Abstract
Protective immune responses to tuberculosis in man are primarily cell-mediated and require the interaction of specific T cells, cytokines and activated macrophages. In the present study, Mycobacterium tuberculosis H37Rv labelled with luciferase reporter enzyme was used to analyse the anti-mycobacterial immunity in man using an in vitro whole blood infection model. Peripheral blood samples obtained from M. bovis bacille Calmette–Guérin (BCG)-vaccinated tuberculin-positive healthy volunteers (n = 23) were cultured with M. tuberculosis H37Rv reporter strain. The growth of bacteria in the whole blood cultures was monitored after 48 and 96 h of infection. The results showed that the growth of M. tuberculosis was significantly inhibited after 96 h (P < 0·029) of culture. Among the cytokines studied, interleukin (IL)-10 and IL-12 were not detected at all, whereas low levels of interferon (IFN)-γ after 96 h (0·4 IU/ml) and tumour necrosis factor (TNF)-α after 48 (135 pg/ml) and 96 h (47 pg/ml) of culture were detected in the supernatants of whole blood infected with M. tuberculosis. The magnitude of bacterial growth correlated directly with the concentration of TNF-α detected after 48 h (r = 0·722) and 96 h (r = 0·747) of culture (P ≤ 0·0001 and P ≤ 0·0001, respectively). However, the addition of monoclonal antibodies specific to TNF-α and IFN-γ to the blood cultures did not alter mycobacterial growth indicating the role of other mechanisms/factors in restricting the growth of M. tuberculosis in whole blood cultures.
Keywords: cytokines, immunity, peripheral blood, Reporter Mycobacterium tuberculosis, tuberculosis
Introduction
The global epidemic of tuberculosis is growing and becoming more dangerous [1]. It has been estimated that for the years 2002–20, 1000 million people will be infected; more than 150 million people will become sick, with 36 million deaths. Although drugs are available for the effective treatment of tuberculosis, long-term and costly therapy emphasizes the need for an effective vaccine to control the disease [1].
The success of M. tuberculosis as a pathogen resides in its ability to replicate and persist in a dormant state within macrophages for long periods of time. Therefore, understanding the mechanisms of protective immunity to M. tuberculosis in humans is essential for the development of immunotherapy and new vaccines. However, there are various difficulties associated with studies investigating anti-mycobacterial immunity, including slow growth of M. tuberculosis and the need of containment facilities [2]. There is also difficulty in demonstrating the effective inhibition of intracellular growth of M. tuberculosis in human macrophages [3]. The use of colony-forming units (CFU) to quantify the number of live organisms and to demonstrate the inhibition of mycobacterial growth is extremely laborious and requires several weeks due to the slow growth of M. tuberculosis [2].
More recently, the problems associated with CFU determinations have been overcome by the measurement of luminescence emitted by recombinant bioluminescent mycobacteria [4]. This development has been particularly useful in evaluating the in vitro immune responses and monitoring the anti-mycobacterial activity of various drugs [2,4,5]. These studies have shown that the light emitted by bioluminescent mycobacteria is dependent on the presence of live organisms and the measurement of luminescence is sensitive, stable and adaptable to the microplate format [5]. Furthermore, a linear relationship existed between CFU counts and bioluminescence [5]. However, all the in vitro studies using bioluminescent M. tuberculosis in humans have been carried out with monocytes/macrophages [4–6], which do not have all the components required for protective immunity against mycobacteria. Ideally, to include all components of the immune system mediating protective immunity to M. tuberculosis, such studies should also be carried out with whole blood.
Recently, a novel in vitro assay has been described to monitor the growth of a bioluminescent reporter strain of M. bovis bacille Calmette–Guérin (BCG) in human peripheral blood cultures [7]. In this assay, the number of viable mycobacteria in infected whole blood cultures, determined by CFU count method, correlated with the luminescence emitted. Compared to the macrophage infection model, in which the growth of M. tuberculosis was not inhibited throughout the culture period [6], the growth of M. bovis BCG was restricted in whole blood cultures within 96 h of infection [7]. Furthermore, anti-mycobacterial immunity, demonstrated by growth inhibition of M. bovis BCG, correlated with tuberculin skin test reactivity of the tested donors [7].
In the present study, for the first time we have used the in vitro peripheral blood infection model with bioluminescent recombinant M. tuberculosis H37Rv to study the growth of virulent M. tuberculosis in the presence of all possible cell types and factors required for protective immunity and analysed the effect of some of the cytokines considered important for protection against M. tuberculosis infection.
