Abstract
Conversion of arginyl to citrullyl residues (citrullination) is essential for the formation of the epitopes recognized by rheumatoid arthritis (RA)-associated autoantibodies to citrullinated proteins (ACPA). ACPA are secreted by plasma cells of the rheumatoid synovial tissue where their major target, citrullinated fibrin, is abundant. Although numerous arguments suggest that ACPA play an important role in RA, their pathological relevance remains to be established. In the present study, we assessed the immunogenicity and arthritogenicity of complete Freund's adjuvant-emulsified autologous citrullinated (C-rFBG) or non-citrullinated (NC-rFBG) fibrinogen in Lewis (LEW) and Brown–Norway rats, which exhibit drastic differences in their susceptibility to induced autoimmune diseases. NC-rFBG induced no antibody response. In contrast, a single injection of C-rFBG induced an IgG response directed mainly to citrullinated determinants of rFBG. However, all rat strains remained devoid of clinical and histological signs of arthritis up to 3 months after C-rFBG inoculation. Next, in LEW rats, we tested whether autoimmunity to C-rFBG could aggravate acute ankle arthritis triggered by intra-articular injection of incomplete Freund's adjuvant (IFA). However, such arthritis evolved identically in the presence or absence of anti-C-rFBG autoantibodies. However, IFA-injected joints were devoid of citrullinated fibrin deposits. Therefore, citrullination allows breakdown of immunological tolerance but the autoimmune response developed is not spontaneously arthritogenic. Whether or not it can aggravate arthritis with citrullinated fibrin deposits remains to be evaluated.
Keywords: anti-citrullinated protein autoantibodies, citrulline, fibrin, post-translational modification, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA), essentially characterized by chronic inflammation of synovial joints with frequent extra-articular manifestations, is the most common human autoimmune disorder. In the serum of ∼ 80% of affected individuals, IgG autoantibodies to ‘citrullinated’ (deiminated) proteins are present and constitute a highly specific serological marker of the disease. These autoantibodies, described initially as two independent autoantibody families, the so-called ‘anti-keratin antibodies’ and the anti-perinuclear factor, were both shown to recognize epitopes borne by several molecular variants of the epithelial differentiation protein filaggrin and thus to constitute a unique family of autoantibodies referred to thereafter as antifilaggrin autoantibodies (AFA) [1–3]. Secondly, it was demonstrated that protein ‘citrullination’ (deimination), i.e. post-translational conversion of arginyl residues into citrullyl residues mediated by a peptidylarginine deiminase (PAD), was crucial for the formation of the epitopes recognized by AFA [4,5]. In addition we demonstrated that citrullinated forms of the α- and β-chains of fibrin correspond to major antigenic targets of AFA in the rheumatoid synovial tissue [6]. Finally, the recent demonstration that citrullinated vimentin corresponds to the target of the RA-associated anti-Sa antibodies [7] showed that anti-Sa antibodies and the AFA/anti-citrullinated fibrin autoantibodies belong to a single family of autoantibodies that can henceforth be generally named ACPA (autoantibodies to citrullinated proteins).
The existence of the RA-like joint disorder of the K/B × N T cell receptor (TCR) transgenic mouse line that depends critically on the development of a B cell reaction to glucose-6-phosphate isomerase [8,9] suggests that the role played by B cells in human RA needs careful consideration. Moreover, sustained clinical improvement obtained following the use of an anti-CD20 antibody (rituximab) as a therapy for RA indicates an important role for B cells in the pathophysiology of the disease [10]. ACPA-producing B cells could therefore play a significant role in RA pathophysiology. Indeed, not only are ACPA largely the most disease-specific of the RA-associated autoantibodies, but also numerous studies have established clearly that a significant positive correlation exists between the titre of these autoantibodies in the serum and clinical, biological and radiological data related to RA activity and/or severity (for a review see [11] and [12]). In addition, several studies have demonstrated that the appearance of ACPA in the serum occurs very early in the course of the disease, before arthritis becomes clinically perceptible (reviewed in [12]). Secretion and concentration of ACPA in the rheumatoid synovial membrane [13] and the presence therein of their specific antigenic target, citrullinated fibrin, constitute a strong additional argument for the involvement of ACPA in the pathophysiology of RA via a disease-specific immunological conflict occurring precisely in the disease-targeted tissue.
The existence of an arthritis model using intra-articular injection of fibrin into rabbits immunized previously with human (heterologous) fibrin supports the idea that immunization against an inflammation product can play a significant role in maintaining a synovitis [14]. However, recent studies performed in mice to investigate the role of the autoimmune response to citrullinated fibrin in RA pathophysiology were disappointing. In these studies, mice were inoculated either with a heterologous antigen, human fibrinogen (hFBG) or with autologous mouse FBG (mFBG), both in either native or citrullinated forms [15,16]. Immunization with hFBG induced an antibody response independently of the state of citrullination of the Ag [15]. In contrast, only inoculation of the citrullinated form of mFBG was associated with production of specific IgG [16]. However, no arthritis signs appeared in the animals that developed an autoimmune response against FBG [15,16].
Mice and rats often exhibit differences in their susceptibilities to stimuli designed to trigger autoimmune disorders. For instance, compared to mice, rats are more susceptible to experimental autoimmune encephalomyelitis [17], experimental autoimmune myasthenia gravis [18] or adjuvant-induced arthritis [19]. This prompted us to evaluate the immunogenic and arthritogenic properties of autologous citrullinated FBG (rFBG) in the rat. In particular, we used Lewis (LEW) and Brown–Norway (BN) rats, which are powerful models of human immunopathological disorders because they show an inverse polarization of their immune responses and susceptibility to experimentally induced immunological disorders [20,21].
Materials and methods
Rats
Female LEW and BN rats (8–9 weeks old) were obtained from the Centre d'Élevage R. Janvier (Le Genest St Isle, France) and maintained in our animal house facility under specific pathogen-free conditions. All procedures were in accordance with national regulations on animal experiments.
