Abstract
Smokers exhibit airway inflammation and increased number of alveolar macrophages (AM), but not all develop chronic obstructive pulmonary disease (COPD). We hypothesized that AMs in COPD patients have an altered functional capacity mirrored in a different phenotype. Sixteen steroid-naive COPD patients [forced expiratory volume in 1 s (FEV1) < 70% of predicted] underwent bronchoalveolar lavage (BAL). Age- and smoking-matched non-obstructive smokers (n = 10) and healthy non-smokers (n = 9) served as controls. Nine COPD patients had a BAL cell yield sufficient for flow cytometry analysis, where expression of AM cell surface markers reflecting various functions was determined. AMs from COPD patients showed decreased expression of CD86 (co-stimulation) and CD11a (adhesion) compared to smokers' AMs (P < 0·05). Furthermore, smokers' AMs showed lower (P < 0·05) expression of CD11a compared to non-smokers. AM expression of CD11c was higher in the COPD and smokers groups compared to non-smokers (P < 0·05). The expression of CD54 (adhesion) was lower in smokers' AMs compared to non-smokers (P < 0·05), whereas CD16 was lower (P < 0·05) in COPD patients compared to non-smokers. The AM expression of CD11b, CD14, CD58, CD71, CD80 and human leucocyte antigen (HLA) Class II did not differ between the three groups. The AM phenotype is altered in COPD and further research may develop disease markers. The lower AM expression of CD86 and CD11a in COPD implies a reduced antigen-presenting function. Some alterations were found in smokers compared to non-smokers, thus indicating that changes in AM phenotype may be associated with smoking per se. The functional relevance of our findings remains to be elucidated.
Keywords: alveolar macrophages, bronchoalveolar lavage, chronic obstructive pulmonary disease, flow cytometry, phenotype
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease associated with chronic inflammation of the airways and lung parenchyma [1,2]. Tobacco smoking is the main aetiological factor of COPD pathogenesis resulting in a four- to sixfold increase in concentration of inflammatory cells in bronchoalveolar lavage (BAL) fluid [3,4].
The predominant cell type in BAL fluid is the alveolar macrophage (AM), which has several important functions. Phagocytosis is a key function in the innate immune response [5,6] and fundamental in the defence against microorganisms, but also in the resolution of inflammation and tissue damage where phagocytosis of apoptotic and necrotic cells is essential. Furthermore, macrophages can act as antigen-presenting cells and may be capable of activating the adaptive host response [7–9]. Additionally, there are several macrophage-derived products of interest in the context of smoke-induced inflammation and tissue destruction of the lungs. The alveolar macrophage products include cytokines and chemokines with the capacity of recruiting other inflammatory cells to the lungs [6]. Furthermore, alveolar macrophages produce cysteine proteinases and matrix metalloproteinases which are involved in tissue destruction preceding emphysema [10].
Consequently, macrophages have a potential role in the pathogenesis of COPD. However, despite the fact that all smokers exhibit increased number of alveolar macrophages, not all of them develop chronic airflow obstruction [11], suggesting a different inflammatory response in COPD patients compared to ‘healthy’ smokers. Thus, a more profound comprehension of the pathogenesis of COPD may be obtained by analysing the profile of inflammatory cells, particularly the macrophages, present in the airways of patients with COPD, and compare this with that of smokers without airways obstruction.
Because an altered functional capacity of AMs may be reflected in cell surface antigen expression, we hypothesized that COPD patients have a different alveolar macrophage phenotype compared to ‘healthy’ smokers and non-smokers. Therefore, the primary aim of this study was to characterize BAL fluid from COPD patients, smokers and non-smokers regarding alveolar macrophage phenotype. Specifically, we wanted to investigate the expression of selected cell surface molecules by alveolar macrophages with flow cytometry. We also wanted to compare BAL fluid regarding cell concentration and differential counts between COPD patients and two groups of control subjects, ‘healthy’ smokers and non-smokers.
Subjects and methods
Patients and controls
Twenty-three patients between 39 and 69 years of age (mean age 57) with moderate to severe COPD were enrolled with intent to undergo bronchoscopy and BAL. However, during bronchoscopy, three patients did not have BAL due to clinical constraints. In three patients BAL fluid contents could not be analysed due to low recovery, and one patient was excluded due to a lower respiratory tract infection. For the remaining 16 patients, demographic and lung function data are given in Table 1. There was no difference in demographic or lung function data between the 16 patients that underwent BAL compared to the 23 patients included initially.
Table 1.
