Abstract
Allograft rejection remains a major cause of morbidity and mortality following lung transplantation and is associated with an increased expression of T cell proinflammatory cytokines. We have shown that CD4+ T cell proinflammatory cytokine production was significantly reduced in peripheral blood and bronchoalveolar lavage (BAL) of stable lung transplant patients, consistent with immunosuppression therapy. However, analysis of inflammatory cytokine profiles of intraepithelial T cells in bronchial brushing (BB) may be more relevant than peripheral blood or BAL T cells for assessing immune graft status. To investigate the immunomodulatory effects of currently used immunosuppressive regimens on bronchial intraepithelial T cell cytokine production, whole blood, BAL and BB from stable lung transplant patients and control volunteers were stimulated in vitro and cytokine production by CD8+ and CD4+ T cell subsets determined using multi-parameter flow cytometry. In bronchial intraepithelial T cell subsets in control subjects and transplant patients there was compartmentalization of interferon (IFN)-γ and tumour necrosis factor (TNF)-α production, a decrease in interleukin (IL)-2 production by CD4+ T cells and CD4 : CD8 inversion compared with blood and BAL. Although there was a decrease in T cell proinflammatory cytokine production in blood of transplant patients, this was not found in BAL or bronchial intraepithelial CD8 T cell subsets, suggesting that the same level of immunosuppression may not occur in the lung of transplant recipients. Drugs that effectively reduce CD8 T cell proinflammatory cytokine production in the lung compartment may improve current protocols for reducing graft rejection in these patients.
Keywords: bronchial brushings, flow cytometry, immunosuppression, intracellular cytokines, intraepithelial T cells, lung transplant
Introduction
Survival after lung transplantation is less than 50% after 5 years. Allograft rejection remains a major cause of morbidity and mortality following lung transplantation and is associated with an increase in proinflammatory cytokine expression in graft-infiltrating T cells [1]. Acute rejection has been shown to be associated with an increase in interleukin (IL)-2 and other proinflammatory cytokines such as interferon (IFN)-γ and tumour necrosis factor (TNF)-α, while chronic rejection or obliterative bronchiolitis (OB) is associated with a modest increase in proinflammatory cytokine production and also transforming growth factor (TGF)-β[2,3].
Immunosuppressive drugs used to reduce proinflammatory cytokine expression levels are closely monitored to their ‘therapeutic range’. We have shown that CD4+ T cell proinflammatory cytokine production was significantly reduced in peripheral blood from stable lung transplant patients consistent with the effects of immunosuppression therapy [4]; however, inhibition of proinflammatory cytokines by CD8+ T cells was less effective. We have also shown recently that there was a significant decrease in T cell proinflammatory cytokine production in bronchoalveolar lavage (BAL) compared with blood in control subjects but not in stable transplant patients [5] suggesting that the pulmonary-derived cells are more active in the latter group. However, analysis of cytokine expression within the graft may be more relevant for assessing lung transplantation and may give the greatest diagnostic and prognostic value [6]. As lung parenchymal biopsies are invasive, an alternative may be the analysis of cells and cytokines in minimally invasive bronchial brushings (BB).
To investigate the immunomodulatory effects of currently used immunosuppressive regimens on bronchial intraepithelial T cell cytokine production, whole blood, BAL and BB from stable lung transplant patients and control volunteers were stimulated in vitro and cytokine production by CD8+ and CD4+ T cell subsets determined using multi-parameter flow cytometry.
Materials and methods
Patient and control groups
Thirteen lung transplant recipients with no clinical or histopathological evidence of current acute or chronic rejection or infection, scheduled for routine surveillance bronchoscopy, were invited to participate in the study and informed consent obtained (Table 1) following institutional ethics approval. All patients were tested to exclude infection including cytomegalovirus (CMV) [histopathologically, rapid viral culture and CMV polymerase chain reaction (PCR) of BAL] and mycoplasma (enzyme immunoassay of BAL). One patient was retested at their next routine surveillance bronchoscopy. All transplant patients were at least 3 months post-transplant. Details of patients and previous acute rejection episodes are presented in Table 2. Immunosuppression therapy comprised combinations of either cyclosporin A (CsA) or tacrolimus (Tac) with prednisolone and azathioprine. Trough plasma drug levels of either CsA or Tac were within recommended therapeutic ranges (Table 2). Ten healthy age-matched volunteers were recruited as controls. Venous blood was collected and added to 10 U/ml preservative free sodium heparin (DBL, Sydney, Australia) and BAL was collected from the lung transplant patients and volunteers and samples were processed within 2 h of collection.