In vitro citrullination of rat fibrinogen
Rat FBG (rFBG) (purified from plasma and containing at least 90% of clottable protein; Sigma, Saint Quentin Fallavier, France) was further purified to eliminate residual contamination by IgG using affinity chromatography on a protein-G column (HiTrap® protein G, 5 ml; Amersham Biosciences, Orsay, France) according to the manufacturer's protocol. After that purification step, the percentage of IgG contamination was estimated as 0·1% or less. Citrullination was then performed with rabbit skeletal muscle PAD enzyme (PAD2, 7 U/mg rFBG, Sigma) in 0·1 M Tris-HCl (pH 7·4), 0·5 M NaCl, 10 mM CaCl2 and 5 mM DTT for 2 h at 37°C at an rFBG concentration of between 1·8 and 3·8 mg/ml (varying from batch to batch). Citrullinated rFBG is designated as C-rFBG. Control non-citrullinated rat fibrinogen (NC-rFBG) was incubated in citrullination buffer alone. After citrullination, buffer exchange to phosphate-buffered saline (PBS) pH 7·4 containing 0·5 M NaCl was performed in regenerated cellulose dialysis tubing (Spectra/Por®3, 3500 MWCO; Spectrum Laboratories, Inc., Interchim, Montluçon, France). Dialysis was pursued until complete reassembly of the six constitutive chains of the rFBG molecule (two Aα-, two Bβ- and two γ-chains), monitored by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing conditions. Efficient citrullination was checked by immunoblotting using an antibody able to recognize any citrullinated protein (purified rabbit IgG to modified citrullyl residues, a generous gift from Dr T. Senshu, Graduate School of Integrated Science, Yokohama City University, Yokohama, Japan), as described previously [6].
Inoculation of rats with autologous fibrinogen
Rats were injected subcutaneously on the back with 10 µg or 50 µg of C-rFBG or NC-rFBG emulsified in complete Freund's adjuvant (CFA) containing 1 mg/ml heat-killed Mycobacterium tuberculosis H37Ra (Difco Laboratories Inc., Detroit, MI, USA). A second inoculation was performed 4 weeks after the first, either intraperitoneally or intradermally with 50 µg of the same antigen emulsified in incomplete Freund's adjuvant (IFA) (Difco Laboratories) or alum adjuvant (Sigma).
Induction of ankle joint inflammation and clinical evaluation
Female LEW rats were injected with 50 µl IFA into the right ankle joint. PBS (50 µl) was injected into the left ankle joint as control. The clinical severity of hind paw inflammation was monitored using a macroscopic scoring system from 0 to 3 according to changes in redness and swelling (0 = no changes; 1 = detectable; 2 = moderate; 3 = severe redness and swelling of the entire paw).
Enzyme-linked immunosorbent assay (ELISA) detection of serum antibodies to C-rFBG, to NC-rFBG and to PAD
Rats inoculated with C-rFBG or NC-rFBG were bled from the retro-orbital sinus at regular intervals after the first inoculation and sera were collected individually. For the detection of Ig specific for C-rFBG, NC-rFBG or PAD enzyme in these sera, microtitre plates (MaxiSorp; NUNC, VWR International, Fontenay-sous-Bois, France) were coated overnight at 4°C with 5 µg/ml of PBS-diluted C-rFBG or NC-rFBG or rabbit skeletal muscle PAD, respectively. The plates were then blocked with PBS containing 2% bovine serum albumin (BSA) (Sigma; PBS–BSA) and 100 µl/well of rat serum diluted to 1 : 50 in PBS–BSA containing 2 M NaCl was added. Bound total IgG or IgM were detected using peroxidase-conjugated in-house-produced polyclonal sheep antibodies to rat IgG (kindly provided by E. Druet, Institut National de la Santé et de la Recherche Médicale U28, Toulouse, France) or polyclonal goat antibodies to rat IgM (SouthernBiotech, Birmingham, AL, USA), respectively. To detect IgA, plates were incubated with a mouse monoclonal antibody (MoAb) to rat IgA (clone MARA-1) (LO/IMEX; University of Louvain, Brussels, Belgium) then with peroxidase-conjugated goat anti-mouse IgG (H + L) (Zymed Laboratories, San Francisco, CA, USA). Alternatively, bound IgG1, IgG2a or IgG2b were detected using biotinylated mouse MoAb to rat IgG1 (clone MARG1-2), to rat IgG2a (clone MARG2a-1) or to rat IgG2b (clone MARG2b-3), respectively (LO/IMEX), followed by incubation with peroxidase-conjugated streptavidin (Amersham, Little Chalfont, Bucks, UK). All incubations were performed for 1 h at 4°C and followed by three washes in PBS containing 0·1% (v/v) Tween-20 (Sigma). Peroxidase activity was revealed by 2 mg/ml orthophenylene diamine dihydrochloride, 0·03% H2O2 in 35 mM trisodium citrate, 40 mM Na2HPO4 at a pH adjusted to 5 with orthophosphoric acid. The reaction was stopped by 6 N H2SO4 and the OD at 492 nm was measured with an automated plate reader (VersAmax; Molecular Devices, Menlo Park, CA, USA).
In competition experiments, the ELISA IgG reactivity to C-rFBG of two representative sera of LEW rats inoculated with C-rFBG (day 53) was assayed in the presence of PAD enzyme. ELISA was performed exactly as mentioned above, except that the sera were tested at a dilution resulting in an OD of ∼ 1·0 on C-rFBG (1 : 150). PAD enzyme was added to the serum dilution 1 h prior to the ELISA testing, at a 0·5, 1, 2, 5 or 10 µg/ml final concentration. As control, one of the sera incubated with increasing concentrations of PAD enzyme was also tested simultaneously by ELISA on PAD. The IgG reactivities obtained in the presence of soluble PAD were expressed as the percentage of those obtained without PAD (considered as 100%).