Characteristics and lung function data for chronic obstructive pulmonary disease (COPD) patients, ‘healthy’ smokers (HS) and non-smokers (NS).
| COPD (n = 16) | HS (n = 10) | NS (n = 9) | |
|---|---|---|---|
| Age (years) | 57 (55–60) | 57 (50–62) | 54 (52–59) |
| Tobacco (pack-years) | 34 (26–43)### | 28 (24–42)††† | 0 |
| Sex (male/female) | 9/7 | 8/2 | 5/4 |
| FEV1/VC (%) | 50 (42–55)***### | 75 (73–83) | 77 (76–80) |
| FEV1 (% of predicted) | 53 (47–61)***### | 95 (90–114) | 108 (99–109) |
| Reversibility (% of predicted FEV1) | 6 (1–7) | 3 (0–5) | 1 (0–3) |
Data are shown as mean and standard deviation for age, median and inter quartile range for all others. Significant difference between groups is marked with * (COPD versus HS), # (COPD versus NS) and † (HS versus NS). The considered levels of significance were P < 0·05 ( *, # or †), P < 0·01 (**, ## or ††) and P < 0·001
,
or
.
FEV1: forced expiratory volume in 1 s, measured post-bronchodilation; VC: vital capacity.
All COPD patients had a smoking history of more than 10 pack-years, and all had a post-bronchodilator forced expiratory volume in 1 s (FEV1)/vital capacity (VC) < 70% and a post-bronchodilator FEV1 < 70% of that predicted. Three of the 16 COPD patients had quit smoking. None had clinical or radiological signs of any lung disease other than COPD. Two groups of controls were included: one group (n = 10) of current smokers (matched with regard to age and pack-years) with normal spirometry values and one group (n = 9) of age-matched healthy non-smokers (Table 1). All subjects in the two control groups had a normal chest X-ray. There was no significant difference in tobacco consumption, assessed as pack-years, between COPD and ‘healthy’ smokers. No participant had a history of allergy or a history suggesting asthma. In addition, in vitro screening for the presence of specific IgE antibodies against common inhaled allergens (Phadiatop®, Pharmacia-Upjohn, Uppsala, Sweden) was negative in all participants, and none of the COPD patients had > 0·5% eosinophils in peripheral blood. None of the patients or control subjects were treated with inhaled or oral steroids within 3 months prior to the study, and all were in a stable clinical condition defined as the absence of exacerbations for the last 3 months. All subjects included in the study gave their informed consent to participate, and the study protocol had the approval of the Ethics Committee, Karolinska University Hospital, Stockholm, Sweden.
Lung function tests
All participants performed a dynamic spirometry in a standardized manner (Vitalograph®, Buckingham, UK). Both slow vital capacity and forced vital capacity were performed, before and 10 min after inhalation with two doses of 0·5 mg terbutalin (Bricanyl® Turbuhaler®; AstraZeneca, Södertälje, Sweden), and reversibility was calculated.
Bronchoscopy and BAL
After premedication with morphine–hyoscine (Morfin-skopolamin, Meda, Solna, Sweden) intramuscularly and topically applied lidocaine (Xylocain®; AstraZeneca), bronchoscopy was performed with a flexible fibreoptic bronchoscope (Olympus F Type P30, Olympus Optical Co. Ltd, Tokyo, Japan). BAL was performed by wedging the bronchoscope into one of the subsegments of the middle lobe. For BAL, five 50-ml aliquots of warmed phosphate-buffered saline (PBS) was instilled and aspirated. The BAL procedure was interrupted if the BAL fluid return appeared to be low or if the patient had extensive coughing, or desaturated below 90% despite oxygen supplement. Of the 16 COPD patients, nine had a complete BAL performed (250 ml), seven had an instilled BAL fluid volume of less than 250 ml due to low recovery (n = 2), or coughing or desaturation (n = 5). The fluid was collected in a silicone-treated bottle kept on ice, which was transported immediately to the laboratory.
Handling of BAL fluid
Bronchoalveolar lavage (BAL) fluid was strained through a Dacron net (Millipore, Cork, Ireland) and the volume of recovered fluid was measured. Cells were pelleted by centrifugation at 394 g, 4°C, for 10 min and the supernatants were poured off. The cell pellets were resuspended in RPMI-1640 medium (Sigma-Aldrich, Irvine, UK). Cells were counted in a Bürker chamber, and total cell viability was determined by Trypan blue exclusion. Smears for differential counts were prepared by cytocentrifugation at 28 g for 3 min (Cytospin 2 Shandon; Southern Products Ltd, Runcorn, UK), whereupon cells were stained with May–Grünwald–Giemsa and 500 cells were counted.
Immunostaining and quenching of autofluorescence
Among the 16 COPD patients in whom a successful BAL procedure could be performed, a sufficient number of cells for flow cytometry analysis were acquired in nine patients. Of these nine COPD patients, all were current smokers. Lung function parameters and demographic data in this group did not differ compared to the 16 included initially.
The cell pellet was distributed to conical polypropylene tubes (1 × 106 cells/tube) and washed (4°C, 394 g, 6 min) in 3 ml cold PBS. An appropriate amount of monoclonal antibodies (mAbs) and 100 µl of PBS were then added to each pellet and incubated in the dark on ice for 30 min. A panel of antibodies reflecting various macrophage functions such as adhesion (CD54, CD11a, CD11b and CD11c), activation (CD14, CD71), phagocytosis (CD16), antigen presentation [human leucocyte antigen (HLA) class II] and co-stimulation (CD86, CD80) were used; details are given in Table 2. Irrelevant mouse IgG antibodies of the same isotype and concentration served as controls.
