooth muscle Ab; LKM1, anti-liver kidney microsomes Ab;
C4, C4 fraction of complement.
Anti-C1q antibodies prevalence in SLE patients was, as expected, higher than in HCV-infected patients [38% (23/60) versus 26% (29/111), P < 0·01] and in the range described by others (34–47%) [10,11].
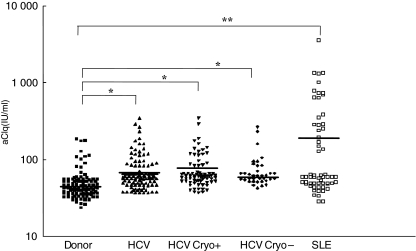
Anti-C1q antibodies titres (Fig. 1) in HCV-infected patients were significantly higher than those observed in healthy donors (mean titre: 83 ± 04 versus 60 ± 30 IU/ml, respectively, P < 0·01). Among HCV-infected patients, no significant difference was observed between cryoglobulin-positive, cryoglobulin-negative or systemic vasculitis groups (mean titre: 86 ± 44 versus 77 ± 49 versus 75 ± 33 IU/ml, respectively) (Fig. 1 and Table 1). Anti-C1q antibody titres were higher in HCV patients with low C4 levels compared to those with normal C4 (mean titre: 137 ± 45 versus 88 ± 33 IU/ml; P < 0·01).
Fig. 1.
C1q-antibodies levels in normal blood donors (Donors) (60 ± 30), in chronically hepatitis C virus-infected patients (HCV) (83 ± 40), in HCV-cryoglobulin positive patients (HCV Cryo+) (86 ± 44), in HCV-cryoglobulin negative patients (HCV Cryo–) (77 ± 49) and in systemic lupus erythematosus (SLE) patients (295 ± 71). *P < 0·01, **P < 0·001 compared to normal blood donors.
Discussion
Our study was designed to evaluate the prevalence of anti-C1q antibodies in HCV infection and to analyse the association of anti-C1q antibodies with clinical and biological features of HCV-related systemic vasculitis. The interaction between the core protein of HCV and the C1q receptor has been shown to suppress the T cell immune response which may have implications in HCV persistence [12]. C1q protein and C1q binding activity are enriched substantially in the cryoprecipitates of HCV-infected patients [13]. The wide expression of C1q receptor on the surface of blood cells and endothelial cells [14] favours their specific binding to immune complexes containing HCV core protein. Efficient engagement of the C1q protein by cryoglobulins may represent an important pathogenetic mechanism in the cryoglobulin-related pathway.
In the present study, we show an overall prevalence of 26% of anti-C1q antibodies in 111 chronically HCV-infected patients. This was significantly higher than the 10% found in healthy blood donors. The prevalence of anti-C1q antibodies was similar whether HCV-infected patients were cryoglobulin-positive or cryoglobulin-negative. Binding of immune complexes to undigested C1q is not abrogated uniformly by high salt [15]. Therefore, a cross-reactivity of cryoglobulins with the anti-C1q assay as used in our study cannot be excluded. However, we found no correlation between the occurrence of cryoglobulins and anti-C1q antibodies, suggesting that such a potential cross-reactivity was of no or only minor significance in our cohort. The lack of a correlation between the occurrence of cryoglobulins and anti-C1q is of interest, because IgM–IgG complexes as found in MC are good receptors for C1q [16]. The presence of cryoglobulin–C1q complexes should have facilitated the generation of autoantibodies against C1q as, at least in SLE patients, anti-C1q antibodies are directed against a neoepitope that is expressed on C1q only in its bound form [17]. However, the precise epitope recognized by anti-C1q antibodies in HCV-infected patients remains to be elucidated.
An important result of our study was the association of anti-C1q antibodies with low C4 fractions of complement. Among HCV–MC patients there was a significantly higher titre and prevalence of anti-C1q antibodies in those with low C4 levels. Hypocomplementaemia is an important finding in MC vasculitis and helps to distinguish this vasculitis from (normo- or hypercomplementaemic) anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides. Most HCV–MC patients showed decreased levels of the early complement components C1, C4 and C2, whereas C3 levels fluctuate with the disease course. Experiments performed to define the mechanism responsible for this complement profile showed that activation of the early complement components in serum was due to the activation of the classical pathway by mixed cryoglobulins [18].
Contrasting with that observed in SLE patients (another IC disease), anti-C1q antibodies were not associated with specific clinical signs of HCV–MC vasculitis. The level of anti-C1q antibodies in SLE patients was markedly higher than in HCV-infected patients. However, as anti-C1q antibodies have been reported to be associated predominantly with nephritis in SLE patients [4], one weakness of our study is that only two of our MC patients had glomerulonephritis. In SLE nephritis, anti-C1q antibodies affects patients not only by increasing the complement activation, but also potentially accelerates the development of anti-nuclear antibodies by interfering with C1q clearance functions [19]. In HCV–MC vasculitis, the defective immune complex clearance may involve preferentially the mononuclear phagocytic system. Cryoglobulinaemic nephritis usually had type II cryoglobulin with IgM kappa (IgM-κ) monoclonal component. The IgM-κ that has rheumatoid activity towards anti-HCV IgG forms mega-complexes that do not bind to the erythrocyte transport system [20], remaining free to circulate and saturate the phagocyte’s ability to remove immune-complexes from the blood. Phagocyte cell blockade is also favoured by HCV infection, which makes cells unable to digest cryoglobulins following phagocytosis [21].