Immunoblotting
C-rFBG and NC-rFBG were separated by SDS-PAGE on over-migrated 12% polyacrylamide gels. Over-migration was monitored using pre-stained molecular weight standards (low range, Bio-Rad Laboratories, SA, Marnes La Coquette, France) and performed until the ovalbumin standard reached the bottom of the gel. The gels were then electrotransferred onto reinforced nitrocellulose membranes (Hybond™C extra, Amersham) and membrane strips (∼ 1·36 µg antigen/strip) were cut and immunodetected by several primary probes diluted in 40 mM Tris-HCl buffer (pH 8·0) containing 150 mM NaCl, 0·05% Tween-20 and 2·5% powdered skimmed milk (blotting buffer). The primary probes included the sera of rats inoculated with C-rFBG or with NC-rFBG (all diluted to 1 : 1000), a pool of ACPA-positive sera or individual ACPA-positive sera from human patients with RA (all diluted to 1 : 1000), a sheep anti-serum directed to the Aα-chain of hFBG and cross-reactive to the Aα-chain of rFBG (1 : 250; Cambio, Cambridge, UK), a rabbit anti-serum directed to the Bβ-chain of hFBG and cross-reactive to the Bβ-chain of rFBG (1 : 100 000; Cambio), a sheep anti-serum directed to the γ-chain of hFBG and cross-reactive to the γ-chain of rFBG (1 : 500; Cambio), an antibody reactive to rabbit PAD2 (1 µg/ml, purified rabbit Ig raised against peptides derived from the sequence of human PAD2 22 and the purified rabbit IgG to modified citrullyl residues. On the membrane strips that were probed with the latter antibodies (0·02 µg/ml), citrullyl residues were chemically modified previously, as described elsewhere [6]. Peroxidase-conjugated secondary probes were used for the detection of all the primary antibodies: sheep polyclonal antibodies to rat IgG, protein-A (Sigma), goat antibodies to rabbit IgG (H + L) (SouthernBiotech, Inc., Clinisciences, Montrouge, France) and rabbit F(ab′)2 fragments to sheep IgG (Southern Biotech. Inc.) for the detection of rat, human, rabbit and sheep IgG, respectively. Peroxidase activity was visualized using ECL™ reagents (Amersham), as suggested by the manufacturer. Negative controls included probing with the secondary probe only.
Histological analysis
Rat hind paws were collected immediately after killing by aorta sectioning under anaesthesia. After removal of the skin, ankles were fixed for 48 h in Bouin's solution, then decalcified in 0·5 M disodium ethylene diamine tetraacetate, pH 7·4 and embedded in paraffin. After sectioning (5-µm), the specimens were stained with haematoxylin and eosin.
Statistical analysis
Results are shown as the mean ± s.d. and overall differences between medians were evaluated by the Mann–Whitney U-test.
Results
Inoculation of LEW and BN rats with autologous citrullinated fibrinogen induces production of specific autoantibodies
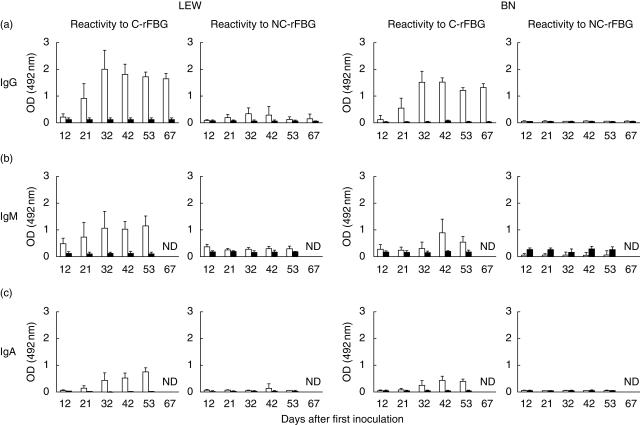
To investigate the effect of citrullination on the immunogenic property of autologous FBG, LEW and BN rats were inoculated with 50 µg of C-rFBG or of NC-rFBG emulsified in CFA (at day 0) followed, 32 days later, by a second challenge with 50 µg of antigen emulsified in IFA. The same antigenic preparations were used in both rat strains. The sera of these animals were collected at different time-points and analysed by ELISA for the presence of Ig directed to C-rFBG or to NC-rFBG (Fig. 1). The sera of rats inoculated with NC-rFBG did not recognize NC-rFBG or C-rFBG, even after the second inoculation of this antigen. In contrast, following a single injection of C-rFBG, all rats developed an IgG, IgM and IgA autoimmune response directed mainly to the citrullinated form of rFBG (Fig. 1a–c). Although the IgG response to C-rFBG was higher in LEW than in BN rats, the time-course of this response was very similar in both rat strains. Maximal levels of IgG reactive with C-rFBG were obtained around day 30, i.e. before the second antigenic challenge. Surprisingly, the second immunization with C-rFBG did not induce a secondary humoral immune response. Indeed, in sera collected after the second inoculation (at days 42, 53 and 67), IgG reactivity to C-rFBG was similar to that obtained at day 32, suggesting that the first challenge with antigen failed to induce the generation of memory immune cells. Interestingly, the IgM and IgA response followed the same course and did not rise following the second antigenic challenge. Moreover, in another independently conducted experiment there was no difference in the mean maximal IgG reactivity to C-rFBG between groups that were re-inoculated or not with C-rFBG at day 32 (data not shown). In LEW rats several protocols of immunization were tested using different doses of antigen at the first inoculation (10 µg or 50 µg) and different adjuvants (IFA or Alum) or different sites of injection (intraperitoneally or intradermally) at the second inoculation. None of these conditions allowed the development of a secondary antibody response after re-inoculation with 50 µg of C-rFBG (data not shown).
Fig 1.