Table 2.
Characterization of fluorescent-labelled antibodies.
| Specificity | Other name | Clone | Isotype | Major function |
|---|---|---|---|---|
| CD11a | LFA-1 | MHM24 | IgG1 | Adhesion |
| CD11b | CR-3 | 2LPM19c | IgG1 | Adhesion |
| CD11c | CR-4 | KB90 | IgG1 | Adhesion |
| CD14 | LPS-receptor | TÜK4 | IgG2a | Activation |
| CD16 | FCγRIII | DJ130c | IgG1 | Fc-receptor |
| CD54 | ICAM-1 | 6·5B5 | IgG1 | Adhesion |
| CD58* | LFA-3 | BRIC-5 | IgG2a | Adhesion |
| CD71 | Transferrin-receptor | BER-T9 | IgG1 | Activation |
| CD80* | B7·1 | DAL-1 | IgG1 | Co-stimulation |
| CD86 | B 7·2 | BU63 | IgG1 | Co-stimulation |
| HLA class II | HLA-DP, DQ, DR | CR3/43 | IgG1 | Antigen presentation |
All antibodies manufactured by Dako AS, Glostrup, Denmark, except
, manufactured by Serotec, Oxford, UK. HLA: human leucocyte antigen; LFA: leucocyte function antigen; LPS: lipopolysaccharide.
After one further wash (4°C, 394 g, 6 min) in 2 ml PBS, an appropriate amount of secondary antibody (phycoerythrin-conjugated rabbit-anti-mouse) was added and incubated on ice for 30 min After one wash (3 ml PBS) the surface-immunostained cells were fixed by incubating them with 4% paraformaldehyde (PFA) for 5 min at 20°C.
In smoking subjects, alveolar macrophage autofluorescence hampers the detection of fluorochrome-labelled antibodies [12]. In order to facilitate flow cytometric analysis, the alveolar macrophage autofluorescence was quenched according to a previously described method [13]. Cells were washed (394 g, 8 min) in 3 ml PBS, whereupon 200 µl of 0·6%n-octyl-α-D-glucopyranoside (OG) (Sigma, St Louis, MO, USA) was added and incubated (5 min, 20°C). Finally, the cells were treated with 200 µl crystal violet for 5 min at 4°C and washed twice in 2 ml PBS.
For the purpose of assessing the effect on AM autofluorescence of the quenching procedure, two additional cell suspensions without antibody labelling were prepared: one without quenching and one with quenching.
Flow cytometry analysis
Each cell suspension was analysed in a flow cytometer (FACScalibur; Becton Dickinson, Mountain View, CA, USA). The instrument was cleaned carefully before every new analysis and was calibrated daily with standardized fluorescent particles. The macrophage population in each sample was gated according to the light scattering properties presented in a two-parameter scatter plot. Fluorescence in the macrophage gate was assessed and quantified in arbitrary units as mean fluorescence intensity (MFI). The MFI was assessed for untreated and quenched alveolar macrophages from each participant. Subsequently, the MFI was assessed for each antibody-labelled cell suspension and the background level, as indicated by the irrelevant IgG mouse antibody of the same isotype, was subtracted.
Statistical methods and data management
Statistical comparisons in order to test differences between the three groups were made by use of the Kruskall–Wallis analysis of variance (anova) and median test. In addition, the Nemenyi test was used in post-hoc analysis. After corrections of P-values according to the Nemenyi test, the 5, 1 and 0·1% levels of significance were considered. In the case of a statistically significant result the probability value (P-value) has been given. Correlations were calculated according to Spearman.
Results
BAL cell recovery and differential counts
BAL characteristics for all patients and controls are given in Table 3. The BAL fluid return (expressed as ml as well as percentage of instilled volume) in the COPD group was lower (P < 0·001) compared to both control groups [14]. Recovery did not differ between the two control groups (Table 3). The ‘healthy’ smokers group had a higher BAL cell yield compared to both COPD (P < 0·01) and non-smokers (P < 0·001), whereas COPD had a higher (P < 0·01) cell yield than non-smokers. The cell concentration in BAL did not differ between COPD and ‘healthy’ smokers, but both these groups had higher concentration compared to the non-smoking group (P < 0·001 for both). The cell viability was lower in COPD compared to both control groups (P < 0·001 for both), but did not differ between the two control groups. The predominant cell type in BAL cell smear was alveolar macrophages, and the macrophage percentage was higher in both COPD and ‘healthy’ smokers (P < 0·05 and P < 0·001, respectively) compared to non-smokers, but did not differ between COPD and ‘healthy’ smokers. Consequently, the lymphocyte percentage was higher in the non-smoking group compared to ‘healthy’ smokers (P < 0·001) and COPD (P < 0·01). The neutrophil percentage was higher in the COPD group as well as in the non-smokers group, compared to the ‘healthy’ smokers group, but no difference was observed between COPD and non-smokers. In addition, macrophage concentration in BAL was higher in COPD and ‘healthy’ smokers (P < 0·001 for both) compared to non-smokers, but did not differ between COPD and ‘healthy’ smokers. Eosinophil concentration in BAL was higher in COPD compared to both ‘healthy’ smokers (P < 0·01) and non-smokers (P < 0·05).
