Abstract
(–)-Epigallocatechin-3-gallate (EGCG) is the major active component of green tea. Increasing evidence has suggested that EGCG exhibits anti-inflammatory, anti-oxidant and immunosuppressive effects. In this study, we investigated the effect of EGCG on concanavalin A (ConA)-induced hepatitis (CIH) in mice, a model of immune-mediated liver injury in humans. We pretreated mice with EGCG before ConA injection, and then measured alanine aminotransferase (ALT) levels in plasma, inflammatory infiltration and hepatocyte apoptosis in liver. Potential therapeutic mechanisms were elucidated further by measuring several inflammatory mediators. Mice pretreated with EGCG exhibited much less increased ALT levels in plasma, reduced inflammatory infiltration and hepatocyte apoptosis in liver compared with control mice pretreated with vehicle solutions. We further investigated the mechanisms of the protective effects of EGCG. In EGCG-pretreated mice, we found abrogated tumour necrosis factor (TNF)-α and interferon (IFN)-γ at both protein levels in plasma and mRNA levels in liver. At the same time, the concentration of nitrite in plasma and inducible nitric oxide synthase production in liver were both down-regulated in these mice. Moreover, IFN-inducible protein-10 and macrophage inflammatory protein-1α expressions in liver were decreased significantly. Therefore, EGCG is capable of regulating immune-mediated liver injury in vivo. The protective effect depended on its suppressive effect on the production of important inflammatory mediators.
Keywords: concanavalin A-induced hepatitis, (–)-epigallocatechin-3-gallate, immune-mediated liver injury
Introduction
Tea (Camellia sinensis) is one of the most popular beverages in the world, and its beneficial effects on health have attracted great attention. Drinking tea, especially green tea, has recently been associated with a lower incidence of human cancer [1]. The most significant groups of tea components are polyphenols, especially the catechin group called flavonols. (–)-Epigallocatechin-3-gallate (EGCG) is the major tea catechin. Many of the known biological effects of green tea are mediated by EGCG.
Many studies have suggested that EGCG exhibits anti-oxidant, anti-inflammatory and immunosuppressive effects. EGCG can inhibit lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α and inducible nitric-oxide synthase (iNOS) production in mice [2,3]. It can also promote apoptosis and cell cycle arrest of transformed cells [4–6]. The underlying mechanisms of EGCG's action involve inhibiting the activation of nuclear factor-kappa B (NF-κB), which regulates the expression of a variety of genes critical for the induction of inflammatory and apoptosis mediators [7]. Several groups have reported a positive effect of EGCG in inflammatory or autoimmune diseases in animal models, such as experimental autoimmune encephalomyelitis (EAE), toxin-induced liver injury and autoimmune diabetes [8–10].
Concanavalin A (ConA)-induced hepatitis (CIH) is an experimental model of immune-mediated liver disease in humans [11]. It is characterized by massive hepatocellular degeneration and lymphoid infiltration in the liver [12]. The hepatitis induced by ConA is mainly dependent on T cell activation [13,14]. T cell activation elicited by ConA results in the elevation of plasma levels of various cytokines, including TNF-α, interferon (IFN)-γ, interleukin (IL)-6 [15]. In particular, TNF-α and IFN-γ are considered to play critical roles in the development of CIH because passive immunization against these cytokines effectively protects animals from hepatic injury [14,16].
In this current study, we found that EGCG protected mice from CIH. Mice pretreated with EGCG exhibited significantly reduced alanine aminotransferase (ALT) in plasma. Histological staining showed that EGCG administration strongly attenuated liver injury and hepatocyte apoptosis. The beneficial effect of EGCG was related to the inhibition of the production of several major inflammatory mediators.
Materials and methods
Mice
C57BL/6 mice, male, 8–10 weeks of age, were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). Mice were housed in the animal care facilities of Shanghai Jiao Tong University School of Medicine under pathogen-free conditions. All experimental procedures received approval by the Institutional Laboratory Animal Care and Use Committees.