Citrullination of autologous fibrinogen induces breakdown of tolerance to this autoantigen in Lewis (LEW) and Brown–Norway (BN) rats. Rats were inoculated subcutaneously with citrullinated rat fibrinogen (C-rFBG) (white bars; LEW: five rats, BN: four rats) or non-C-rFBG (NC-rFBG) (black bars; LEW: four rats, BN: five rats) in complete Freund's adjuvant (CFA) (day 0). A second inoculation was performed intraperitoneally with antigen emulsified in incomplete Freund's adjuvant (IFA), 32 days after the first injection. The titres of total IgG (a), IgM (b) and IgA (c) reactive to C-rFGB and NC-rFBG were measured by enzyme-linked immunosorbent assay at different times after the first inoculation as indicated. Results (LEW rats: left panels, BN rats: right panels) are expressed as the mean OD at 492 nm obtained with the different rat sera diluted to 1 : 50 (± s.d.).
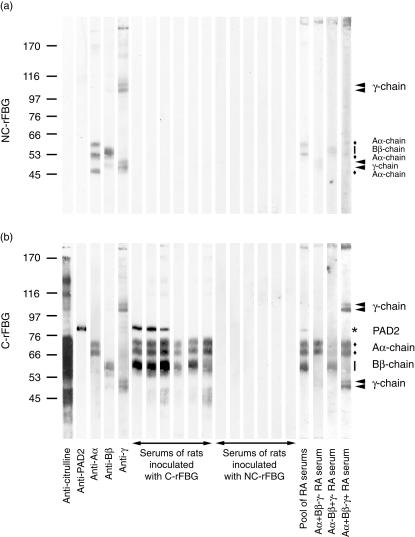
The IgG reactivity to C-rFBG and to NC-rFBG was also tested by immunoblotting using sera of LEW rats inoculated with C-rFBG or with NC-rFBG obtained from two independent experiments (Fig. 2). With the sera of rats inoculated with NC-rFBG, we did not find antibodies reactive with C-rFBG or NC-rFBG. In contrast, the sera of rats inoculated with C-rFBG, exhibited reactivity with the citrullinated forms of the Aα- and of the Bβ-chain of rFBG. Similarly to what is generally observed with ACPA-positive human RA sera [6], the rat sera tested exhibited no reactivity to the citrullinated form of the γ-chain. Using these immunoblotting conditions, no reactivity to NC-rFBG was detected. Overall, the results of this immunoblotting analysis are in accordance with those of the ELISA analyses.
Fig 2.
The serum of Lewis (LEW) rats inoculated with citrullinated rat fibrinogen (C-rFBG) is strongly reactive to the Aα- and Bβ-chains of C-rFGB. LEW rats were inoculated with C-rFGB or with non-C-rFBG (NC-rFGB). The serum of these rats, obtained from two independent experiments at around day 45 after inoculation, was analysed by immunoblotting on NC-rFBG (a) and on C-rFBG (b). The rabbit skeletal muscle peptidylarginine deiminase (PAD) enzyme present in the C-rFBG preparation was detected using PAD2-specific antibodies (anti-PAD2). An antibody able to recognize any citrullinated protein (anti-citrulline) was used to check efficient citrullination of the three Aα- Bβ- and γ-chains constitutive of rFBG. These three constitutive chains (and bands probably corresponding to their degradation or polymerization products) were identified using specific anti-sera (anti-Aα, anti-Bβ and anti-γ) and are shown with filled diamonds, vertical bars and filled arrowheads, respectively. Identification of the three Aα- Bβ- and γ-chains constitutive of C-rFBG was confirmed using a pool of anti-citrullinated protein autoantibody (ACPA)-positive sera from human rheumatoid arthritis (RA) patients reactive with both the Aα- and the Bβ-chain of in vitro citrullinated human fibrinogen (hFBG), and individual sera from three ACPA-positive RA patients characterized previously as being differently reactive to the Aα-, Bβ- and γ-chains of citrullinated hFBG [6]: a serum reactive only to the Aα-chain (Aα + Bβ-γ- RA serum), a serum reactive only to Bβ-chain (Aα-Bβ + γ- RA serum) and a serum reactive to both the Aα- and the γ-chain (Aα + Bβ-γ + RA serum). Apparent molecular masses (kDa) are indicated on the left.
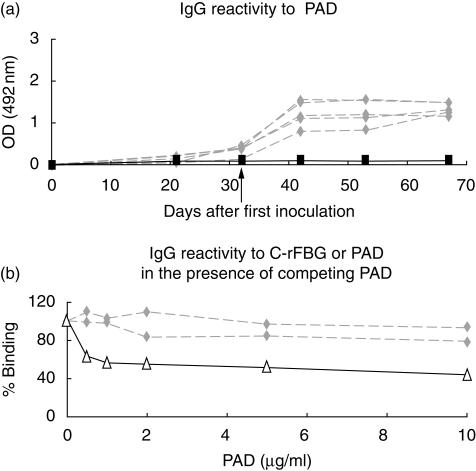
Because the C-rFBG preparation contained the rabbit skeletal muscle PAD enzyme that was used for citrullination (representing ∼1% of the total protein content), the IgG immune response to this PAD was also evaluated by ELISA. In the sera of LEW rats inoculated with C-rFBG, IgG reactive with PAD were clearly detected while, as expected, they were absent from the sera of rats inoculated with NC-rFBG (Fig. 3a). IgG reactivity to PAD enzyme was also detectable by immunoblotting (Fig. 2). Interestingly, the IgG response to PAD increased rapidly after day 32 corresponding to the second inoculation of C-rFBG (Fig. 3a) showing that, in this case, the first inoculation led to the development of PAD-specific memory B cells. In order to assess the contribution of the reactivity to PAD in the OD obtained in the ELISA using C-rFBG as immunosorbent, PAD enzyme was used as a competitor. Whereas the addition of PAD in sera of LEW rats inoculated with C-rFBG was able to strongly inhibit the IgG reactivity against PAD, it virtually did not affect the reactivity against C-rFBG (Fig. 3b).
Fig 3.