Table 3.
BAL characteristics for patients with chronic obstructive pulmonary disease (COPD), ‘healthy’smokers (HS) and non-smokers (NS). Differential counts are also given.
| COPD (n = 16) | HS (n = 10) | NS (n = 9) | |
|---|---|---|---|
| Recovery (ml) | 87 (39–108)***### | 144 (130–168) | 178 (147–198) |
| (% of instilled volume) | 35 (23–45)***### | 59 (52–67) | 71 (59–79) |
| Viability (%) | 80 (75–87)***### | 95 (92–98) | 94 (91–95) |
| Total cell yield (× 106) | 23 (17–48)**## | 81 (52–109)††† | 13 (12–14) |
| Cell concentration (× 106/l) | 340 (199–605)### | 585 (357–776)††† | 73 (68–91) |
| Macrophages (× 106/l) | 279·7 (188·0–547·5)### | 567·9 (307·4–783·8)††† | 63·1 (53·7–86·5) |
| (%) | 96·7 (91·9–98·3)# | 97·6 (97·2–98·9)††† | 92·3 (86·8–95·0) |
| Lymphocytes (× 106/l) | 5·5 (2·4–11·1) | 5·9 (3·2–11·5) | 4·6 (4·0–7·2) |
| (%) | 1·2 (0·7–4·7)## | 1·8 (0·9–2·4)††† | 6·6 (4·2–9·0) |
| Neutrophils (× 106/l) | 2·8 (1·1–7·9) | 2·4 (1·6–3·3) | 0·7 (0·7–2·6) |
| (%) | 1·1 (0·6–1·8)* | 0·4 (0·3–0·6)†† | 1 (1·0–3·3) |
| Eosinophils (× 106/l) | 0·8 (0–1·6)***# | 0 (0–0·2) | 0 |
| (%) | 0·2 (0·1–0·5)** | 0 (0–0·2)† | 0·1 |
Data are shown as median and interquartile range. Significant differences between groups are marked with
(COPD versus HS)
(COPD versus NS)
(HS versus NS).
The considered levels of significance were P < 0·05 (*, # or †), P < 0·01
,
or
P < 0·001
,
or
.
Macrophage autofluorescence
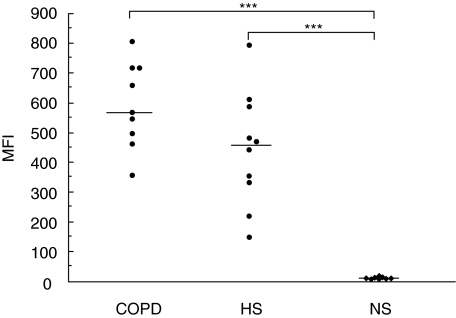
Autofluorescence, assessed as MFI, in the COPD group (567, range 496–714) did not differ from the ‘healthy’ smokers group (455, range 333–585) (median and inter quartile range). Both these smoke-exposed groups had a higher (P < 0·001) autofluorescence compared to the non-smokers (11, range 7–15) (Fig. 1). After quenching, autofluorescence was reduced in all three groups but still showed no difference between the COPD group (15, range 10–23) and the ‘healthy’ smokers group (16, range 13–21). Both smoke-exposed groups had higher post-quenching autofluorescence (P < 0·01) compared to non-smokers (3, range 3–5).
Fig 1.
Flow cytometry analysis of alveolar macrophage autofluorescence. Autofluorescence of alveolar macrophages, before quenching, is given as mean fluorescence intensity (MFI) for the three groups: chronic obstructive pulmonary disease (COPD), ‘healthy’ smokers (HS) and non-smokers (NS). Each point depicts the result from one subject and the median of the group is marked with a horizontal bar. Significant difference between groups is shown as ***P < 0·001. Statistical analysis was performed using Kruskal–Wallis and Nemenyi tests.