EGCG administration
Mice received EGCG (Sigma-Aldrich, St Louis, MO, USA) dissolved in phosphate-buffered saline (PBS). EGCG (5 mg/kg each time) or PBS was administered by gavage twice daily for 10 days before the induction of CIH.
Induction and evaluation of CIH
Mice were injected via the tail vein with a single dose of ConA (Vector Laboratories, Burlingame, CA, USA) (15 mg/kg of body weight). Plasma was obtained about 20 h later, and ALT levels were determined using the ALT detection kit (Shanghai Yihua Medical Science and Technology, Shanghai, China).
Histology
Livers were removed after perfusion with PBS, fixed with 4% phosphate-buffered paraformaldehyde and embedded in paraffin. Tissue sections (5 µm) were prepared and stained with H&E; sections were then examined by light microscopy. A total of 10 tissue sections were analysed for each animal.
TUNEL staining
Paraffin embedded liver tissues were assayed for DNA fragmentation using a terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labelling (TUNEL) reaction, according to the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN, USA); sections were then examined by light microscopy. A total of 10 tissue sections were analysed for each animal.
Analysis of plasma cytokines
The plasma concentrations of TNF-α and IFN-γ were determined with the help of a specific enzyme-linked immunosorbent assay kit (ELISA) (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions.
Measurement of nitrite
NO is converted rapidly to nitrite in plasma. In our study, plasma were deproteinized and nitrite was measured by using the Griess reaction, as described previously [17]. Briefly, 100 µl pretreated plasma were mixed with 100 µl of Griess reagent at room temperature for 10 min. Absorbance was measured at 540 nm in an automated microplate reader. The concentration of nitrite was determined by reference to a standard curve of sodium nitrite.
Immunochemistry
Sections were immunostained with anti-serum to iNOS by using the biotin–avidin–peroxidase method. Briefly, endogenous peroxidase activity was blocked by immersing the sections in 3% hydrogen peroxide for 5 min at room temperature. The sections were permeabilized in 0·1% tyrosine in 10 mM Tris-HCL PH8·0. The sections were incubated overnight at 4°C, with rabbit polyclonal iNOS antibody (Upstate Biotechnology, Lake Placid, NY, USA) and diluted at 1 : 50 in phosphate-buffered saline containing 1% bovine serum albumin (BSA). Sections were washed three times in PBS and then incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) at a dilution of 1 : 100 for 1 h at 37°C. The sections were further washed. Finally, the peroxidase was visualized by immersing in 0·05% diaminobenzidine containing 0·03% hydrogen peroxide in Tris-HCl buffer (pH 7·5) for 5 min. Positive staining was indicated by a brown colour. Control sections were stained with 1% BSA.
RNA extraction and reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was isolated from liver tissues using RNeasy Mini Kit (Qiagen, Hilden, Germany). Genomic DNA was removed from total RNA prior to cDNA synthesis using the RNase-free DNase set for DNase digestion during RNA purification (Qiagen, Hilden, Germany). RNA was stored at −80°C. First-strand cDNA synthesis was performed for each RNA sample using the Sensiscript RT kit (Qiagen, Hilden, Germany). Random hexamers were used to prime cDNA synthesis.
Real-time PCR
Gene expression of iNOS and chemokines [monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, interferon-inducible protein-10 (IP-10)] mRNA were performed by real-time PCR using SYBR Green master mix (Applied Biosystems, Foster City, CA, USA). Thermocycler conditions comprised an initial holding at 50°C for 2 min, then at 95°C for 10 min. This was followed by a two-step PCR program consisting of 95°C for 15 s and 60°C for 60 s for 40 cycles. Data were collected and analysed quantitatively on an ABI Prism 7900 sequence detection system (Applied Biosystems). The α-actin gene was used as an endogenous control to normalize for differences in the amount of total RNA in each sample. All quantities were expressed as number of folds relative to the expression of α-actin, as follows. α-actin: sense 5′-TGTCCACCTTCCAGC AGATGT-3′, anti-sense 5′-AGCTCAGTAACAGTCCGCCT AGA-3′; TNF-α: sense 5′-GACGTGGAACTGGCAGAAG AG-3′, anti-sense 5′-GCCACAAGCAGGAATGAGAAG-3′; IFN-γ: sense 5′-TCAAGTGGCATAGATGTGGAAGAA-3′, anti-sense 5′-TGGCTCTGCAGGATTTTCATG-3′; iNOS: sense 5′-GCCACCAACAATGGCAACA-3′, anti-sense 5′-CG TACCGGATGAGCTGTGAA-3′; MIP-1α: sense 5′-CACC CTCTGTCACCTGCTCAA-3′, anti-sense 5′-ATGGCGCTG AGAAGACTTGGT-3′; IP-10: sense 5′-GCCGTCATTT TCTG CCTC-3′, anti-sense 5′-ATGGCGCTGAGAAGACTT GGT-3′.
