Abstract
Mannan-binding lectin (MBL) is an important component of the first-line defence against infections. Evidence has shown that MBL deficiency, reducing phagocytosis and internalization of intracellular pathogens may protect the host against intracellular infections such as leprosy. In this study, we speculated whether genetically determined low MBL serum levels confer protection against Mycobacterium leprae infection. One hundred and ninety-one patients with leprosy, presenting lepromatous (n = 118), tuberculoid (n = 31), dimorph (n = 30) and indeterminate (n = 12) clinical forms and 110 healthy controls matched with the patients according to sex, age and ethnic background were investigated. MBL concentrations were measured in a double-antibody enzyme immune assay and C-reactive protein (CRP) serum levels by nephelometry. A significant negative association of MBL low values (< 100 ng/ml) was observed with lepromatous patients when comparing with controls and tuberculoid patients [10/118, 8·47% versus 21/110, 19·09% P = 0·03 χ2 with Yates' correction, odds ratio (OR) 0·39, confidence interval (CI) 0·18–0·88 and 8/31, 25·81%, P = 0·02, OR 0·27, CI 0·09–0·75, respectively]. There was no significant difference in the distribution of MBL levels between patients and controls or among the clinical forms. The concentration of CRP was significantly increased in the patients (P = 0·0002) and in the lepromatous form (P = 0·0001) when compared to controls. A weak positive correlation between MBL and CRP levels was observed in the patients (P = 0·010, R = 0·255). These data suggest a protective role for MBL deficiency against the development of the most severe and multi-bacillary form of leprosy.
Keywords: complement, leprosy, M. leprae, mannan-binding lectin
Introduction
Leprosy is an ancient chronic disease caused by Mycobacterium leprae, an obligate intracellular pathogen that mainly infects macrophages and Schwann cells. Despite the widely available and effective multi-drug therapy, the disease continues to be endemic in several developing countries. After India, Brazil presents the highest endemicity of leprosy, with a prevalence of 4·6 cases/10 000 habitants in 2004 [1].
Most individuals exposed to M. leprae are resistant to infection and do not develop clinical leprosy. Only susceptible individuals develop the disease and progress to the different clinical manifestations that range from localized to severe and systemic disease, depending on the host response to the M. leprae. At one pole of this spectrum is the lepromatous form, which is the most severe manifestation, characterized by the absence of specific cellular immunity leading to widely disseminated disease and extended multi-bacillary lesions. At the other extreme, the tuberculoid form presents a vigorous cellular response to M. leprae, which results in a few localized paucibacillary lesions [2,3]. Characteristic deformities and disabilities, due to damage to peripheral nerves and skin in untreated patients, still remain a cause of social stigma and discrimination of patients and their families [3,4].
There is evidence that the variability in host response to M. leprae infection is determined largely by genetic factors, which have an effect on both the development of leprosy per se and on the pattern of clinical manifestation [4,5].
Mannan-binding lectin (MBL) is an important component of innate immunity, which acts as a pattern recognition molecule of a wide range of infectious agents [6,7]. MBL recognizes sugar moieties such as mannose, N-acetylglucosamine, fucose and glucose present on the surface of several microorganisms, leading to their phagocytosis and activation of complement. More recent evidence shows that MBL can induce inflammatory responses by binding to receptors on phagocytes [6–8].
The presence of mutations in the promoter and exon 1 regions of MBL2 gene strongly affects the levels of circulating MBL, with pronounced interindividual variation. MBL deficiency due to the presence of MBL2 variant alleles is very frequent in different populations and is considered to be the most common immunodeficiency [7]. MBL plasma levels are also known to be affected by other factors, including growth hormone [9], and increase up to threefold during an acute phase response [10].