A wide range of NOSA can be detected in HCV infection, the most frequent being ANA, anti-cardiolipin, anti-thyroglobulin and SMA antibodies found in 10–40% of patients [2]. However, no correlation was found between anti-C1q antibodies and NOSA. The occurrence of anti-C1q antibodies did not correlate with specific virological features (i.e. HCV genotypes, viral load or liver cirrhosis) of HCV infection. Such results contrast with a report on NOSA showing a correlation between autoantibody positivity and cirrhosis [22].
In conclusion, this study demonstrates for the first time an increased prevalence of anti-C1q antibodies in HCV-infected patients. Anti-C1q antibodies were associated with low C4 levels. No association, however, was found between anti-C1q antibodies and HCV–MC vasculitis, or between anti-C1q antibodies and cryoglobulinaemia.
Acknowledgments
This work was supported by the Swiss National Sciences Foundation 0032-66708-01.
References
- 1.Gorevic PD, Kassab HJ, Levo Y, et al. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med. 1980;69:287–308. doi: 10.1016/0002-9343(80)90390-3. [DOI] [PubMed] [Google Scholar]
- 2.Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients.The GERMIVIC Groupe d'Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l'Hepatite C. Medicine (Balt) 2000;79:47–56. doi: 10.1097/00005792-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 4.Seelen MA, Trouw LA, Daha MR. Diagnostic and prognostic significance of anti-C1q antibodies in systemic lupus erythematosus. Curr Opin Nephrol Hypertens. 2003;12:619–24. doi: 10.1097/00041552-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 6.Siegert CE, Daha MR, van der Voort EA, Breedveld FC. IgG and IgA antibodies to the collagen-like region of C1q in rheumatoid vasculitis. Arthritis Rheum. 1990;33:1646–54. doi: 10.1002/art.1780331107. [DOI] [PubMed] [Google Scholar]
- 7.Trendelenburg M, Marfurt J, Gerber I, Tyndall A, Schifferli JA. Lack of occurrence of severe lupus nephritis among anti-C1q autoantibody-negative patients. Arthritis Rheum. 1999;42:187–8. doi: 10.1002/1529-0131(199901)42:1<187::AID-ANR24>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775–88. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 9.Bedossa P, Poynard T for the French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 10.Siegert C, Daha M, Westedt ML, van der Voort E, Breedveld F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18:230–4. [PubMed] [Google Scholar]
- 11.Wener MH, Uwatoko S, Mannik M. Antibodies to the collagen-like region of C1q in sera of patients with autoimmune rheumatic diseases. Arthritis Rheum. 1989;32:544–51. doi: 10.1002/anr.1780320506. [DOI] [PubMed] [Google Scholar]
- 12.Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 2000;106:1239–49. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansonno D, Lauletta G, Nisi L, et al. Non-enveloped HCV core protein as constitutive antigen of cold-precipitable immune complexes in type II mixed cryoglobulinaemia. Clin Exp Immunol. 2003;133:275–82. doi: 10.1046/j.1365-2249.2003.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng X, Tonnesen MG, Peerschke EI, Ghebrehiwet B. Cooperation of C1q receptors and integrins in C1q-mediated endothelial cell adhesion and spreading. J Immunol. 2002;168:2441–8. doi: 10.4049/jimmunol.168.5.2441. [DOI] [PubMed] [Google Scholar]
- 15.Kohro-Kawata J, Wener MH, Mannik M. The effect of high salt concentration on detection of serum immune complexes and autoantibodies to C1q in patients with systemic lupus erythematosus. J Rheumatol. 2002;29:84–9. [PubMed] [Google Scholar]
- 16.Sansonno D, Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect Dis. 2005;5:227–36. doi: 10.1016/S1473-3099(05)70053-0. [DOI] [PubMed] [Google Scholar]
- 17.Golan MD, Burger R, Loos M. Conformational changes in C1q after binding to immune complexes: detection of neoantigens with monoclonal antibodies. J Immunol. 1982;129:445–7. [PubMed] [Google Scholar]
- 18.Haydey RP, Patarroyo de Rojas M, Gigli I. A newly described control mechanism of complement activation in patients with mixed cryoglobulinemia (cryoglobulins and complement) J Invest Dermatol. 1980;74:328–32. doi: 10.1111/1523-1747.ep12543575. [DOI] [PubMed] [Google Scholar]
- 19.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- 20.Roccatello D, Morsica G, Picciotto G, et al. Impaired hepatosplenic elimination of circulating cryoglobulins in patients with essential mixed cryoglobulinaemia and hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;110:9–14. doi: 10.1046/j.1365-2249.1997.4751383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roccatello D, Isidoro C, Mazzucco G, et al. Role of monocytes in cryoglobulinemia-associated nephritis. Kidney Int. 1993;43:1150–5. doi: 10.1038/ki.1993.161. [DOI] [PubMed] [Google Scholar]
- 22.Squadrito G, Previti M, Lenzi M, et al. High prevalence of non-organ-specific autoantibodies in hepatitis C virus-infected cirrhotic patients from southern Italy. Dig Dis Sci. 2003;48:349–53. doi: 10.1023/a:1021991813586. [DOI] [PubMed] [Google Scholar]