Materials and methods
Human subjects
Peripheral blood (30 ml) was collected in ethylenediamine tetraacetic acid (EDTA) tubes by vein-puncture from 23 M. bovis BCG-vaccinated and purified protein derivative (PPD)-positive healthy adult volunteers with skin test ≥ 10 mm in diameter. None of the donors had any signs and symptoms of tuberculosis at the time of blood collection or had a history of active tuberculosis. Informed consent was obtained from all the subjects and the study was approved by the Ethical Committee of the Faculty of Medicine, Kuwait University, Kuwait.
Reporter M. tuberculosis
The construction and properties of the reporter strain, luciferase-tagged M. tuberculosis H37Rv, have been described previously by Snewin et al. [6]. In brief, M. tuberculosis H37Rv was transformed with the shuttle plasmid pSMT1, which carried the lux A and B genes from Vibrio harveyi, under the control of the BCG (hsp60) promotor.
Recombinant M. tuberculosis were grown into the logarithmic phase in Middlebrook 7H9 culture medium (Difco, Detroit, MI, USA) with 10% albumin dextrose catalase (ADC) supplement (Becton Dickinson, Oxford, UK), hygromycin B (50 µg/ml; Clontech, Meadow Circle, CA, USA) and amphotericin B (10 µg/ml; Gibco laboratories, Grand Island, NY, USA) at 37°C using a shaker incubator. The culture was centrifuged at 1200 g for 30 min. The resulting bacterial pellet was resuspended in fresh 7H9 medium. Washed and autoclaved 4·5–5·5 mm diameter glass beads (BDH Chemicals Ltd, Poole, UK) were added to the bacterial culture, vortexed for 5 min and centrifuged at 200 g for 10 min. Mycobacteria in the supernatant were dispensed into vials; glycerol was added to a final concentration of 25% and the vials were frozen at −70°C. Quantification of bacteria in these vials was performed by growing them on Middlebrook 7H10 agar with 10% oleic acid albumin dextrose catalase (OADC) enrichment (Becton Dickinson), hygromycin B (50 µg/ml) and amphotericin B (10 µg/ml) in order to count the bacteria by the colony-forming unit (CFU) method.
Prior to each experiment a vial was defrosted, added to 10 ml of Middlebrook 7H9 culture medium supplemented with 10% ADC, hygromycin B and amphotericin B, and incubated with shaking at 37°C for 72 h. To prepare a single bacillus inoculum and allow for accurate quantification of bacteria, cultures of mycobacteria were processed following the procedure described by Nilsson et al. [8]. In brief, cultures of M. tuberculosis were washed with phosphate-buffered saline (PBS) and then broken into single bacilli by adding washed and autoclaved 4·5–5·5 mm diameter glass beads to the culture. The culture was then vortexed for 5–10 min with glass-beads and the supernatant was collected after standing for 5 min. Quantification of bacteria in the supernatant was performed by counting the CFU of plated serial dilutions and measurement of luminescence in 10-fold serial dilutions.
Counting by CFU
Three spots each of 10 µl of three different dilutions of the M. tuberculosis cultures were plated on Middlebrook 7H10 agar. The plates were incubated at 37°C for 3 weeks and colonies were counted [9]. Results were expressed as mean number of CFU ± s.e.
Whole blood assays
The procedure described previously by Kampmann et al. [7] was followed with slight modifications. In brief, the peripheral blood was diluted with an equal volume of RPMI 1640 (gibco Laboratories) supplemented with 1% l-glutamine and heparin (20 IU/ml; Leo Pharmaceutical Products, Ballerup, Denmark). Aliquots of diluted anti-coagulated whole blood (0·8 ml) in 7 ml screw-top, endotoxin-free Bijou tubes (Scientific Laboratory Supplies, Nottingham, UK) were inoculated with 0·2 ml of luciferase-labelled M. tuberculosis H37Rv prepared as described above (single bacilli inoculum) to obtain a final concentration of 5 × 104 relative light units (RLU)/ml. All tubes were incubated at 37°C in a shaker incubator set at 20 rev/min. Triplicate samples were analysed for the viability of M. tuberculosis at the time of inoculation (0 time), and after 48 and 96 h of culture. Aliquots of reporter M. tuberculosis H37Rv were also inoculated into tubes containing RPMI-1640–10% fetal calf serum (FCS), plasma or Middlebrook 7H9 broth.