There is increasing evidence that MBL plays a complex role in disease, with MBL deficiency being associated with increased susceptibility to several infections, notably by extracellular pathogens [11–14], and on the other hand conferring protection against certain intracellular microorganisms [15–17]. It has been speculated that low MBL concentrations may confer a selective advantage to those individuals carrying the variant alleles. Because some microorganisms use C3 opsonization and C3 receptors to enter the cell, such as M. leprae, M. tuberculosis and Leishmania species, any reduction in the complement-activating function of the host may reduce the possibility of intracellular uptake of these organisms [18]. Such evidence that increased levels of MBL are associated with leprosy has already been seen by Garred et al. [15], who found significantly higher levels of MBL in 36 Ethiopian patients with lepromatous/borderline lepromatous leprosy. The authors also showed that MBL binds to mannose residues of M. leprae as strongly as pure mannan.
In this study, the levels of MBL was reported in Brazilian patients presenting different clinical manifestations of leprosy and the results showed that MBL deficiency might protect against lepromatous but not tuberculoid leprosy.
Materials and methods
Patients and controls
One hundred and ninety-one leprosy patients (LP) (median age of 52·06 years, range 19–94 years, 112 male and 79 female) from Curitiba, Southern Brazil were studied (Table 1). Patients were classified according to the criteria of Ridley and Jopling [19]. One hundred and eighteen (61·8%) patients were diagnosed as presenting the lepromatous form (LL) of the disease, 31 (16·2%) the tuberculoid (TL), 30 (15·7%) the borderline or dimorph form (DL) and 12 (6·3%) the indeterminate form (IDL). Type 2 reaction or erythema nodosum leprosum (ENL) was present in 33·05% (39/118) of patients with the lepromatous form. For some analyses TL, DL and IDL patients were combined in one group, named TDI. The patients were selected from out-patient clinics of the Clinical Hospital of the Federal University of Paraná and Health State Department of Paraná and among in-patients from the Sanitary Dermatology Hospital of Paraná. As healthy controls (HC), 110 healthy individuals from the same geographical region (median age of 47·82, range 19–78 years, 68 male and 43 female), matched to patients according to ethnic background, were studied. Informed consent was obtained from each patient and control and the project was approved by the local medical ethical committee.
Table 1.
Clinical and demographic profile of patients with leprosy and controls.
| Group | n | Gender male/female (%) | Age mean ± s.d. (years) | Ethnic group Caucas/other (%) | Type 2 reaction yes/no (%) |
|---|---|---|---|---|---|
| Lepromatous | 118 | 80/38(67·80/32·20) | 53·97 ± 16·37 | 93/25(78·80/21·20) | 39/79(33·00/67·00) |
| Dimorph | 30 | 21/9(70·00/30·00) | 50·17 ± 14·73 | 22/8(73·30/26·70) | – |
| Tuberculoid | 31 | 10/21(32·30/67·70) | 44·71 ± 14·24 | 23/8(74·20/25·80) | – |
| Indeterminate | 12 | 1/11(8·30/91·70) | 45·08 ± 12·59 | 10/2(83·30/16·70) | – |
| TDI | 73 | 32/41(43·80/56·20) | 47·01 ± 14·25 | 55/18(75·30/24·70) | – |
| Patients total | 191 | 112/79(58·60/41·40) | 51·31 ± 15·92 | 148/43(77·50/22·50) | 39/152(20·40/79·60) |
| Controls | 110 | 67/43(60·90/39·10) | 47·82 ± 12·79 | 87/23(79·10/20·90) | – |
TDI = tuberculoid/dimorph/indeterminate; caucas: caucasoids.
Methods
Ten ml of venous blood were collected from each subject and separated by centrifugation. Serum was kept at −20°C until tested for MBL and C-reactive protein (CRP).
MBL concentrations were measured in a double-antibody enzyme-linked immunosorbent assay (ELISA), using microplates coated with monoclonal antibody against MBL carbohydrate-binding domain (Antibody Shop, Staten Serum Institute, Copenhagen, Denmark). Bound MBL was detected by a second antibody labelled with biotin, followed by incubation with streptavidin-conjugated horseradish peroxidase (HRP) and tetramethylbenzidine (TMB). Colour intensity was read at 450 nm in an ELISA reader. MBL levels were considered as low or deficient (< 100 ng/ml), medium (100–1000 ng/ml) and high (> 1000 ng/ml), and the assay cut-off was 10 ng/ml.