The peptidylarginine deiminase (PAD) included in the citrullinated rat fibrinogen (C-rFBG) preparation induces primary and secondary IgG responses that do not significantly contribute to the OD obtained by enzyme-linked immunosorbent assay (ELISA) using C-rFBG as immunosorbent. Lewis (LEW) rats were inoculated subcutaneously with C-rFGB (grey diamonds; five rats) or non-C-rFBG (black squares; four rats) in complete Freund's adjuvant (CFA) (day 0). A second inoculation was performed intraperitoneally with antigen emulsified in incomplete Freund's adjuvant (IFA) 32 days after the first injection. The titres of IgG reactive to PAD enzyme were measured by ELISA on days 12, 21, 32, 42, 53 and 67 after the first inoculation (a). Results are expressed as OD at 492 nm of serum diluted to 1 : 50. The sera of two LEW rats obtained at day 32 after inoculation of C-rFGB were also tested in a competition assay using PAD enzyme as competitor (b). The ELISA plates were coated with C-rFBG (also containing minute amounts of PAD) (grey diamonds) or PAD enzyme (white triangles, only one serum tested). Results are expressed as the percentage of the OD obtained in the absence of competing PAD enzyme (considered as 100%).
On the whole, the results obtained demonstrate clearly that inoculation of C-rFBG induces production of IgG antibodies directed mainly to citrullinated determinants of rFBG, but that antigen-specific mechanisms regulate antibody production.
In LEW and BN rats, autologous citrullinated fibrinogen induces production of different IgG subclasses
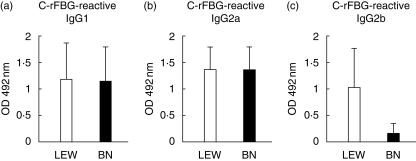
In the rat, IgG1 and IgG2a production are associated with type 2 cytokines while IgG2b depends on type 1 immune responses [20,23,24]. Because LEW and BN rats differ markedly in the polarization of their autoimmune responses [20,21], we determined the isotype profile of the IgG reactive with C-rFBG obtained in both strains after inoculation of this antigen. As shown in Fig. 4, at day 32 after the first challenge with 50 µg of C-rFBG the titres of IgG1 and IgG2a directed to C-rFBG were similar in both strains, whereas the titres of Ag-specific IgG2b were higher in LEW rats. Similar profiles of IgG subclasses were obtained at different times after the second inoculation of C-rFBG (days 42, 53 and 67) (data not shown). These results demonstrate that C-rFBG induces a different polarization of the immune response in LEW and BN rats: in LEW rats, both Th1- and Th2-type immune responses were generated, whereas in BN rats a Th2-type immune response was preferentially induced.
Fig 4.
In Lewis (LEW) and Brown–Norway (BN) rats, inoculation of citrullinated rat fibrinogen (C-rFBG) induces production of different IgG subclasses. LEW (white bars; five rats) and BN (black bars; five rats) rats were inoculated subcutaneously with C-rFGB at day 0. The titres of IgG1 (a), IgG2a (b) and IgG2b (c) reactive to C-rFGB were measured by enzyme-linked immunosorbent assay on day 32 after the inoculation. Results are expressed as the mean OD at 492 nm obtained with the different rat sera diluted to 1 : 50 (± s.d.).
The autoimmune response to autologous citrullinated fibrinogen is not spontaneously arthritogenic
LEW (n = 28) and BN (n = 5) rats that had been inoculated with C-rFBG following our ‘standard’ immunization protocol (50 µg of C-rFBG emulsified in CFA followed, 32 days after, by a second challenge with 50 µg of antigen emulsified in IFA) were examined daily for clinical signs of arthritis (redness and swelling of the paws). All these animals were still free of arthritis 3 months after the first sensitization with C-rFGB. Moreover, none of the other different immunization protocols that were tested in LEW rats (see above) induced clinical signs of joint inflammation within the 2–3 months following the first sensitization. We also looked for histological signs of inflammation by examination of haematoxylin and eosin-stained sections of the hind paw of four LEW and four BN rats, 3 months after immunization with C-rFBG following our standard protocol. This histological approach confirmed that no signs of inflammation were induced in animals immunized with C-rFBG. Together, these findings show that the autoimmune response to C-rFBG is not spontaneously arthritogenic both in LEW and in BN rat strains.
The autoimmune response to autologous citrullinated fibrinogen is not able to aggravate acute arthritis
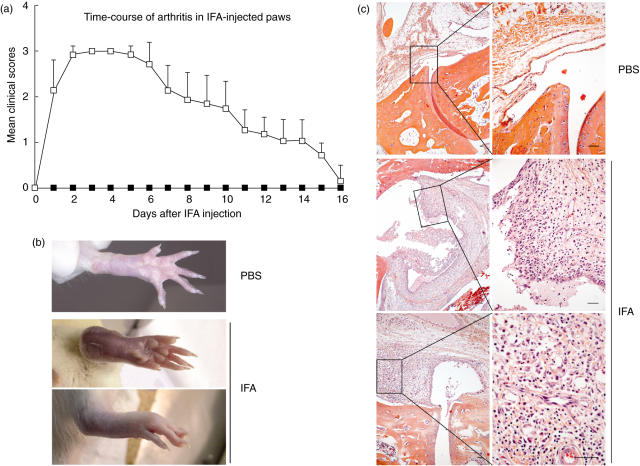
We then tested the capacity of the autoimmune response to C-rFBG to aggravate non-specifically induced joint inflammation. A previous study had shown that intra-articular injection of mineral oils such as squalene (50 µl) or IFA (10 µl) in female DA rats induced moderate joint inflammation by day 6, with oedema of synovial tissues containing many polymorphs and monocytes/macrophages, followed by induction of a severe arthritis, reaching a peak on day 21, with marked hyperplasia of the synovial tissue and recruitment of pathogenic T cells [25]. We tested the effect of intra-articular injection of IFA in female LEW rats. This rat strain was chosen because, in comparison with the BN rats, LEW rats exhibited a higher IgG response when challenged with C-rFBG (Fig. 1). IFA (50 µl) was injected into the right ankle joint and, as control, PBS was injected into the left ankle joint. Intra-articular injection of IFA induced very transitory acute inflammation: the first clinical signs, including redness and swelling of the injected paw, occurred 1 day after injection, with maximal severity at days 2–5, then progressive recession and complete disappearance at around day 18 (Fig. 5a). Animals were followed-up until 25 days after the intra-articular injection and no signs of joint inflammation relapse were observed. The presence of severe inflammation (redness and swelling) at day 3 after IFA injection can be seen clearly in the right ankle photographs presented in Fig. 5b. At that time-point, histological analysis after haematoxylin and eosin staining indicated marked hyperplasia of the synovial tissue with thickening of the lining layer and dense infiltration with polynuclear cells, macrophages and lymphocytes (Fig. 5c). In contrast, intra-articular injection of PBS induced no clinical or histological signs of inflammation (Fig. 5).