Statistical analysis
All results are expressed as mean ± s.d. Statistical comparisons were made using Student's t-test after analysis of variance. The level of significance was set to α= 0·05. All tests were two-sided.
Results
Oral administration of EGCG inhibited ConA-induced ALT release
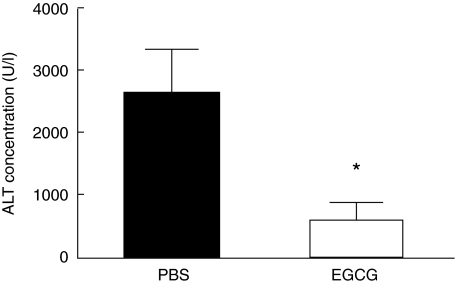
C57BL/6 mice, four mice per group, were pretreated with oral administration of PBS or EGCG (5 mg/kg) twice per day for 10 continuous days, then received ConA (15 mg/kg) injection via tail vein. Twenty hours later, plasma were obtained and ALT was measured. As shown in Fig. 1, pretreatment of mice with 5 mg/kg EGCG significantly inhibited release of ALT into plasma (about 80% reduction).
Fig 1.
Plasma alanine aminotransferase (ALT) levels. C57BL/6 mice, four mice per group, were pretreated with oral administration of phosphate-buffered saline (PBS) or (–)epigallocatechin-3-gallate (EGCG) (5 mg/kg) twice per day for 10 continuous days, then received concanavalin-A (ConA) (15 mg/kg) injection via tail vein. Twenty hours later, plasma were obtained and ALT were measured. Results shown are mean ± s.d. Data are representative of five experiments. *P < 0·05.
Lower doses of EGCG (2·5 mg/kg) did not significantly inhibit ConA-induced ALT release (data not shown). Moreover, higher doses of EGCG (up to 10 mg/kg) did not inhibit ConA-induced ALT release more efficiently than the 5 mg/kg feeding described above (data not shown).
Oral administration of EGCG protected mice from liver injury in CIH
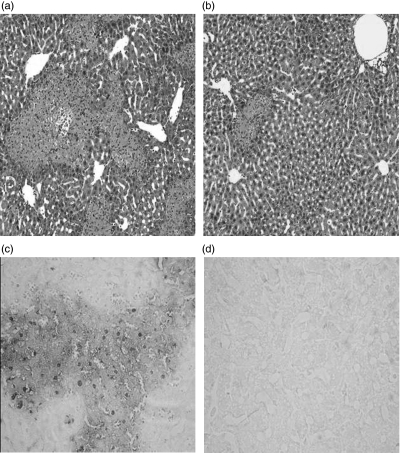
In CIH, ConA administration caused severe liver damage exhibiting as inflammatory infiltration around the central veins and large areas of centrilobular necrosis and hepatocyte apoptosis (Fig. 2a for H&E staining, and Fig. 2c for TUNEL staining). In contrast, in mice treated with EGCG before ConA administration, liver damage was minimal: fewer areas of intralobular necrosis or significant inflammatory infiltration were observed (Fig. 2b, H&E staining); only a few hepatocytes exhibited TUNEL-positive nuclei (Fig. 2d, TUNEL staining), indicating that apoptosis was markedly reduced in EGCG-pretreated mice exposed to ConA administration. Therefore, EGCG administration protected mice from ConA-induced liver inflammation and injury.