At each time-point, red blood cells were lysed by adding 10 ml sterile distilled water containing 0·1% Triton X-100 (Sigma Chemical Co., St Louis, MO, USA) and the tubes were incubated at room temperature for 10 min. The samples were centrifuged at 1200 g for 10 min. The remaining pellet of bacteria and cells were suspended in 10 ml sterile PBS and washed again. The cell pellet was resuspended in 200 µl PBS. Samples were read for luminescence over a 20-s period, using white 96-well plates in a MLX microtitre plate luminometer (Dynex Technologies, Chantilly, VA, USA). To quantify bacterial luciferase activity, the substrate, 1% n-decyl aldehyde (Sigma), was prepared in absolute ethanol and injected automatically by the luminometer. Results were expressed as the mean of luminescence signals (RLU/ml) of triplicate wells.
Cytokine assays
To determine the concentrations of secreted cytokines, the blood culture tubes after 0, 48 and 96 h of culture were centrifuged at 1200 g for 10 min; the supernatants were collected and frozen immediately at −70°C for subsequent cytokine measurement. Commercially available enzyme-linked immunosorbent assay (ELISA) kits (Coulter Immunotech, Fullerton, CA, USA) were used to measure the concentration of the cytokines, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-12 and IL-10 according to the manufacturer's instructions. The sensitivities of these assays were as follows: 15·6 pg/ml for TNF-α, 0·39 IU/ml for IFN-γ, 16 pg/ml for IL-12 and 16 pg/ml for IL-10.
For cytokine inhibition assays, neutralizing monoclonal antibodies (mAbs) specific for IFN-γ (cat. no. 14–7318), TNF-α (cat. no. 14–7348), IL-12 (cat. no. 14–7129) and isotype control mouse IgG1 (cat. no. 14–4714) at 5 µg/ml (Bioscience, San Diego, CA, USA) were added to aliquots of diluted blood prior to infection with single cell inoculum of luciferase-tagged M. tuberculosis (200 µl) prepared as described above.
Statistical analysis
Statistical analyses were performed using spss version 13·0 for Windows software package. The differences between paired values were determined by the paired Student's t-test and P < 0·05 was considered significant. Correlation analyses were performed by the non-parametric Spearman's product–method analysis.
Results
Growth of reporter M. tuberculosis H37Rv in a whole blood infection model
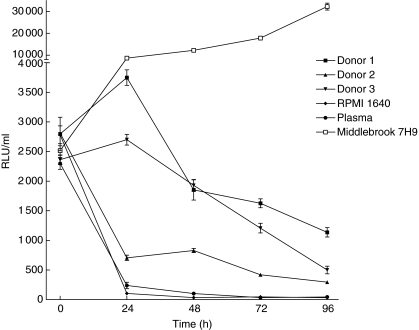
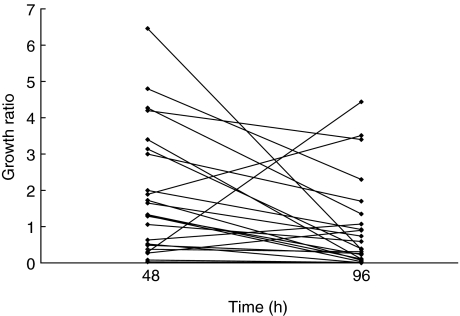
To monitor the growth of the reporter M. tuberculosis in whole blood, aliquots of fresh anti-coagulated peripheral blood from BCG-vaccinated healthy tuberculin-positive volunteers (n = 23) were cultured with 5 × 104 RLU/ml of luciferase-labelled M. tuberculosis H37Rv. A reading of 10 RLU was found to correspond to approximately 1 CFU (data not shown). The initial experiments were performed with whole blood obtained from three different donors and RLU/ml values were determined at 0, 24, 48, 72 and 96 h of culture. The results showed that compared to the start of culture (0 h), the RLU/ml values were consistently reduced after 96 h of culture (Fig. 1). However, the growth of reporter M. tuberculosis in Middlebrook 7H9 culture medium was greatly enhanced within the 96-h period (Fig. 1) and continued to grow for a period of 2 weeks of observation (Fig. 1, data shown for up to 96 h of culture). The reporter M. tuberculosis failed to grow in plasma samples and RPMI-1640–10% FCS cultures (Fig. 1). The results of the growth of reporter M. tuberculosis in whole blood cultures for all the donors (n = 23) are expressed as a growth ratio (GR) = (RLU/ml of reporter M. tuberculosis H37Rv at 48 h (or 96 h)/RLU/ml of reporter M. tuberculosis H37Rv at 0 h) (Fig. 2). The overall results further confirmed that the luminescent signal emitted by reporter M. tuberculosis H37Rv was significantly lower at 96 h (median GR = 0·39; range, 0·002–4·4) than at 48 h of culture (median GR = 1·3; range, 0·029–6·5) (P < 0·029).