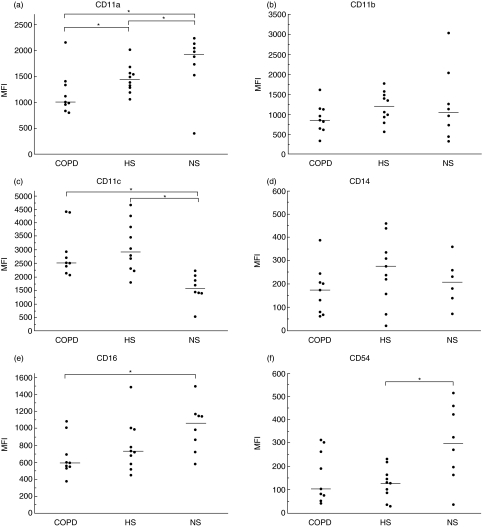
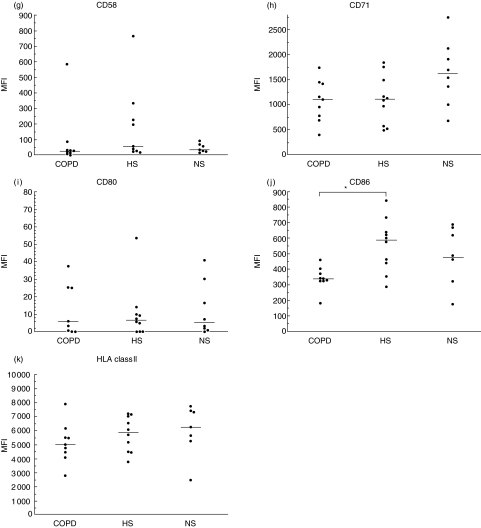
Macrophage expression of cell surface molecules
Results are illustrated in Fig. 2. Due to technical reasons, flow cytometry data are missing in one subject of non-smoking controls. The expression of the co-stimulatory molecule CD86 was lower (P < 0·05) in the COPD group compared to the ‘healthy’ smokers, but did not differ between COPD and non-smokers, nor between ‘healthy’ smokers and non-smokers. CD11a, which is thought to reflect cell adhesion, had a lower expression in COPD compared to both control groups (P < 0·05 for both). Additionally, the ‘healthy’ smokers were found to express a lower (P < 0·05) level of CD11a compared to non-smokers. The adhesion molecule CD54 was found to be less expressed in ‘healthy’ smokers compared to non-smokers (P < 0·05), but it did not differ between COPD and ‘healthy’ smokers. Expression of the adhesion molecule CD11c was higher in COPD and ‘healthy’ smokers compared to non-smokers (P < 0·05 for both), but there was no difference between COPD and ‘healthy’ smokers. The Fc-receptor, CD16, which is associated with phagocytosis, was found to be less expressed in COPD compared to non-smokers (P < 0·05), but no difference was found between COPD and ‘healthy’ smokers, nor between the two control groups, even though ‘healthy’ smokers had a tendency of less expression. In the remaining analyses (CD11b, CD14, CD58, CD71, CD80 and HLA Class II) there were no statistically significant differences between groups.
Fig 2.
Flow cytometry analysis of cell surface molecules of alveolar macrophages. A short description of surface molecules is given in Table 2. Results are presented as mean fluorescence intensity (MFI) for the three groups: chronic obstructive pulmonary disease (COPD), ‘healthy’ smokers (HS) and non-smokers (NS). Each point depicts the result from one subject and the median of the group is marked with a horizontal bar. Significant difference between groups is shown as *P < 0·05. Statistical analysis was performed using Kruskal–Wallis and Nemenyi tests.
Results for the markers of CD11a, CD16, CD54, CD71 and HLA class II showed a common pattern, although statistically significant only for CD11a, CD16 and CD54, of gradually decreasing expression with highest expression in the non-smoking group, an intermediate result in the ‘healthy’ smokers group and lowest expression in the COPD group.
Correlations between lung function and macrophage expression of cell surface molecules
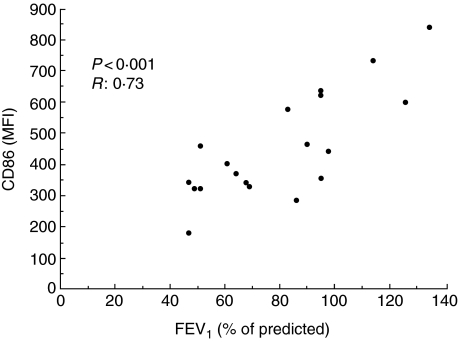
When pooling the three groups of participants (COPD, ‘healthy’ smokers and non-smokers), the results show a positive Spearman correlation between lung function (assessed as FEV1% of predicted) and alveolar macrophage expression of CD86 (P < 0·001, R: 0·65), CD11a (P < 0·01, r = 0·57), CD58 (P < 0·05, r = 0·59) and CD16 (P < 0·05, r = 0·38). When pooling the two smoke-exposed groups (COPD and HS), FEV1 (% of predicted) correlates positively with CD86 (P < 0·001, r = 0·73), CD11a (P < 0·05, r = 0·56) and CD58 (P < 0·05, r = 0·59). The correlation between FEV1 (% of predicted) and CD86 is presented in Fig. 3.
Fig 3.
The expression of CD86 in alveolar macrophages, presented as mean fluorescence intensity (MFI), correlates to the lung function assessed as forced expiratory volume in 1 s (FEV1) (% of predicted) (P < 0·001, r = 0·73). The figure presents data from chronic obstructive pulmonary disease (COPD) patients and ‘healthy’ smokers, and each point depicts the result from one participant. Statistical calculation performed using Spearman' test.