Fig 2.
Liver haematoxylin and eosin (H&E) and dUTP-biotin nick end labelling (TUNEL) staining. C57BL/6 mice, four mice per group, were pretreated with oral administration of phosphate-buffered saline (PBS) or (–)epigallocatechin-3-gallate (EGCG) (5 mg/kg) twice per day for 10 continuous days, then received concanavalin-A (ConA) (15 mg/kg) injection via tail vein. Twenty hours later, livers were obtained. (a) In PBS-pretreated mice, liver sections were stained with H&E. (b) In EGCG- pretreated mice, liver sections were stained with H&E. (c) In PBS-pretreated mice, liver sections were stained with TUNEL staining. (d) In EGCG- pretreated mice, liver sections were stained with TUNEL staining. Ten sections were observed in each sample. Results are representative of four experiments (magnification × 200).
Effect of EGCG on cytokine production
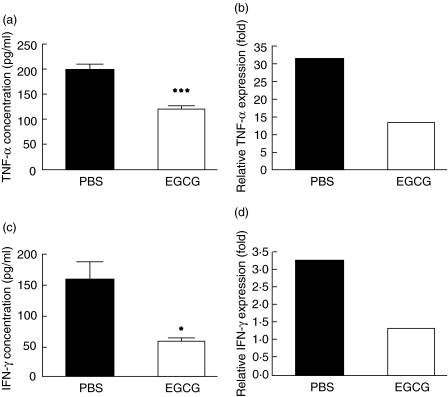
In CIH-induced mice immune cells are activated, and consequently inflammatory cytokines which promote liver cell damage are released. TNF-α and IFN-γ are among these inflammatory cytokines and have been found critical in triggering liver injury [18,19]. In evaluating the effect of EGCG pretreatment on the development of CIH, we analysed the levels of TNF-α and IFN-γ in plasma and liver. As shown in Fig. 3a and b, TNF-α at both protein level in plasma and mRNA level in liver were suppressed by EGCG administration (about 30% reduction for protein expression and 50% reduction for mRNA expression). In addition, we analysed the expression of IFN-γ at 20 h after ConA administration. As shown in Fig. 3c,d, the expression of IFN-γ at both protein level in plasma and mRNA level in liver of EGCG-pretreated mice were significantly decreased in comparison to that of PBS-pretreated mice (about 50% reduction and 55% reduction, respectively). Therefore, EGCG effectively suppressed the production of TNF-α and IFN-γ at both mRNA and protein levels, thereby reducing the damage to liver tissue caused by these inflammatory factors.
Fig 3.
Cytokine profiles. C57BL/6 mice were pretreated with oral administration of phosphate- buffered saline (PBS) or (–)epigallocatechin-3- gallate (EGCG) (5 mg/kg) twice per day for 10 continuous days, then received concanavalin-A (ConA) (15 mg/kg) via tail vein. Two hours later (a) plasma were obtained and tumour necrosis factor (TNF)-α were measured by enzyme-linked immunosorbent assay (ELISA); (b) liver RNA were extracted and relative TNF-α mRNA expression were measured by real-time polymerase chain reaction (PCR). Twenty hours later (c) plasma were obtained and interferon (IFN)-γ were measured by ELISA; (d) liver RNA were extracted and relative IFN-γ mRNA were measured by real-time PCR. Data are representative of three or four experiments. Results are shown by mean ± s.d. *P < 0·05, ***P < 0·001.