Fig 1.
Kinetics of the growth of the reporter Mycobacterium tuberculosis H37Rv in the whole blood cultures. Two hundred µl suspensions of reporter M. tuberculosis H37Rv containing 5 × 104 relative light units (RLUs)/ml were inoculated into 0·8 ml of whole blood from bacille Calmette–Guérin-vaccinated tuberculin-positive healthy subjects and the RLUs were measured over 0–96 h at 24-h intervals. Light emitted (RLU/ml) readings are expressed as mean ± s.e.m. of the results obtained from three donors. Growth of reporter M. tuberculosis H37Rv in cultures containing plasma, RPMI-1640–10% fetal calf serum and Middlebrook 7H9 culture medium for 96 h is also indicated.
Fig 2.
Growth of reporter Mycobacterium tuberculosis H37Rv in whole blood cultures after 48 and 96 h. Whole blood cultures of bacille Calmette–Guérin-vaccinated tuberculin-positive healthy subjects (n = 23) were infected with reporter M. tuberculosis H37Rv and examined for growth at 48 and 96 h of infection by measuring relative light units (RLU)/ml as described in Materials and methods. Paired values from the individual subjects are connected. Growth is expressed as growth ratio (GR) = (RLU of reporter M. tuberculosis H37Rv at 48 h (or 96 h)/RLU of reporter M. tuberculosis H37Rv at 0 time. Each point represents the mean of triplicate determinations; paired values from the individual subjects are connected. At 48 h, the median of GR = 1·33, range = 0·29–6·46; at 96 h, median of GR = 0·39, range = 0·002–4·4 (P < 0·029 by paired t-test).
Cytokine secretion in infected whole blood cultures
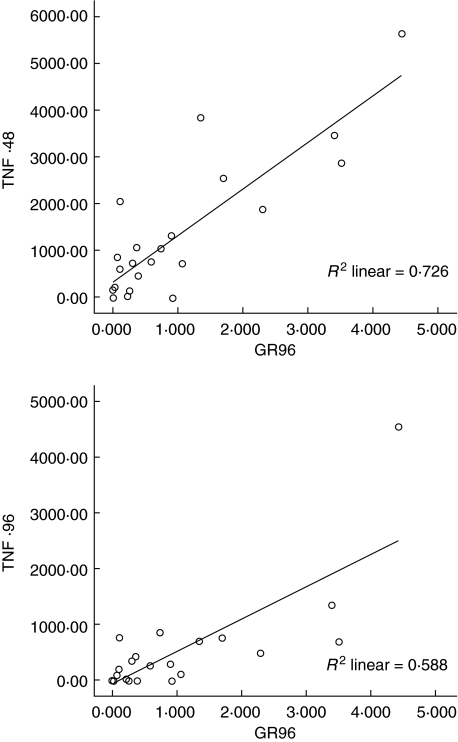
The secretion of cytokines IFN-γ, TNF-α, IL-12 and IL-10 in whole blood cultures was studied by quantification of the cytokines in the culture supernatants after 0, 48 and 96 h of infection with reporter M. tuberculosis (Table 1). The results showed that, compared to 0 h, only TNF-α was secreted at significantly higher concentrations at 48 h (mean ± s.e. = 135 ± 30 pg/ml, P < 0·000) and 96 h (mean ± s.e. = 47 ± 19 pg/ml, P < 0·027) of culture (Table 1). However, analysis of the results for correlation between M. tuberculosis growth and concentration of TNF-α showed that M. tuberculosis growth at 96 h of culture correlated directly with the concentrations of TNF-α at 48 h (r = 0·722, P ≤ 0·0001) as well as at 96 h of culture (r = 0·747, P ≤ 0·0001) (Fig. 3).
Table 1.