Fig 5.
Intra-articular injection of incomplete Freund's adjuvant (IFA) in the ankle of Lewis (LEW) rats induces a very transitory acute inflammation. Naive LEW rats (n = 7) were injected with 50 µl of IFA into the right ankle joint (day 0). As a control, phosphate-buffered saline (PBS) (50 µl) was injected into the left ankle joint. The right and left hind paws of all animals were examined daily for clinical signs of inflammation. The results are expressed in (a) as the mean daily clinical score (± s.d.) of the IFA-injected and of the PBS-injected ankles (white and black symbols, respectively) and correspond to one representative out of two independent experiments. Three days after the intra-articular injections, the left (PBS) and right (IFA) hind paws of the rats were photographed (b) and analysed histologically after haematoxylin and eosin staining of tissue sections (c, scale bars = 50 µm).
Intra-articular injection of IFA was also performed in rats inoculated previously with C-rFBG when the titre of autoantibodies had reached the maximal level (at day 38 after the inoculation). As controls, the intra-articular injection of IFA was performed on age-matched naive rats or on age-matched rats inoculated previously with NC-rFBG. The time-course of inflammation was very similar between naive rats and age-matched rats inoculated previously with CFA only (data not shown), indicating that previous exposure to the mineral oil that enters into the composition of both the CFA and IFA emulsions has no effect on the kinetics of the inflammation it induces when injected into the ankle joint. The course of ankle inflammation in rats inoculated with C-rFBG was similar to that observed in control rats (Fig. 6). These data show that the transitory inflammation induced by intra-articular injection of IFA in female LEW rats follows an identical course in the absence or presence of an immune response to citrullinated fibrin.
Fig 6.
In Lewis (LEW) rats, pre-immunization with citrullinated rat fibrinogen (C-rFBG) does not aggravate the arthritis induced by intra-articular injection of incomplete Freund's adjuvant (IFA). Age-matched naive LEW rats (grey symbols or bars; 12 rats) and LEW rats inoculated with C-rFGB (white symbols or bars; 14 rats) or with non-C-rFBG (black symbols or bars; 12 rats) were injected with IFA into the ankle joint 38 days after the first antigen inoculation, and examined daily for clinical signs of inflammation. The results are expressed as the mean daily clinical score of the right hind paw in each experimental group (a), and as the mean cumulative clinical score ± s.d. (calculated as the sum of the daily clinical scores from day 1 to day 13) in each experimental group (b). Results represent the pooled data of three independent experiments.
Discussion
In the present study, we have shown that FBG can become immunogenic upon citrullination, allowing the development of an IgG autoimmune response directed essentially to citrullinated determinants. In comparison with the mouse studies already mentioned, it should be stressed that in the rat, much lower amounts of autologous citrullinated FBG were immunogenic as a single injection of 50 µg of antigen induced a specific IgG reactivity detectable 12 days after inoculation. This can be compared with four inoculations of 50 µg of citrullinated mFBG in CFA that did not induce any significant antibody response in B10.S mice [15] and with 50 µg per week during 10 weeks in the presence of adjuvant (CFA or LPS), necessary for efficient immunization of Balb/c mice [16]. Moreover, whereas the majority of the autoantibodies cross-reactive with mFBG obtained after immunization of mice with hFBG are IgG1 [26], a Th2-dependent IgG subclass that cannot activate the classical pathway of complement [27,28], in both LEW and BN rats, both complement- and FcγR-fixing C-rFBG-reactive IgG were generated, holding the prospect of multiple potentially pathogenic effector mechanisms. This is similar to what is observed in human RA where ACPA are mainly IgG1 [29], i.e. can both activate FcγR- and classical complement-dependent pathways.
Another recent study showed that, in rats, citrullination of an abundant circulating autologous protein, albumin, made it able to elicit an IgG antibody response upon inoculation with IFA whereas the native form was not immunogenic [30]. The ability of FBG or albumin to break tolerance depending on whether it is or is not citrullinated is probably related to differences in the degree of exposure of the immune system to these two molecular forms. The native forms are very abundant (in mammals the blood FBG and albumin concentration is in the range of g/l and of 101 g/l, respectively) and the very important physiological functions they fulfil call for immunological tolerance. On the other hand, in the case of albumin the conditions necessary for generating the citrullinated forms of these proteins are either totally unknown (its in vivo occurrence actually remains to be established), or in the case of fibrin are thought to be associated with tissue inflammation [31,32]. This suggests a much lower pressure for tolerance of the citrullinated forms of these autoantigens.
Even though we were able to readily induce an autoimmune response to C-rFBG, we could not find a way to elicit a secondary immune response to it. This probably arises from the existence of mechanisms able to exert strong negative control on the developing immune response. The establishment of such mechanisms could be related to the concomitant development of a response to NC-rFBG, as indicated by the presence of a low reactivity to this antigen in the sera of LEW rats inoculated with C-rFBG. It was indeed shown that memory cells able to mount a strong secondary response do not persist in mice, where the immune response elicited with hFBG is the most strongly cross-reactive with autologous FBG [26]. This was shown to be due to an unknown antigen-specific regulation process, apparently not related to exhaustion of autoimmune reactive T cells or to the presence of antigen-specific regulatory T cells [26]. In our study, the absence of a secondary response following the second antigenic challenge concerned only C-rFBG and not the PAD present in the antigen preparation. This also points to the existence of antigen-specific down-modulation mechanisms. Alternatively, it could be that a secondary B cell response to C-rFBG cannot be generated because of the lack of appropriate cognate T cell help, even though the generation of IgG and IgA reactive to C-rFBG following the first immunization is highly suggestive of specific T cell help available at least in the primary response.