Discussion
In this study we specifically investigated alveolar macrophage phenotype, cell recovery and differential counts in BAL fluid. The analysis of macrophage phenotype showed a lower expression of the co-stimulatory molecule CD86 and of the adhesion molecule CD11a in COPD compared to ‘healthy’ smokers. Additionally, the Fc-receptor associated CD16 was less expressed in COPD compared to non-smokers. We also found that the expression of the adhesion molecules CD11c was higher and CD54 was lower in ‘healthy’ smokers compared to non-smokers. Furthermore, in concordance with previous studies [3,4], we found an increase in BAL cell number in COPD and smoking controls compared to non-smokers. In addition, the neutrophil percentage in COPD was higher compared to smokers.
Resting AMs have few HLA class II molecules and few co-stimulatory B7 molecules expressed on their cell surface [15,16]. Under normal conditions, AMs are considered to be poor antigen-presenting cells [17,18], and the principal mechanism of inducing an adaptive immune response is regarded to be T cell activation via dendritic cells in the regional lymph nodes. In contrast, AMs seem to have the capacity to present antigens to T cells under inflammatory conditions [19,20], and have been reported to stimulate specific T cell clones in vitro [8,9]. In addition to presentation of antigenic peptides, a co-stimulatory signal provided by B7 molecules is necessary for the induction of a proper immune response [16]. Our finding of a lower expression of AM CD86 (also known as the co-stimulatory molecule B 7·2) in COPD suggests an impaired capacity to activate T cells. A potentially reduced capacity to activate the adaptive immune system is interesting, as an increased exacerbation frequency seems to be associated with a more rapid decline in FEV1 [21,22]. Impaired alveolar macrophage cytokine response to bacterial antigen stimulation has been reported recently in COPD [23], and our results of decreased CD86 are in line with an impaired immune response in COPD, and thus a susceptibility to chronic bacterial colonization as well as an increased exacerbation frequency.
We believe that our findings regarding lower expression of CD11a and CD16 in COPD further support the hypothesis that a reduced host defence against airway infection in COPD may be reflected in alveolar macrophage phenotype. CD11a is a component of leucocyte function antigen (LFA)-1, which is of importance, e.g. in cell–cell adhesion during antigen presentation. A lower expression of CD11a in COPD compared to ‘healthy’ smokers as well as non-smokers may reflect a decreased adhesion capacity of alveolar macrophages in COPD. Similarly, the observation of a lower expression of the Fc-receptor CD16 in COPD compared to non-smokers may imply a decreased capacity of Fc-mediated phagocytosis.
In a previously published study [24], AM phenotype in COPD was studied with flow cytometry using a repertoire of markers partly overlapping the repertoire in this study. Interestingly, that group also observed a lower AM expression of a co-stimulatory molecule (CD80) in COPD, potentially implying an impaired antigen-presenting function. However, a straightforward comparison of results is not possible, due to both differences in study populations considering concomitant lung nodules on X-ray and inhaled corticosteroids and methodological differences.
The increased susceptibility to bacterial infection in COPD may be associated with a general deficiency in the functions of the innate immune system. Recently, the AM expression of Toll-like receptor 2 (TLR2) was reported to be lower in COPD patients and non-obstructed smokers compared to non-smokers, suggesting a reduced capacity to respond to bacterial infection [25].
Smoking per se has been found previously to be associated with an altered alveolar macrophage phenotype. Our result regarding a higher expression of the adhesion molecule CD11c in smokers compared to non-smokers is in line with some previous reports [26,27], even though immunocytochemistry analysis has reported contrasting data [28]. Similarly, the lower expression of the adhesion molecule CD54 in ‘healthy’ smokers compared to non-smokers are in line with previous data [26].
Several alterations in the AM phenotype seem to be present in COPD [24,25,29]. Some alterations may contribute functionally to the pathogenesis of COPD but some may, without possessing pathogenetic potential of their own, merely occur in parallel with functionally relevant alterations. Smoke-induced alterations in AM phenotype with actual influence on the pathogenetic process may occur with or without differences between COPD patients and ‘healthy’ smokers: on one hand, a smoke-induced alteration of the immunological response could be enhanced further in a subpopulation, thus contributing to the development of airways obstruction. This could potentially be valid for our results regarding CD11a, for which the expression is lower in ‘healthy’ smokers than in non-smokers, and reduced even further in COPD patients. Similar tendencies, although not statistically significant, were observed for several markers. On the other hand, a smoking-related immunological alteration could be a potential pathogenetic factor despite no difference being observed between the obstructed smokers group (COPD) and the non-obstructed smokers (‘healthy’ smokers), as for our results regarding CD11c and CD54. In this latter case, the immunological alteration would be necessary, but not sufficient in itself for the development of disease. In other words, one particular immunological alteration may contribute to a progressive decline in lung function only in subjects who possess other concomitant (host or environmental) pathogenetic factors.