Concentration of cytokines detected in the supernatants of whole blood cultures of healthy subjects following infection with reporter Mycobacterium tuberculosis H37Rv.
| Median cytokine concentration (range) in the culture supernatants at | |||
|---|---|---|---|
| 0 h | 48 h | 96 h | |
| TNF-α (pg/ml) | < 15·6 (< 15·6–59) | 763 (< 15·6–5626)a | 269 (< 15·6–4545)b,c |
| IFN-γ (IU/ml) | 2·3 (< 0·39–16) | 2·88 (< 0·39–18) | 3·9 (< 0·39–99) |
| IL-12 (pg/ml) | 41 (< 16–865) | 38 (< 16–1355) | 74 (< 16–1251) |
| IL-10 (pg/ml) | < 16 (< 16–2376) | 9·3 (< 16–2612) | < 16 (< 16–1767) |
P < 0·001, compared to the value at 0 h
P < 0·0017, compared to the value at 0 h
P < 0·001, compared to the value at 48 h. IFN: interferon; IL: interleukin; TNF: tumour necrosis factor.
Fig 3.
The correlation between the concentration of tumour necrosis factor (TNF)-α and the growth of Mycobacterium tuberculosis H37Rv in whole blood cultures. The secretion of TNF-α in whole blood cultures was studied by quantification of the cytokine in the culture supernatants after 0, 48 and 96 h of infection with reporter M. tuberculosis H37Rv. The analysis of the results for correlation, using spss version 13·0, between M. tuberculosis H37Rv growth and concentration of TNF-α showed that M. tuberculosis H37Rv growth measured as growth ratio (GR) at 96 h of culture correlated directly with the concentrations of TNF-α at 48 h (r = 0·722, P ≤ 0·0001) and at 96 h of culture (r = 0·747, P ≤ 0·0001)
The effect of anti-cytokine antibodies on the growth of reporter M. tuberculosis
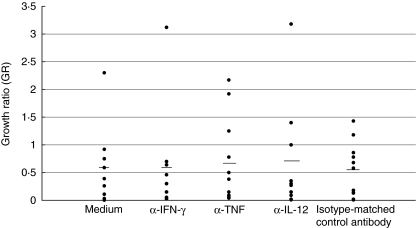
To evaluate further the effect of cytokines in inhibiting or enhancing the growth of reporter M. tuberculosis H37Rv in whole blood cultures, monoclonal antibodies (mAbs) to TNF-α, IFN-γ, IL-12 and their isotype control were added to the blood cultures of 11 donors prior to the infection with reporter M. tuberculosis H37Rv. An additional control for each donor included blood cultures infected with reporter M. tuberculosis H37Rv but lacking the mAbs. The overall results showed that the addition of anti-cytokine antibodies did not affect the growth of M. tuberculosis in whole blood cultures (P > 0·05 between cultures with and without mAbs) (Fig. 4).
Fig 4.
The effect of neutralizing monoclonal anti-cytokine antibodies on the growth of Mycobacterium tuberculosis H37Rv in whole blood cultures. Monoclonal antibodies (mAb) specific to interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interkeukin (IL)-12 were added to the whole blood cultures of tuberculin-positive healthy subjects (n = 11) prior to the infection with the reporter M. tuberculosis H37Rv. Control blood cultures from the same donors containing the reporter M. tuberculosis H37Rv with medium or isotype-matched control mAb were included. Results are expressed as the growth ratio (GR): relative light units (RLU) in the mAb-containing cultures (or control cultures) at 96 h/RLU in the mAb-containing cultures (or control cultures) at 0 h. Each point represents the mean of triplicate determinations; horizontal lines show median values. For the untreated cultures, the median GR was 0·56 and for cultures treated with α-IFN-γ, median GR = 0·53; α-TNF, median GR = 0·57; α-IL-12, median GR = 0·64; isotype-matched control, median GR = 0·54.
Discussion
In the present study, anti-coagulated whole blood cultures were used to test the ability of peripheral blood to restrict the growth of virulent M. tuberculosis in an in vitro infection model. For this purpose, venous blood samples obtained from M. bovis BCG-vaccinated and PPD-positive healthy volunteers were infected with bioluminescent recombinant M. tuberculosis H37Rv. M. bovis BCG non-vaccinated and tuberculin-negative donors are not available in Kuwait, and therefore could not be included in this study. We used EDTA tubes to collect peripheral blood and heparin to dilute it further for the whole blood assay; however, most previous studies used heparin for both steps [10]. The results showed that the whole blood from M. bovis BCG-vaccinated and PPD-positive healthy donors exerted a significant inhibitory effect on the growth of reporter M. tuberculosis within 96 h of culture compared to the enhanced growth detected in Middlebrook 7H9 culture medium supplemented with 10% ADC, hygromycin B and amphotericin B. Furthermore, plasma samples and RPMI-1640–10% FCS failed to support the growth of the reporter M. tuberculosis.