Concerning the arthritogenic character of C-rFBG, the study we performed in the rat agrees with the mouse studies in finding that the autoimmune response to native or citrullinated autologous FBG cannot induce joint inflammation spontaneously. Fibrin deposition in the synovial tissue is secondary to the inflammation. Citrullination of fibrin deposits in the tissue also occurs as a consequence of the synovitis [31,32]. We proposed recently that ACPA play an important role in the chronic character of human rheumatoid synovitis arguing that, in the synovial tissue, the immunological conflict between ACPA and citrullinated fibrin is self-fuelling, as the inflammation it induces leads to the formation of new fibrin deposits that will become citrullinated secondarily by a locally expressed PAD enzyme [32]. Following this hypothesis, one can speculate that the lack of arthritogenicity of the autoimmune response to C-rFBG is related to the absence of its antigenic target, citrullinated fibrin, in the synovial tissue of the immunized rats. We tested whether the presence of an autoimmune response to citrullinated fibrin would aggravate joint inflammation induced by intra-articular injection of IFA. Injection of 50 µl of IFA into the ankle joints of female LEW rats, however, induced arthritis that followed an identical course in the presence or absence of autoantibodies to C-rFBG. However, immunochemical analysis of protein extracts of the synovial tissue of IFA-injected ankle joints obtained at the time of maximal arthritis severity (3 days after the intra-articular injection) actually showed the presence of small amounts of fibrin but not of its citrullinated form (data not shown). This observation is probably related to the short duration of the arthritis we induced, briefer than that obtained by intra-articular injection of 5-times lower amounts of IFA into the ankle joint of female Dark Agouti (DA) rats [25]. Indeed, it was shown that the presence of citrullinated proteins in the joints of DA rats with arthritis induced by autologous collagen type II (CII) is correlated with the degree of joint inflammation, appearing only at least 7 days after the onset of clinical and histological signs of arthritis and still rising 24 days after and paralleling the increase in arthritis severity [30]. On the other hand, in our arthritis model 7 days after onset, the arthritis is already markedly waning and, 24 days later, it has disappeared completely.
Interestingly, in the rat strain LEW.1AV1 it was shown that the arthritis induced by autologous citrullinated CII had an earlier onset and a higher incidence than that induced by the non-citrullinated form of this antigen [30]. Moreover, it was shown very recently that mice with CIA develop IgG antibodies specific for the citrullinated forms of proteins and peptides (notably the citrullinated form of the β-chain of hFBG), appearing early after immunization with bovine CII, even before joint swelling is observed [33]. This is in drastic contrast with previous reports on the absence of such citrulline-specific antibodies in the same strain of mice with CIA and could be related to subtle differences in the immunization protocols [31,34]. Moreover and importantly, although the biochemical nature of the autoantigen(s) that these antibodies recognize in arthritic mice is still elusive, their arthritogenic role was demonstrated clearly. First, mice tolerized with a citrulline-containing peptide fail to develop these autoantibodies upon challenge with CII and demonstrate reduced severity and incidence in their CIA. Secondly, murine MoAbs reactive with citrullinated hFBG derived from mice with CIA enhance arthritis when co-administered with a submaximal dose of a pool of anti-CII antibodies [33]. However, it remains to be evaluated whether the course taken by arthritis presenting abundant citrullinated fibrin deposits is influenced by previous immunization with autologous C-FBG. For instance, if DA rats can develop an autoimmune response to C-rFBG, it would be interesting to use this strain to evaluate the influence of this response on the arthritis induced by intrarticular injection of IFA because, as mentioned, in comparison with the LEW rat strain, DA rats develop an arthritis that lasts longer [25] and is therefore associated more probably with formation of citrullinated fibrin deposits. Evaluation of the effect of previous sensitization with C-rFBG on collagen-induced arthritis (CIA) can also be proposed. Indeed, although it has not been demonstrated formally, it is highly probable that at least part of the extracellular citrullinated proteins present in the joints of DA rats with arthritis induced with autologous CII correspond to citrullinated fibrin [30]. Moreover, the presence of citrullinated fibrin-related proteins in the arthritic joints of mice with CIA or streptococcal cell-wall-induced arthritis has been demonstrated clearly [31].
In conclusion, our study has shown for the first time in the rat that an autoimmune response to autologous fibrinogen can be induced by specific immunization with its citrullinated form but that this response is neither spontaneously arthritogenic nor able to aggravate arthritis that is not associated with the formation of citrullinated fibrin deposits. However, the pathogenic role of an induced autoimmune response to citrullinated fibrin in animal models with citrullinated fibrin deposits remains to be established formally. This would strongly support the hypothesis of the role of ACPA in the maintenance of human rheumatoid synovitis. Moreover, such models should prove very useful not only for dissection of the pathophysiological pathways involved but also for the assessment and validation of new therapeutic approaches for human RA.
Acknowledgments
This study was supported by grants from the Toulouse III University, the CNRS, the INSERM and the ‘Association pour la Recherche sur la Polyarthrite’. The technical assistance of Ms R. Llobera is gratefully acknowledged. We also thank M. Calize, P. Aregui and A. Boyer (Service de zootechnie, IFR-30, Toulouse) for taking care of the animal house and Dr T. Al Saati and Ms F. Capilla (Plateau technique d'histopathologie expérimentale, IFR-30) for their assistance in histological analyses.