Concentration of CRP, as inflammatory marker, was measured in 100 patients and in 50 controls by nephelometry in a BN* System (Dade Behring, Marburg, Germany) using polystyrene particles coated with monoclonal antibodies specific to human CRP. The concentration of CRP was considered normal when < 0·5 mg/dl.
Statistical analysis
Comparisons of MBL levels between the groups were performed using Mann–Whitney or Kruskal–Wallis tests. Tests of independence between cases and controls were performed using the χ2 test with Yates' correction, and the odds ratio (OR) and 95% confidence limits (CI) were calculated. Spearman's rank test was used for MBL and CRP correlation studies. P-values less than 5% were considered significant.
Results
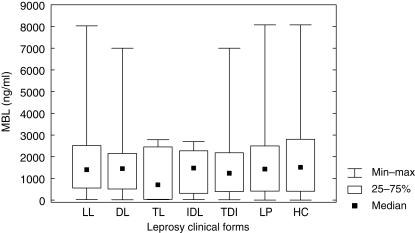
The distribution of MBL plasma levels observed in the leprosy patients and controls is shown in Fig. 1 and Table 2. MBL concentration ranged from 15·20 to 8030·40 ng/ml, median 1377·50 ng/ml, in the leprosy patients and 2·0–8076·50 ng/ml, median 1508·60 ng/ml, in controls. The tuberculoid patients presented the lowest MBL mean values when compared to those with the lepromatous form (median 743·50 ng/ml versus 1400·30 ng/ml, P = 0·09), dimorph (median 1449·55, P = 0·76) and controls (median 1508·60, P = 0·36). There was no significant difference in the overall distribution of MBL levels between patients (LP) and controls (P = n.s.) or among the clinical forms and the controls (P = n.s.). A significant increase in MBL levels was observed in male individuals in relation to females in LP (median 1592·20 ng/ml versus 876·45 ng/ml, P = 0·03) and in LL patients (median 1800·85 ng/ml versus 802·75 ng/ml, P = 0·03).
Fig 1.
Distribution of mannan-binding lectin (MBL) serum concentration in the controls and the different clinical forms of leprosy. LL = lepromatous form; DL = dimorph form; TL = tuberculoid form; IDL = indeterminate form; TDI = TL + DL + IDL form; LP = leprosy patients (total) and HC = healthy controls.
Table 2.
MBL and CRP serum concentration in patients with leprosy and controls.
| Group | n | MBL (ng/ml) (min–max) | n | CRP (mg/dl) median |
|---|---|---|---|---|
| Lepromatous | 118 | 1400·30(19·10–8030·40) | 61 | 0·51(0·32–12·90) |
| Dimorph | 30 | 1449·55(15·20–6997·90) | 18 | 0·66(0·32–8·06) |
| Tuberculoid | 31 | 743·50(19·00–2783·50) | 9 | 0·32(0·32–0·81) |
| Indeterminate | 12 | 1475·00(20·90–2697·30) | 12 | 0·32(0·32–33·50) |
| Patients total | 191 | 1377·50(15·20–8030·40) | 100 | 0·40(0·32–33·50) |
| Healthy controls | 110 | 1508·60(2·00–8076·50) | 50 | 0·32(0·32–1·29) |
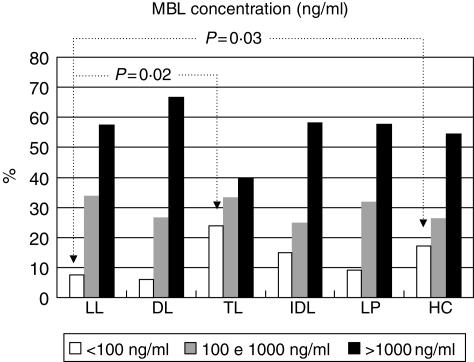
In addition, MBL deficiency (< 100 ng/ml) was decreased significantly among patients with the lepromatous form (LL) when compared to tuberculoid patients (TL) (10/118, 8·47% versus 8/31, 25·81%, P = 0·02) and controls (10/118, versus 21/110, 19·09%, P = 0·03) (Fig. 2). No significant differences were observed according to age, ethnic group and low, medium and high MBL levels (> 100, 100–1000 and > 1000 ng/ml, respectively) among patients and controls. Among the lepromatous patients with ENL reaction (39/118), three showed MBL deficiency. There was no significant difference in the presence of MBL deficiency in patients with or without ENL (3/39, 7·7% versus 7/79, 8·9%, P = 0·89). The serum concentration of CRP was significantly increased in the patients (LP) and in the lepromatous form (LL) when compared with controls (median 0·40 mg/dl versus 0·32 mg/dl, P = 0·0002, and median 0·51 mg/dl versus 0·32 mg/dl, P = 0·0001, respectively) (Table 2). A weak positive correlation in the concentrations of MBL and CRP was observed in the patients (LP) (P = 0·010 and R = 0·255).