It has been shown recently that whole blood cultures infected with reporter M. bovis BCG provides a reproducible and practical method for the read-out of mycobacterial viability [7,10,11]. However, ours is the first report on the evaluation of mycobacterial immunity using reporter M. tuberculosis in whole blood cultures. A major advantage of this experimental system is that whole blood assays allow the interaction of M. tuberculosis with both cellular and humoral components of the blood, which may be important for optimal anti-mycobacterial immunity. For example, the presence of serum enhances complement-mediated uptake and hence the restriction of intracellular growth of M. tuberculosis by macrophages [12]. Furthermore, in whole blood culture a single bacillus inoculum prior to infection is prepared by breaking the clumps by the glass beads and the cultures are shaken continuously during incubation. This procedure minimizes mycobacterial clumping during the infection, which mimics conditions in vivo [13].
The exact contribution of various components of innate and acquired immunity in providing protection against M. tuberculosis is still unknown [14]. Therefore, there is a need to understand the role of various effector cells/molecules in anti-mycobacterial immunity as this could help in the development of effective prophylactic and therapeutic vaccines against tuberculosis. The in vitro assays using whole blood allow analysis of the interaction of M. tuberculosis with human monocytes/macrophages, non-adherent cells and other possible humoral mediators of protective immunity including cytokines.
Several cytokines have been suggested previously to have an inhibitory or enhancing effect on the growth of M. tuberculosis in human macrophages [15–25]. Among these, TNF-α and IFN-γ have been studied extensively [15–25]. In the present work, to study the effect of TNF-α, IFN-γ, IL-12 and IL-10 on M. tuberculosis growth in whole blood cultures, we have used two approaches. In the first approach, we determined the concentrations of these cytokines in the supernatants of cultures infected with reporter M. tuberculosis in order to determine the correlation, if any, between M. tuberculosis growth and the concentration of cytokines. In the other approach, we added neutralizing antibodies to TNF-α, IFN-γ and IL-12 to study their effect on the growth of M. tuberculosis in whole blood cultures. The results showed that significantly elevated concentrations of only TNF-α were present in the supernatants after 48 and 96 h of culture and the intracellular growth of M. tuberculosis H37Rv correlated directly with the concentration of TNF-α after 48 (r = 0·722) and after 96 h (r = 0·747) of culture (P < 0·001). Furthermore, the addition of neutralizing antibodies to IFN-γ, TNF-α and IL-12 did not affect the growth of M. tuberculosis in whole blood cultures. Although their effectiveness in blocking the relevant cytokines had not been tested directly, the lack of a significant effect of the neutralizing antibodies on the growth of M. tuberculosis confirms a previous finding using a model of M. tuberculosis-infected macrophages [16].
Previous studies performed by several groups to delineate the effect of cytokines on the growth of M. tuberculosis in human macrophages in vitro have shown controversial results [14–16,20,22]. Some of these studies have shown only weak inhibition [20,22], whereas others have shown enhancement [15,22] of the growth of virulent M. tuberculosis in human monocytes/macrophages activated with IFN-γ. Furthermore, in whole blood cultures using the CFU method, the inhibition of TNF-α production via the addition of methylprednisolone or pentoxifylline significantly enhanced the growth of avirulent M. tuberculosis H37Ra but not the virulent clinical isolate of M. tuberculosis (MP-28) in the blood of PPD-positive donors [26]. In addition, it has been shown that TNF-α alone does not activate anti-mycobacterial immunity in murine or human macrophages [21,27]. However, the presence of both TNF-α and IFN-γ stimulated anti-mycobacterial immunity in mouse macrophages [24,27] but the combination of TNF-α and IFN-γ did not induce killing of M. tuberculosis in human monocytes [24]. Therefore, it is suggested that effector molecules in mice and man are distinct and that TNF-α accelerates the proliferation of the bacilli and may serve as an evasion mechanism of virulent M. tuberculosis in man [21]. This is supported by our finding, which showed that the concentration of TNF-α in the culture supernatants correlated with the growth of M. tuberculosis H37Rv in whole blood cultures.