References
- 1.Girbal E, Sebbag M, Gomès-Daudrix V, Simon M, Vincent C, Serre G. Characterisation of the rat oesophagus epithelium antigens defined by the so-called ‘antikeratin antibodies’, specific for rheumatoid arthritis. Ann Rheum Dis. 1993;52:749–57. doi: 10.1136/ard.52.10.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon M, Girbal E, Sebbag M, et al. The cytokeratin filament-aggregating protein filaggrin is the target of the so-called ‘antikeratin antibodies’, autoantibodies specific for rheumatoid arthritis. J Clin Invest. 1993;92:1387–93. doi: 10.1172/JCI116713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebbag M, Simon M, Vincent C, et al. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1995;95:2672–9. doi: 10.1172/JCI117969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girbal-Neuhauser E, Durieux JJ, Arnaud M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–94. [PubMed] [Google Scholar]
- 6.Masson-Bessière C, Sebbag M, Girbal-Neuhauser E, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 7.Vossenaar ER, Desprès N, Lapointe E, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–50. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–5. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 9.Maccioni M, Zeder-Lutz G, Huang H, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195:1071–7. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards JC, Leandro MJ, Cambridge G. B lymphocyte depletion in rheumatoid arthritis: targeting of CD20. Curr Dir Autoimmun. 2005;8:175–92. doi: 10.1159/000082103. [DOI] [PubMed] [Google Scholar]
- 11.Vossenaar ER, van Venrooij WJ. Anti-CCP antibodies, a highly specific marker for (early) rheumatoid arthritis. Clin Appl Immunol Rev. 2004;4:239–62. [Google Scholar]
- 12.Vincent C, Nogueira L, Clavel C, Sebbag M, Serre G. Autoantibodies to citrullinated proteins. ACPA Autoimmun. 2005;38:17–24. doi: 10.1080/08916930400022582. [DOI] [PubMed] [Google Scholar]
- 13.Masson-Bessière C, Sebbag M, Durieux JJ, et al. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin Exp Immunol. 2000;119:544–52. doi: 10.1046/j.1365-2249.2000.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumonde DC, Glynn LE. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962;43:373–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin B, Sonderstrup G. Citrullination of self-proteins and autoimmunity. Scand J Immunol. 2004;60:112–20. doi: 10.1111/j.0300-9475.2004.01457.x. [DOI] [PubMed] [Google Scholar]
- 16.Hida S, Miura NN, Adachi Y, Ohno N. Influence of arginine deimination on antigenicity of fibrinogen. J Autoimmun. 2004;23:141–50. doi: 10.1016/j.jaut.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Swanborg RH. Experimental autoimmune encephalomyelitis in the rat: lessons in T-cell immunology and autoreactivity. Immunol Rev. 2001;184:129–35. doi: 10.1034/j.1600-065x.2001.1840112.x. [DOI] [PubMed] [Google Scholar]
- 18.Link H, Xiao BG. Rat models as tool to develop new immunotherapies. Immunol Rev. 2001;184:117–28. doi: 10.1034/j.1600-065x.2001.1840111.x. [DOI] [PubMed] [Google Scholar]
- 19.Holmdahl R, Lorentzen JC, Lu S, et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184–202. doi: 10.1034/j.1600-065x.2001.1840117.x. [DOI] [PubMed] [Google Scholar]
- 20.Saoudi A, Bernard I, Hoedemaekers A, et al. Experimental autoimmune myasthenia gravis may occur in the context of a polarized Th1- or Th2-type immune response in rats. J Immunol. 1999;162:7189–97. [PubMed] [Google Scholar]
- 21.Fournié GJ, Cautain B, Xystrakis E, et al. Cellular and genetic factors involved in the difference between Brown Norway and Lewis rats to develop respectively type-2 and type-1 immune-mediated diseases. Immunol Rev. 2001;184:145–60. doi: 10.1034/j.1600-065x.2001.1840114.x. [DOI] [PubMed] [Google Scholar]
- 22.Nachat R, Méchin MC, Takahara H, et al. Peptidylarginine deiminase isoforms 1–3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J Invest Dermatol. 2005;124:384–93. doi: 10.1111/j.0022-202X.2004.23568.x. [DOI] [PubMed] [Google Scholar]
- 23.Saoudi A, Kuhn J, Huygen K, et al. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993;23:3096–103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- 24.Gracie JA, Bradley JA. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217–21. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 25.Yoshino S, Yoshino J. Recruitment of pathogenic T cells to synovial tissues of rats injected intraarticularly with nonspecific agents. Cell Immunol. 1994;158:305–13. doi: 10.1006/cimm.1994.1278. [DOI] [PubMed] [Google Scholar]
- 26.Rubin B, Matron C. The mouse immune response to human fibrinogen reveals an autoimmune component against mouse fibrinogen. Cell Immunol. 2005;233:41–52. doi: 10.1016/j.cellimm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Klaus GG, Pepys MB, Kitajima K, Askonas BA. Activation of mouse complement by different classes of mouse antibody. Immunology. 1979;38:687–95. [PMC free article] [PubMed] [Google Scholar]
- 28.Neuberger MS, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–6. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- 29.Chapuy-Regaud S, Nogueira L, Clavel C, Sebbag M, Vincent C, Serre G. IgG subclass distribution of the rheumatoid arthritis-specific autoantibodies to citrullinated fibrin. Clin Exp Immunol. 2005;139:542–50. doi: 10.1111/j.1365-2249.2004.02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundberg K, Nijenhuis S, Vossenaar ER, et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther. 2005;7:R458–67. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vossenaar ER, Nijenhuis S, Helsen MM, et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489–500. doi: 10.1002/art.11229. [DOI] [PubMed] [Google Scholar]
- 32.Chapuy-Regaud S, Sebbag M, Baeten D, et al. Fibrin deimination in synovial tissue is not specific for rheumatoid arthritis but commonly occurs during synovitides. J Immunol. 2005;174:5057–64. doi: 10.4049/jimmunol.174.8.5057. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn KA, Kulik L, Tomooka B, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–73. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vossenaar ER, van Boekel MA, van Venrooij WJ, et al. Absence of citrulline-specific autoantibodies in animal models of autoimmunity. Arthritis Rheum. 2004;50:2370–2. doi: 10.1002/art.20296. [DOI] [PubMed] [Google Scholar]