The issue of decreased viability in COPD versus controls needs to be considered. The percentage of viable cells in our study is on the same level as in one previous report [30], although others have reported higher cell viability [24]. To our knowledge, there are no previous studies reporting an altered phenotype in BAL cells due to low viability. In addition, non-viable cells with major abnormalities in cell surface structure may exhibit changes in granularity and size, thus falling out of the macrophage gate. Furthermore, an analysis of covariance was performed in order to control for differences in viability between groups, and no changes in significance levels in the tests were noticed. Taken together, we do not believe that our findings of altered expression of cell surface molecules in COPD are due to lower viability, although a certain influence cannot be ruled out.
The intracellular autofluorescence of AM is a marker of endocytosed fluorescent particles from cigarette smoke [31,32]. Furthermore, the AM autofluorescence in smokers who quit show a decline rate that has been found to correlate negatively with the tobacco consumption assessed as pack-years [33]. As expected in our study, autofluorescence in the COPD group did not differ from the ‘healthy’ smokers group.
The finding of a higher fraction of neutrophils in BAL fluid from COPD patients compared to ‘healthy’ smokers, but not compared to non-smokers, contrasts with the finding regarding the neutrophil concentration in BAL fluid which did not differ between groups. We observed a BAL fluid neutrophil concentration in the COPD group that is lower than some previous reports [3,4], but on the same level as others [24]. However, the phenomenon of variability in BAL fluid return in COPD [14,34] renders the BAL fluid analysis difficult to interpret. This problem has been addressed by the European Respiratory Society (ERS) taskforce group on BAL, which recommends the results of cellular BAL analysis to be expressed preferably as a percentage of total inflammatory cell content [35]. Differences in COPD patients' BAL neutrophil content between studies may be influenced by clinical differences in the studied COPD and control subject populations. The fact that all patients in this study were in a stable clinical condition, and none of them had a history of respiratory tract infection within 3 months prior to the investigation, might explain a lower neutrophil concentration in this study compared to some previous studies. Other parameters with potential influence on BAL neutrophil count that may differ between studies are the degree of chronic bronchitis, the level of airflow obstruction and medication with inhaled corticosteroids.
In conclusion, this study describes differences in airway inflammatory cell profile in COPD compared to ‘healthy’ smokers and non-smokers. In addition, the alveolar macrophage phenotype is altered in COPD patients and further studies may lead to a development of disease markers. Changes in AM phenotype may, however, be an effect of smoking per se. Functional studies of the innate immune response as well as of the capacity to induce adaptive immune responses in COPD are of interest to better understand the pathogenesis.
Acknowledgments
The authors would like to acknowledge Heléne Blomqvist, Margitha Dahl, Benita Dahlberg and Gunnel de Forest for excellent technical assistance. The authors would also like to thank Per Näsman for assistance regarding statistical methods. The study was supported by grants from the Swedish Heart Lung Foundation, King Oscar II Jubilee Fund, the Cancer and Allergy Foundation, the Swedish Foundation for Health Care Sciences and Allergy Research, Karolinska Institutet and Boehringer-Ingelheim/Pfizer.
References
- 1.Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–67. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 2.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:258–66. doi: 10.1513/pats.200504-045SR. discussion 290–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson AB, Daughton D, Robbins RA, Ghafouri MA, Oehlerking M, Rennard SI. Intraluminal airway inflammation in chronic bronchitis. Characterization and correlation with clinical parameters. Am Rev Respir Dis. 1989;140:1527–37. doi: 10.1164/ajrccm/140.6.1527. [DOI] [PubMed] [Google Scholar]
- 4.Linden M, Rasmussen JB, Piitulainen E, et al. Airway inflammation in smokers with nonobstructive and obstructive chronic bronchitis. Am Rev Respir Dis. 1993;148:1226–32. doi: 10.1164/ajrccm/148.5.1226. [DOI] [PubMed] [Google Scholar]
- 5.Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002;121:156S–159S. doi: 10.1378/chest.121.5_suppl.156s. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–88. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–21. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 8.Lem VM, Lipscomb MF, Weissler JC, et al. Bronchoalveolar cells from sarcoid patients demonstrate enhanced antigen presentation. J Immunol. 1985;135:1766–71. [PubMed] [Google Scholar]