Fig 2.
Distribution of mannan-binding lectin (MBL) concentration and median of clinical forms of leprosy. LL = lepromatous form; DL = dimorph form; TL = tuberculoid form; IDL = indeterminate form; TDI = TL + DL + IDL form; LP = leprosy patients (total) and HC =healthy controls (LL versus HC, P = 0·03 and LL versus TL, P = 0·02).
Discussion
It is known that clinical leprosy develops in only a small proportion of individuals infected with M. leprae, who progress slowly to the different clinical manifestations of the disease. The incubation period between infection and clinical disease can vary from some months to up to 30 years, with a mean period of development being 4 years for the tuberculoid and 10 years for the lepromatous forms [2]. The tuberculoid and lepromatous forms constitute opposite poles of the spectrum, being clinically, immunologically and histologically distinct from each other. More recently, it was shown that the gene expression profile also correlates accurately with the clinical forms of the disease [20].
It is well established that the host response to M. leprae varies considerably among individuals and determines leprosy phenotypes [2–5]. Elucidation of the immunological process underlying the clinical spectrum of leprosy is essential for better understanding of leprosy pathogenesis and for improvement in prevention and therapeutic approaches. Predisposition to leprosy per se and to the progression to different clinical forms may be modulated by several factors, which include early components of the immune response involved in the interaction of M. leprae with target cells. MBL plays a central role in the early interaction of pathogens with phagocytes, mediating their opsonophagocytosis directly and by the activation of complement [6,7]. The process of opsonization and phagocytosis of the M. leprae and its destruction or survival in the interior of phagocytic cells is crucial for the establishment of the disease [2,3,21].
Based on previous findings, which involved a small number (n = 36) of Ethiopian patients with lepromatous/lepromatous borderline leprosy, it has been speculated that high levels of MBL were associated with leprosy by facilitating M. leprae uptake by macrophages [14]. In this study we investigated a larger number of patients (n = 191) presenting the full spectrum of clinical forms of leprosy. We have demonstrated here that irrespective of the clinical forms, MBL levels in leprosy patients were similar to those observed in controls. When the clinical forms were considered, a significantly lower median levels of MBL was observed in patients with tuberculoid leprosy when compared with lepromatous patients. On the other hand, MBL plasma concentrations in lepromatous patients showed no significant differences from indeterminate or dimorph leprosy, or from the controls.
These results suggest that MBL levels are not associated with leprosy per se. However, by examining MBL deficiency (< 100 ng/ml), a negative association with the lepromatous form (OR = 0·39, P = 0·02) but not with the tuberculoid form or leprosy per se was observed. Patients presenting with multi-bacillary leprosy are prone to develop the type 2 reaction ENL, which is a systemic inflammatory reaction due to the extravascular deposition of immune complexes resulting in neutrophil infiltration and complement activation. Although ENL has been associated with the deficiency of complement C4B in lepromatous leprosy [22], MBL deficiency was not related to the development of ENL in this group of lepromatous patients.