Effect of EGCG on iNOS-derived nitrite oxide production
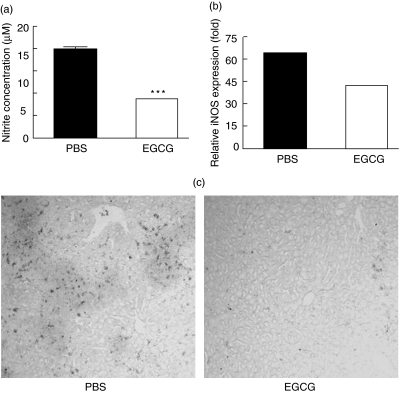
During CIH development, iNOS-derived nitric oxide production was found to act increasingly as an important inflammatory mediator [19,20]. To investigate whether the inhibition effect of EGCG on CIH might also involve the NO-mediated pathway, we examined nitrite concentration in plasma, quantitative iNOS mRNA expression in liver and iNOS expression in liver tissue sections.
As shown in Fig. 4a, compared with PBS-pretreated mice, the nitrite concentration in plasma decreased more than 50% in EGCG-pretreated mice, indicating that much less NO had been released into plasma in these mice. At the same time, the expression level of iNOS mRNA was significantly reduced in mice that received EGCG (Fig. 4b).
Fig 4.
Nitric oxide and inducible nitric-oxide synthase (iNOS). C57BL/6 mice were pretreated with oral administration of phosphate-buffered saline (PBS) or (–)epigallocatechin-3-gallate (EGCG) (5 mg/kg) twice per day for 10 continuous days, then received concanavalin-A (ConA) (15 mg/kg) via tail vein. Twenty hours later (a) plasma were obtained and NO were measured; (b) liver RNA was obtained and relative iNOS expression were measured by real-time PCR; (c) livers were obtained and sectioned. iNOS-positive cells were detected by immunohistochemistry. Results are representative of three experiments. ***P < 0·001.
The effect of EGCG on iNOS expression in liver was confirmed further by immunohistochemical staining of iNOS-positive cells. As shown in Fig. 4c, cells bearing iNOS protein were apparent in the inflammatory areas of the liver in PBS-pretreated mice. In contrast, mice pretreated with EGCG showed a significant reduction in the number of iNOS-positive cells. Therefore, EGCG administration also inhibited CIH through affecting the NO-mediated inflammation pathway.
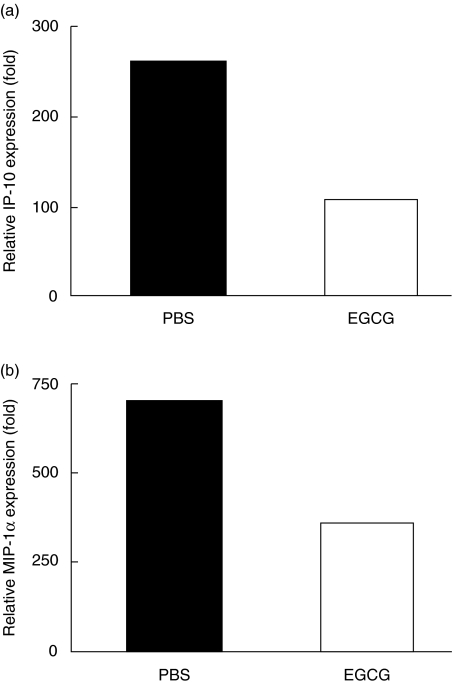
Effects of EGCG on chemokine expression
Augmentations of chemokine productions such as IP-10 and MIP-1α have been found to play critical roles in the development of CIH [21,22]. Therefore, we further studied the changes of IP-10 and MIP-1α after EGCG pretreatment. Twenty hours after CIH induction liver RNA were obtained, and quantitative mRNA expression of IP-10 and MIP-1α was measured. As shown in Fig. 5, the levels of IP-10 mRNA and MIP-1α mRNA were both reduced in EGCG-pretreated mice compared with PBS-pretreated mice. Thus, the production of IP-10 and MIP-1α in CIH was inhibited by EGCG administration.
Fig 5.
Chemokine mRNA expressions. C57BL/6 mice, four mice per group, were pretreated with oral administration of phosphate-buffered saline (PBS) or (–)epigallocatechin-3-gallate (EGCG) (5 mg/kg) twice per day for 10 continuous days, then received concanavalin-A (ConA) (15 mg/kg) via tail vein. Twenty hours later (a) liver RNA were extracted and relative IP-10 expression were measured by real-time polymerase chain reaction (PCR); (b) liver RNA were extracted and relative macrophage inflammatory protein (MIP)-1α expression were measured by real-time PCR. Results are representative of three experiments.