There are only very few reports in the published literature regarding the effects of IL-12 and IL-10 on the growth of M. tuberculosis H37Rv in human monocytes/macrophages [16,17]. These studies have shown that the growth inhibition of M. tuberculosis H37Rv in monocytes, mediated by non-adherent cells from PPD-positive subjects, was not reversed by the addition of neutralizing antibodies to IL-12 [16], and there was no correlation between IL-10 production and the growth of M. tuberculosis H37Rv in human monocytes [17]. In our study, the concentrations of both of these cytokines were very low in whole blood cultures at all time-points (Table 1), and the addition of anti-IL-12 antibodies did not affect the growth of M. tuberculosis H37Rv in these cultures. Thus, our results also do not provide any information on the role of these cytokines on restricting or enhancing the growth of M. tuberculosis H37Rv in whole blood cultures.
The difficulty in identifying a dominant cytokine capable of activating mononuclear phagocytes to control the growth of M. tuberculosis is attributed partly to the fact that some other cytokines, e.g. granulocyte–macrophage colony-stimulating factor (GM-CSF) are suggested to influence the growth of M. tuberculosis H37Rv in mononuclear phagocytes [18,28]. Thus, it is possible that a synergistic effect of multiple cytokines is required to control the growth of M. tuberculosis in human macrophages. In addition, Silver et al. [16] suggested that activation of monocytes to restrict the growth of M. tuberculosis is attributed only partly to soluble mediators and may involve contact-mediated mechanisms. Therefore, in addition to the role of cytokines, T cells may have a direct effect in regulating the growth of M. tuberculosis. This idea is supported by several studies in which αβT cells were activated to regulate the growth of M. tuberculosis by direct interaction with macrophages, thereby contributing to the production of a protective immune response [25,29,30].
In conclusion, immunity to M. tuberculosis may be induced by the interplay of multiple lymphocyte subsets and cytokines which then decides if the infection will result in progression of the disease or clearance of the organism [31]. The in vitro whole blood culture model described in this work can facilitate studies to investigate cellular and humoral mechanisms involved in the control of M. tuberculosis growth. Such models could also play a role in the evaluation of potential vaccines for the ability to induce protective responses against M. tuberculosis infection.
Acknowledgments
The work was supported by the Kuwait Foundation for the Advancement of Sciences (KFAS), grant no. 2002-07-04. We are grateful to Dr Valerie A. Snewin and Professor Douglas B. Young at Imperial College, UK for providing us with the reporter strain, luciferase-tagged M. tuberculosis H37Rv.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Hickey MA, Arain TM, Shawar RM, et al. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob Agents Chemother. 1996;40:400–7. doi: 10.1128/aac.40.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rook G, Bloom B. Mechanisms of pathogenesis in tuberculosis. In: Bloom B, editor. Tuberculosis: pathogenesis, protection and control. Washington, DC: ASM Press; 1994. pp. 485–501. [Google Scholar]
- 4.Arain TM, Resconi AE, Singh DC, Stover K. Reporter gene technology to assess activity of antimycobacterial agents in macrophages. Antimicrob Agents Chemother. 1996;40:1542–4. doi: 10.1128/aac.40.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonay M, Bouchonnet F, Pelicic V, et al. Effect of stimulation of human macrophages on intracellular survival of Mycobacterium bovis Bacillus Calmette–Guérin. Evaluation within a mycobacterial reporter strain. Am J Respir Crit Care Med. 1999;159:1629–37. doi: 10.1164/ajrccm.159.5.9807021. [DOI] [PubMed] [Google Scholar]
- 6.Snewin V, Gares MP, Gaora P, Hasan Z, Brown I, Young D. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 1999;67:4586–93. doi: 10.1128/iai.67.9.4586-4593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampmann B, Gaora PO, Snewin VA, et al. Evaluation of human antimycobacterial immunity using recombinant reporter mycobacteria. J Infect Dis. 2000;182:895–901. doi: 10.1086/315766. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson LE, Anschn S, Hoffner SE, et al. Susceptibility testing of M. tuberculosis. In: Stanley PE, McCarthy BJ, Smith R, et al., editors. ATP luminescence, rapid methods in microbiology. Oxford: Blackwell Scientific Publications Ltd; 1988. pp. 208–9. [Google Scholar]