MBL concentrations lower than 100 ng/ml are considered as deficient and usually associated with the presence of variant alleles [23–25]. MBL deficiency has been reported to confer partial protection against some intracellular pathogens, such as M. leprae and M. tuberculosis [15–18,23,26].
Lepromatous leprosy is the most severe clinical manifestation of leprosy due to the almost complete absence of specific cellular mediated immunity and consequent dissemination of M. leprae in most organs of the body. On the other hand, patients suffering from tuberculoid leprosy present an effective cellular immune response against M. leprae that controls the dissemination of the bacteria leading to paucibacillary lesions. Thus MBL deficiency might exert a protective role against the development of the lepromatous form but not the tuberculoid form, or leprosy per se. In fact, experimental data have indicated that susceptibility to leprosy per se and to the leprosy phenotype is controlled by different group of genes [27]. In this case, MBL2 variant alleles, which are associated with MBL deficiency, might have a role in the progression to the multi-bacillary form of leprosy, supporting the hypothesis that MBL could facilitate the uptake of M. leprae into target cells, contributing to its spread into lepromatous cases.
Because MBL deficiency is observed frequently in the general population, it has been speculated how MBL deficiency could be advantageous to the host. It seems plausible that under certain circumstances, MBL binding to pathogens would lead to excessive activation of complement being harmful to the host. Supporting this hypothesis, Takahashi et al. [28] have shown that lack of MB-LA enhances survival in a mouse model of acute septic peritonitis. In addition, MBL deficiency could be advantageous against intracellular pathogens that exploit complement deposition on their surface to enhance uptake into phagocytes, such as the case of M. leprae. Various studies corroborate this hypothesis [15–18,26,29].
Moreover, it has been reported that the promoter region of the MBL2 gene encloses several elements involved in the acute phase response [30,31]. Indeed, MBL was shown to be an acute-phase reactant whose circulating levels increase 1·5–3-fold during trauma or infections [10]. However, in comparison to CRP, a classical inflammatory marker, the increase in MBL levels during acute phase response is considered weak. This tendency was observed in the present study, with CRP values significantly higher in patients (LP) and in lepromatous cases in comparison to controls. In conclusion, these data suggest a role for MBL deficiency in susceptibility to lepromatous leprosy, warranting genetic studies of patients presenting different clinical forms of the disease.
Acknowledgments
We are grateful to the Brazilian National Research Council CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the Research Fellowship awarded to Iara Messias-Reason and to the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the Research Fellowship awarded to Lilian Pereira-Ferrari.
References
- 1.World Health Organization. Global leprosy situation. Weekly Epidemiological Record. 2005;80:285–96. [ http://www.who.int/Lep/]
- 2.Britton WJ, Lockwood DNJ. Leprosy. Lancet. 2004;363:1209–19. doi: 10.1016/S0140-6736(04)15952-7. [DOI] [PubMed] [Google Scholar]
- 3.Goulart IMB, Penna GO, Cunha G. Immunopathology of Leprosy: the complexity of the mechanisms of host immune response to Mycobacterium leprae. Rev Soc Bras Med Trop. 2002;35:365–75. doi: 10.1590/s0037-86822002000400014. [DOI] [PubMed] [Google Scholar]
- 4.Alcaïs A, Mira M, Casanova JL, et al. Genetic dissection of immunity in leprosy. Curr Opin Immunol. 2005;17:44–8. doi: 10.1016/j.coi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Araújo MG. Leprosy in Brazil. Rev Soc Bras Med Trop. 2003;36:373–82. [PubMed] [Google Scholar]
- 6.Jack DL, Klein N, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 7.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–9. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 8.Casanova JL, Abel L. Human mannose-binding lectin in immunity: friend, foe, or both? J Exp Med. 2004;199:1295–9. doi: 10.1084/jem.20040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grayholt CH, Leth-Larsen R, Lauridsen AL, et al. The effects of GH and hormone replacement therapy on serum concentrations of mannan-binding lectin, surfactant protein D and vitamin D binding protein in Turner syndrome. Eur J Endocrinol. 2004;150:355–62. doi: 10.1530/eje.0.1500355. [DOI] [PubMed] [Google Scholar]