Discussion
In the present study, we demonstrated that EGCG protected mice from CIH induction, which were exhibited as inhibited ALT levels in plasma, as well as reducing inflammatory infiltration and hepatocytes apoptosis in liver. In CIH-induced mice that received EGCG, the levels of TNF-α and IFN-γ were decreased significantly at both mRNA and protein levels. At the same time, EGCG down-regulated iNOS expression in liver, thus inhibiting the production of NO. Moreover, mRNA expressions of IP-10 and MIP-1α in liver were both decreased significantly. Therefore, EGCG administration protected mice from CIH through inhibiting the production of several major inflammatory mediators.
The elevated levels of TNF-α in plasma in CIH mice were suppressed by EGCG administration. Many previous studies have reported that TNF-α, as an important inflammatory cytokine, plays a critical role in the pathogenesis of ConA-induced liver injury [22,23]. In CIH, TNF-α is secreted from liver macrophage (Kupffer cells) and acts as a major mediator in inflammation-induced hepatocyte death, which can cause hepatic damage through binding its receptor-inducing apoptosis cascade [15]. Pretreatment of mice with anti-mouse TNF-α anti-serum protected them from CIH [14]. Yang et al. found that EGCG inhibits LPS-induced TNF-α production in mice [2]. EGCG has a markedly suppressive activity on murine macrophages infected with the intracellular bacterium Legionella pneumophila (Lp), an effect mediated by enhanced production of TNF-α [24]. Moreover, it has been reported that EGCG can inhibit the expression of TNF-α both in collagen-induced experimental arthritis and EAE [8,25]. Our observation of reduced TNF-α production after EGCG administration suggests that EGCG suppresses disease severity by abrogating TNF-α-induced hepatocytic injury and inflammation response.
As well as TNF-α, IFN-γ is another essential key regulator in the development and progression of CIH. Depletion of IFN-γ resulted in a marked reduction in ConA-induced liver injury and inflammation [18,26]. The important roles of IFN-γ perhaps rely on the fact that it can synergize with TNF-α to induce production of several chemokines and adhesion molecules [27]. Previous studies have also suggested that IFN-γ/signal transducer and activator of transcription-1 (STAT-1) contributes to CIH via induction of pro-apoptotic genes [28,29]. In our study, we also found that administration of EGCG inhibited the augmentation of IFN-γ. This finding is consistent with previous reports that EGCG suppresses IFN-γ production in infection and inflammation [25]. However, the underlying mechanisms of the suppression effect of EGCG on IFN-γ induction need further investigation.
We found that the levels of nitrite concentration in plasma and expression of iNOS mRNA and protein in liver were all reduced by EGCG administration in CIH mice. As previous studies have reported, in CIH-induced mice the release of NO into plasma and the expression of iNOS mRNA in liver was found to be increased [18,19]. Excessive NO production derived from iNOS has also been found to play an important role in the induction of toxin-induced liver injury [30]. The involvement of the toxic effect of iNOS-derived NO in the development of CIH has been emphasized recently [30,31]. NO mediates tissue injury through pathways including inhibition of mitochondrial respiration, inactivation of proteinase inhibitors and formation of free radicals [32]. Lin et al. reported that EGCG inhibited NO generation and iNOS expression in peripheral macrophages stimulated with LPS [3]. Chen et al. [9] have pointed out that EGCG inhibited toxin-induced hepatotoxicity partly through inhibition of NO expression and down-regulation of the production of inflammatory mediators resulting from the induction of iNOS. Therefore, the protective effect of EGCG on CIH may also involve inhibiting NO production, thus decreasing its toxicity to hepatocytes.