- 9.Grunewald J, Eklund A, Wigzell H, Van Meijgaarden KE, Ottenhoff TH. Bronchoalveolar lavage cells from sarcoidosis patients and healthy controls can efficiently present antigens. J Intern Med. 1999;245:353–7. doi: 10.1046/j.1365-2796.1999.00482.x. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro SD. Proteinases in chronic obstructive pulmonary disease. Biochem Soc Trans. 2002;30:98–102. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skold CM, Eklund A, Hallden G, Hed J. Autofluorescence in human alveolar macrophages from smokers: relation to cell surface markers and phagocytosis. Exp Lung Res. 1989;15:823–35. doi: 10.3109/01902148909069629. [DOI] [PubMed] [Google Scholar]
- 13.Hallden G, Skold CM, Eklund A, Forslid J, Hed J. Quenching of intracellular autofluorescence in alveolar macrophages permits analysis of fluorochrome labelled surface antigens by flow cytofluorometry. J Immunol Meth. 1991;142:207–14. doi: 10.1016/0022-1759(91)90108-r. [DOI] [PubMed] [Google Scholar]
- 14.Lofdahl JM, Cederlund K, Nathell L, Eklund A, Skold CM. Bronchoalveolar lavage in COPD: fluid recovery correlates with the degree of emphysema. Eur Respir J. 2005;25:275–81. doi: 10.1183/09031936.05.00033504. [DOI] [PubMed] [Google Scholar]
- 15.Janeway A, Travers P, Walport M, Shlomchik M. Immunobiology. 6. New York: Garland Publishing; 2001. pp. 319–335. [Google Scholar]
- 16.Chelen CJ, Fang Y, Freeman GJ, et al. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 co-stimulatory cell surface molecules. J Clin Invest. 1995;95:1415–21. doi: 10.1172/JCI117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipscomb MF, Lyons CR, Nunez G, et al. Human alveolar macrophages: HLA-DR-positive macrophages that are poor stimulators of a primary mixed leukocyte reaction. J Immunol. 1986;136:497–504. [PubMed] [Google Scholar]
- 18.Lyons CR, Ball EJ, Toews GB, Weissler JC, Stastny P, Lipscomb MF. Inability of human alveolar macrophages to stimulate resting T cells correlates with decreased antigen-specific T cell-macrophage binding. J Immunol. 1986;137:1173–80. [PubMed] [Google Scholar]
- 19.Agea E, Forenza N, Piattoni S, et al. Expression of B7 co-stimulatory molecules and CD1a antigen by alveolar macrophages in allergic bronchial asthma. Clin Exp Allergy. 1998;28:1359–67. doi: 10.1046/j.1365-2222.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 20.Jansen HM. The role of alveolar macrophages and dendritic cells in allergic airway sensitization. Allergy. 1996;51:279–92. doi: 10.1111/j.1398-9995.1996.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 21.Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358–64. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berenson CS, Wrona CT, Grove LJ, et al. Impaired alveolar macrophage response to haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 24.Pons AR, Noguera A, Blanquer D, Sauleda J, Pons J, Agusti AG. Phenotypic characterisation of alveolar macrophages and peripheral blood monocytes in COPD. Eur Respir J. 2005;25:647–52. doi: 10.1183/09031936.05.00062304. [DOI] [PubMed] [Google Scholar]
- 25.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skold CM, Lundahl J, Hallden G, Hallgren M, Eklund A. Chronic smoke exposure alters the phenotype pattern and the metabolic response in human alveolar macrophages. Clin Exp Immunol. 1996;106:108–13. doi: 10.1046/j.1365-2249.1996.d01-805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaberg T, Lauer C, Lode H, Fischer J, Haller H. Increased number of alveolar macrophages expressing adhesion molecules of the leukocyte adhesion molecule family in smoking subjects. Association with cell-binding ability and superoxide anion production. Am Rev Respir Dis. 1992;146:1287–93. doi: 10.1164/ajrccm/146.5_Pt_1.1287. [DOI] [PubMed] [Google Scholar]
- 28.Hoogsteden HC, van Hal PT, Wijkhuijs JM, Hop W, Verkaik AP, Hilvering C. Expression of the CD11/CD18 cell surface adhesion glycoprotein family on alveolar macrophages in smokers and non-smokers. Chest. 1991;100:1567–71. doi: 10.1378/chest.100.6.1567. [DOI] [PubMed] [Google Scholar]
- 29.Domagala-Kulawik J, Maskey-Warzechowska M, Kraszewska I, Chazan R. The cellular composition and macrophage phenotype in induced sputum in smokers and ex-smokers with COPD. Chest. 2003;123:1054–9. doi: 10.1378/chest.123.4.1054. [DOI] [PubMed] [Google Scholar]
- 30.Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–76. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 31.Skold CM, Andersson K, Hed J, Eklund A. Short-term in vivo exposure to cigarette-smoke increases the fluorescence in rat alveolar macrophages. Eur Respir J. 1993;6:1169–72. [PubMed] [Google Scholar]
- 32.Skold CM, Eklund A, Hed J, Hernbrand R. Endocytosis of cigarette-smoke condensate by rabbit alveolar macrophages in vitro measured as fluorescence intensity. Eur Respir J. 1992;5:53–8. [PubMed] [Google Scholar]
- 33.Skold CM, Hed J, Eklund A. Smoking cessation rapidly reduces cell recovery in bronchoalveolar lavage fluid, while alveolar macrophage fluorescence remains high. Chest. 1992;101:989–95. doi: 10.1378/chest.101.4.989. [DOI] [PubMed] [Google Scholar]
- 34.Klech H, Hutter C. Clinical guidelines and indications for bronchoalveolar lavage (BAL) Eur Respir J. 1990;3:937–76. Report of the European Society of Pneumology Task Group on BAL. [PubMed] [Google Scholar]
- 35.Haslam PL, Baughman RP. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J. 1999;14:245–8. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]