- 10.Thiel S, Holmskov U, Hviid L, et al. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summerfield JA, Ryder S, Sumiya M, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886–9. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 12.Summerfield JA, Sumiya M, Levin M, et al. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Super M, Thiel S, Lu J, et al. Association of low levels of mannan-binding protein with a common defect of opsonization. Lancet. 1989;2:1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 14.Turner MW. Deficiency of mannan binding protein – a new complement deficiency syndrome. Clin Exp Immunol. 1991;86:53–6. doi: 10.1111/j.1365-2249.1991.tb06208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garred P, Harboe M, Oettinger T, et al. Dual role of mannan-binding protein in infections: another case of heterosis? Eur J Immunogenet. 1994;21:125–31. doi: 10.1111/j.1744-313x.1994.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 16.Bonar A, Chmiela M, Rudnicka W, et al. Mannose-binding lectin enhances the attachment and phagocytosis of mycobacteria in vitro. Arch Immunol Ther Exp. 2005;53:437–41. [PubMed] [Google Scholar]
- 17.Madsen HO, Satz ML, Hogh B, et al. Different molecular events result in low protein levels of mannan-binding lectin in populations from Southeast Africa and South America. J Immunol. 1998;161:3169–75. [PubMed] [Google Scholar]
- 18.Soborg C, Madsen HO, Andersen AB, et al. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188:777–82. doi: 10.1086/377183. [DOI] [PubMed] [Google Scholar]
- 19.Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 20.Bleharski JR, Li H, Meinken C, et al. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301:1527–30. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- 21.Remus N, Alcaïs A, Abel L. Human genetics of common Mycobacterium infections. Immunol Res. 2003;28(2):109–29. doi: 10.1385/IR:28:2:109. [DOI] [PubMed] [Google Scholar]
- 22.Messias IJT, Santamaria J, Brenden M, et al. Association of C4B deficiency (C4B*Q0) with erythema nodosum in leprosy. Clin Exp Immunol. 1993;92:284–7. doi: 10.1111/j.1365-2249.1993.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilpatrick DC. Mannan-binding lectin. clinical significance and applications. Biochim Biophy Acta. 2002;1572:104–13. doi: 10.1016/s0304-4165(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 24.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496–505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 25.Fiane AE, Ueland T, Simonsen S, et al. Low mannose-binding and increased complement activation correlate to allograft vasculopathy, ischaemia, and rejection after human heart transplantation. Eur Heart J. 2005;26:1660–5. doi: 10.1093/eurheartj/ehi198. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosio AR, Messias-Reason IJ. Leishmania (Viannia) braziliensis: interaction of mannose-binding lectin with surface glycoconjugates and complement activation. An antibody-independent defense mechanism. Parasite Immunol. 2005;27:333–40. doi: 10.1111/j.1365-3024.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 27.Mira MT. Genetic host resistance and susceptibility to leprosy. Microbes Infect. 2006;8:1124–31. doi: 10.1016/j.micinf.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Gordon J, Liu H, et al. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 2002;4:773–84. doi: 10.1016/s1286-4579(02)01597-6. [DOI] [PubMed] [Google Scholar]
- 29.Bonar A, Chmiela M, Rozalska B. Levels of mannose-binding lectin (MBL) in patients with tuberculosis. Pneumonol Alergol Pol (Pneumonologia Polska) 2004;72:201–5. [PubMed] [Google Scholar]
- 30.Ezekowit RAB, Day LE, Herman GAA. Human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J Exp Med. 1988;167:1034–46. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor M, Brickell PM, Craig RK, et al. Structure and evolutionary origin of the gene encoding a human serum mannose-binding protein. Biochem J. 1989;262:736–71. doi: 10.1042/bj2620763. [DOI] [PMC free article] [PubMed] [Google Scholar]