We found that EGCG inhibited IP-10 and MIP-1α mRNA expression in liver in CIH mice. During the first hours after challenge, the production of proinflammatory cytokines are followed by activation of chemotactic factors such as IP-10 and MIP-1α, which attract more leucocytes to the liver to amplify local inflammation and damage [20,21]. IP-10 is a selective chemoattractant for activated T lymphocytes. In addition, IP-10 plays an important role in hepatic neutrophil influx in CIH [20,33]. While CIH is developed, IP-10 expression has been found to increase and is modulated by the IFN-γ/STAT1 pathway [34]. MIP-1α has been suggested to promote the recruitment of various leucocyte subtypes, including T cells [35,36]. As previous reports have documented, hepatic macrophages (Kupffer cells) have been discovered as a major source of MIP-1α during hepatic inflammation [37]. In MIP-1α–/– mice CIH has been shown to be markedly decreased, indicating that MIP-1α is significantly involved in the pathogenesis of the disease [38]. In this study, we found for the first time that that EGCG inhibited IP-10 and MIP-1α mRNA expression in the inflammatory sites. We propose that EGCG inhibits the expression of IP-10 and MIP-1α, which in turn inhibits the migration of inflammatory cells into liver, thus reducing liver injury severity. Previous studies have demonstrated that TNF-α and IFN-γ can regulate the expression of IP-10 and MIP-1α [20,21]. Whether EGCG modulated these chemokines directly or indirectly through interfering TNF-α and IFN-γ production needs further investigation.
In conclusion, in this current study we found that EGCG suppressed CIH in mice. The suppressant effect was associated with inhibition of several inflammatory mediators, including TNF-α, IFN-γ, NO and chemokines. The protective effect of EGCG on CIH and its detailed mechanism will be studied further. However, results from this study suggest new perspectives for employing EGCG in the pretreatment of immune-mediated liver disease.
Acknowledgments
This work was supported by grants from the Knowledge Innovation Program of the Chinese Academy of Sciences (J0171-1903), Shanghai Leading Academic Discipline Project (T0206) and Shanghai Rising-Star Program (04QMX1423).
References
- 1.Suganuma M, Okabe S, Sueoka N, et al. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339–44. doi: 10.1016/s1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]
- 2.Yang F, de Villiers WJ, McClain CJ, et al. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334–40. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- 3.Lin YL, Lin JK. (–)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol Pharmacol. 1997;52:465–72. [PubMed] [Google Scholar]
- 4.Yang GY, Liao J, Kim K, et al. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–6. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 5.Liang YC, Lin-Shiau SY, Chen CF, et al. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (–)-epigallocatechin-3-gallate. J Cell Biochem. 1999;75:1–12. [PubMed] [Google Scholar]
- 6.Lu YP, Lou YR, Xie JG, et al. Topical applications of caffeine or (–)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci USA. 2002;99:12455–60. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang F, Oz HS, Barve S, Varilek GW, et al. The green tea polyphenol (–)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol. 2001;60:528–33. [PubMed] [Google Scholar]
- 8.Aktas O, Prozorovski T, Smorodchenko A, et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 9.Chen JH, Tipoe GL, Liong EC, et al. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am J Clin Nutr. 2004;80:742–51. doi: 10.1093/ajcn/80.3.742. [DOI] [PubMed] [Google Scholar]
- 10.Song EK, Hur H, Han MK. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch Pharm Res. 2003;26:559–63. doi: 10.1007/BF02976881. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko Y, Harada M, Kawano T, et al. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–14. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazawa Y, Tsutsui H, Mizuhara H, et al. Involvement of intrasinusoidal hemostasis in the development of concanavalin A-induced hepatic injury in mice. Hepatology. 1998;27:497–506. doi: 10.1002/hep.510270225. [DOI] [PubMed] [Google Scholar]