Table 1.
Demographic details of the populations studied; lung transplant patients and control subjects (mean ± s.d.).
| Subjects | Control | Transplant |
|---|---|---|
| No. of subjects | 10 | 9* |
| Age (years) | 44 (±8) | 41 (±13) |
| FEV1, % pred | 100·4 (±24) | 72·3 (±24) |
| FVC, % pred | 99 (±14) | 78·0 (±14) |
| FEV1, % FVC | 96·4 (±26) | 77·3 (±17) |
Nine patients, 14 episodes. FEV: forced expiratory volume in 1 s; FVC: forced vital capacity.
Table 2.
Lung transplant patients and previous acute cellular rejection (ACR) episodes.
| Patient | Predisposing pathology | Time post-transplant at FOB† | Grade ACR‡ | No. prior ACR episodes | Grade prior ACR episodes | Time post-transplant (current) | CsA/Tac levels* |
|---|---|---|---|---|---|---|---|
| 1 | Cystic fibrosis | 6 m | A0B0 | 1 | A2Bx | 20 m | Tac 14·5 |
| 1 | Cystic fibrosis | 12 m | A0B0 | 1 | A2Bx | ||
| 2 | Bronchiectasis | 6 m | A0B0 | 0 | 6 m | Tac 9 | |
| 3 | Pulmonary hypertension | 3 m | A0BX | 0 | 19 m | CsA 276 | |
| 3 | 9 m | A0B0 | 0 | CsA 216 | |||
| 4 | Congenital bronchial webbs | 12 m | A0BX | 2 | A3B0, A2B0 | 30 m | CsA 185 |
| 4 | Congenital bronchial webbs | 18 m | A0B0 | 2 | A3B0, A2B0 | CsA 235 | |
| 5 | Emphysema | 3 m | A0B0 | 0 | 18 m | CsA 195 | |
| 6 | Pulmonary hypertension | 6 m | A0BX | 0 | 19 m | CsA 276 | |
| 7 | Emphysema | 6 m | A0B1 | 1 | A2B0 | 13 m | CsA 152 |
| 7 | 10 m | A0B0 | 1 | A2B0 | CsA 180 | ||
| 8 | Pulmonary fibrosis | 9 m | A0BX | 0 | 12 m | Tac 15 | |
| 8 | 6 m | A0B1 | 1 | Tac 12 | |||
| 9 | Cystic fibrosis | 9 m | A0B0 | 0 | 18 m | CsA 120 |
Therapeutic range for cyclosporin A (CsA) (80–250 µg/l) and tacrolimus (Tac) (5–20 µg/l).
Time in months post-transplant at fibreoptic bronchoscopy.
Acute rejection grade.
Leucocyte counts
Full blood counts, including white cell differential counts, were determined on blood specimens using a CELL-DYN 4000 (Abbot Diagnostics, Sydney, Australia). Blood films were stained by the May–Grunwald–Giemsa method and white cell differential counts checked by morphological assessment microscopically. BAL and BB cell counts were determined using a haemocytometer using standard techniques.
Bronchoalveolar lavage (BAL)
Aspirated BAL samples (3 × 50 ml aliquots) were collected as described previously [7] and transferred to 50 ml polypropylene tubes. For each collection from an individual, BAL specimens 2 and 3 were pooled and cell counts determined as described above and cells were then pelleted by centrifugation at 500 g for 5 min. Supernatant was discarded and cells resuspended at 4 × 105 cells/ml in Roswell Park Memorial Institute (RPMI)-1640 media (Gibco, New York, USA) supplemented with 125 U/ml penicillin and 125 U/ml streptomycin (Gibco).