- 9.Yoneda T, Ellner JJ. CD4+ T cell and natural killer cell-dependent killing of Mycobacterium tuberculosis by human monocytes. Am J Respir Crit Care Med. 1998;158:395–403. doi: 10.1164/ajrccm.158.2.9707102. [DOI] [PubMed] [Google Scholar]
- 10.Tena GN, Young DB, Eley B, et al. Failure to control growth of mycobacteria in blood from children infected with HIV and its relationship to T cell function. J Infect Dis. 2003;187:1544–51. doi: 10.1086/374799. [DOI] [PubMed] [Google Scholar]
- 11.Kampmann B, Tena GN, Mzazi S, et al. A novel human in vitro system to evaluate antimycobacterial vaccines. Infect Immun. 2004;72:6401–7. doi: 10.1128/IAI.72.11.6401-6407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch CS, Ellner JJ, Russel DG, Rich EA. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–53. [PubMed] [Google Scholar]
- 13.Zhang M, Gong J, Lin Y, Barnes PF. Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect Immun. 1998;66:794–9. doi: 10.1128/iai.66.2.794-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toossi Z, Mayanja-Kizza H, Kanost A, et al. Protective responses in tuberculosis: induction of genes for interferon-gamma and cytotoxicity by Mycobacterium tuberculosis and during human tuberculosis. Scand J Immunol. 2004;60:299–306. doi: 10.1111/j.0300-9475.2004.01478.x. [DOI] [PubMed] [Google Scholar]
- 15.Douvas GS, Locher DL, Vatter AE, et al. Gamma interferon activates human macrophages to become tumoricidal and leishmaniacidal but enhances replication of macrophages-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver RF, Li Q, Boom WH, Ellner JJ. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–17. [PubMed] [Google Scholar]
- 17.Silver RF, Li Q, Ellner JJ. Expression of virulence of Mycobacterium tuberculosis within human monocytes: virulence correlates with intracellular growth and induction of tumor necrosis factor alpha but not with evasion of lymphocyte-dependent monocyte effector functions. Infect Immun. 1998;66:1190–9. doi: 10.1128/iai.66.3.1190-1199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denis M, Ghadirian E. Granulocyte–macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol Lett. 1990;24:203–6. doi: 10.1016/0165-2478(90)90049-v. [DOI] [PubMed] [Google Scholar]
- 19.Denis K. Killing of Mycobacterium tuberculosis within human monocytes: activation by cytokines and calcitriol. Clin Exp Immunol. 1991;84:200–6. doi: 10.1111/j.1365-2249.1991.tb08149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho de Sousa JP, Rastogi N. Comparative ability of human monocytes and macrophages to control the intracellular growth of Mycobacterium avium and Mycobacterium tuberculosis: effect of interferon-gamma and indomethacin. FEMS Microbial Immunol. 1992;4:329–34. doi: 10.1111/j.1574-6968.1992.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 21.Engele M, Stoβel E, Castiglione K, et al. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J Immunol. 2002;168:1328–37. doi: 10.4049/jimmunol.168.3.1328. [DOI] [PubMed] [Google Scholar]
- 22.Rook GAW, Steele J, Ainsworth M, Champion BR. Activation of macrophages to inhibit proliferation of M. tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunol. 1986;59:333–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Fulton SA, Johnsen JM, Wolf SF, Sieburth DS, Boom WH. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–31. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoma-Uszynski S, Stenger S, Takeuchi O, et al. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science. 2001;291:1544–7. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 25.Bonecini-Almeida MG, Chitale S, Bousikakis I, et al. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-gamma and primed lymphocytes. J Immunol. 1998;160:4490–9. [PubMed] [Google Scholar]
- 26.Cheon SH, Kampmann B, Hise AG, et al. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin Diagn Lab Immunol. 2002;9:901–7. doi: 10.1128/CDLI.9.4.901-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flesch IE, Kaufmann SH. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis. 2004;84:93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 30.Canaday D, Wilkinson RJ, Li Q, et al. CD4+ and CD8+ T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167:2734–42. doi: 10.4049/jimmunol.167.5.2734. [DOI] [PubMed] [Google Scholar]
- 31.Bai X, Wilson SE, Chmura K, Feldman NE, Chan ED. Morphometric analysis of Th (1) and Th (2) cytokine expression in human pulmonary tuberculosis. Tuberculosis. 2004;84:375–85. doi: 10.1016/j.tube.2004.05.001. [DOI] [PubMed] [Google Scholar]