- 13.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuhara H, O'Neill E, Seki N, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529–37. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gantner F, Leist M, Lohse AW, et al. Concanavalin A-induced T cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21:190–8. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 16.Mizuhara H, Uno M, Seki N, et al. Critical involvement of interferon gamma in the pathogenesis of T cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996;23:1608–15. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- 17.Moshage H, Kok, Huizenga B, JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41:892–6. [PubMed] [Google Scholar]
- 18.Kusters S, Gantner F, Kunstle G. Interferon-ã plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–71. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 19.Yoneda M, Wada K, Katayama K, et al. A novel therapy for acute hepatitis utilizing dehydroepiandrosterone in the murine model of hepatitis. Biochem Pharmacol. 2004;68:2283–9. doi: 10.1016/j.bcp.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 20.Tamaru M, Nishioji K, Kobayashi Y, et al. Liver-infiltrating T lymphocytes are attracted selectively by IFN-inducible protein-10. Cytokine. 2000;12:299–308. doi: 10.1006/cyto.1999.0560. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Okada M, Yoneda M, et al. Macrophage inflammatory protein-2 induced by TNF-alpha plays a pivotal role in concanavalin A-induced liver injury in mice. J Hepatol. 2001;35:217–24. doi: 10.1016/s0168-8278(01)00109-x. [DOI] [PubMed] [Google Scholar]
- 22.Trautwein C, Rakemann T, Brenner DA, et al. Concanavalin A-induced liver cell damage: activation of intracellular pathways triggered by tumor necrosis factor in mice. Gastroenterology. 1998;114:1035–45. doi: 10.1016/s0016-5085(98)70324-5. [DOI] [PubMed] [Google Scholar]
- 23.Wolf D, Schumann J, Koerber K, et al. Low molecular-weight hyaluronic acid induces nuclear factor-κB-dependent resistance against tumor necrosis factor α-mediated liver injury in mice. Hepatology. 2001;34:535–47. doi: 10.1053/jhep.2001.27218. [DOI] [PubMed] [Google Scholar]
- 24.Rogers J, Perkins I, van Olphen A, et al. Epigallocatechin gallate modulates cytokine production by bone marrow-derived dendritic cells stimulated with lipopolysaccharide or muramyldipeptide, or infected with Legionella pneumophila. Exp Biol Med. 2005;230:645–51. doi: 10.1177/153537020523000906. [DOI] [PubMed] [Google Scholar]
- 25.Haqqi TM, Anthony DD, Gupta S, et al. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci USA. 1999;96:4524–9. doi: 10.1073/pnas.96.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siebler J, Wirtz S, Klein S, et al. A key pathogenic role for the STAT1/T-bet signaling pathway in T cell-mediated liver inflammation. Hepatology. 2003;38:1573–80. doi: 10.1016/j.hep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Neish AS, Read MA, Thanos D, et al. Endothelial interferon regulatory factor 1 cooperates with NF-κB as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 1995;15:2558–69. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong F, Jaruga B, Kim WH, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–13. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streetz K, Fregien B, Plumpe J, et al. Dissection of the intracellular pathways in hepatocytes suggests a role for Jun kinase and IFN regulatory factor-1 in ConA-induced liver failure. J Immunol. 2001;167:514–23. doi: 10.4049/jimmunol.167.1.514. [DOI] [PubMed] [Google Scholar]
- 30.Sass G, Koerber K, Bang R, et al. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest. 2001;107:439–47. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto T, Masuda Y, Kawasaki T, et al. Aminoguanidine prevents concanavalin A-induced hepatitis in mice. Eur J Pharmacol. 2000;396:125–30. doi: 10.1016/s0014-2999(00)00186-2. [DOI] [PubMed] [Google Scholar]
- 32.Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidant in the regulation of gene expression. FASEB J. 2000;14:1889–900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- 33.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 34.Jaruga B, Hong F, Kim WH, et al. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1044–52. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ajuebor MN, Das AM, Virag L, et al. Regulation of macrophage inflammatory protein-1 alpha expression and function by endogenous interleukin-10 in a model of acute inflammation. Biochem Biophys Res Commun. 1999;255:279–82. doi: 10.1006/bbrc.1999.0196. [DOI] [PubMed] [Google Scholar]
- 36.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 37.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajuebor MN, Hogaboam CM, Le T, et al. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004;34:2907–18. doi: 10.1002/eji.200425071. [DOI] [PubMed] [Google Scholar]