Bronchial brushings (BB)
Fibreoptic bronchoscopy was performed as described previously [7]. Bronchial epithelial cells were obtained with several passages of the brush into each airway in order to avoid bleeding. Cells were deposited by washing the brush in 10 ml RPMI-1640 in 10 ml conical polypropylene tubes (Johns Professional Products, Sydney, Australia) and kept on ice. Cells were processed within 1 h of collection.
Absolute lymphocyte and CD4+ and CD8+ T cell counts
One hundred µl of peripheral blood, BAL and BB was stained with appropriately diluted fluorescently conjugated monoclonal antibodies to CD8 fluorescein isothiocyanate (FITC) (BD Biosciences, Sydney, Australia), CD4 phycoerythrin (PE) (BD) and CD3 PC5 (Beckman Coulter, Sydney, Australia) as described previously [4,5]. Samples were analysed by gating using forward scatter (FSC) versus side scatter (SSC) to exclude platelets and debris. Gated cells were analysed with CD45/CD14 to ascertain that cells were of lymphoid origin, as reported previously [4]. Control staining of leucocytes with anti-mouse IgG1–FITC/IgG1a–PE/IgG1–PC5 was performed on each sample and background readings of < 2% were obtained. A minimum of 5000 CD3+ cells from blood and BAL and 3000 CD3+ T cells from BB was acquired in list mode format for analysis.
Leucocyte stimulation
Two ml aliquots of prepared BAL or BB or one ml aliquots of blood (diluted 1 : 2 with RPMI-1640 medium) were placed in a 10-ml sterile conical polyvinyl chloride (PVC) tubes (Johns Professional Products). Phorbol myristate (25 ng/ml) (Sigma, Sydney, Australia) and ionomycin (1 µg/ml) (Sigma) was added for T cell cytokine stimulation. Brefeldin A (10 µg/ml) was added as a ‘Golgi block’ (Sigma) and the tubes reincubated in a humidified 5% CO2/95% air atmosphere at 37°C for 24 h.
Cytokine determination
Cytokine determination was performed as reported previously [4,5]. Briefly, at 24 h 100 µl 20 mm ethylenediamine tetraacetic acid (EDTA)/phosphate-buffered saline (PBS) was added to the culture tubes which were vortexed vigorously for 20 s to remove adherent cells. To lyse red blood cells in the blood cultures, 2 ml of FACSlyse solution (BD) was added and tubes incubated for 10 min at room temperature in the dark. After centrifugation at 500 g for 5 min and decanting, 0·5 ml 1 : 10 diluted FACSperm (BD) was added to each blood, BAL and BB culture tube, mixed, and incubated for a further 10 min at room temperature in the dark. Two ml 0·5% bovine serum albumin (Sigma)/Isoton II (Beckman Coulter) was then added and the tubes centrifuged at 300 g for 5 min. After decanting supernatant, Fc receptors were blocked with 10 µl human immunoglobulin (Intragam, CSL, Parkville, Australia) for 10 min at room temperature. Five µl of appropriately diluted anti-CD8 (BD) and anti-CD3 PC5 (Coulter/Immunotech) PE-conjugated anti-cytokine monoclonal antibodies to IL-2, IL-4, IFN-γ, TNF-α (BD) and TGF-β (IQ Products, Groningen, the Netherlands) or isotype control monoclonal antibody was added for 15 min in the dark at room temperature. Two ml of 0·5% bovine serum albumin (Sigma)/Isoton II (Beckman Coulter) was then added and the tubes centrifuged at 300 g for 5 min. After decanting, cells were analysed within 1 h on a FACSCalibur flow cytometer using CellQuest software (BD). Samples were analysed by live gating using FL3 staining versus side scatter (SSC). A minimum of 5000 CD3+ low SSC events from blood and BAL and 3000 CD3+ low SSC events from BB were acquired in list-mode format for analysis. The CD3+ T cell gating strategy also included cells that had FSC and SSC characteristics of viable cells [8]. Control staining of cells with anti-mouse IgG1–PE/IgG–PC5 was performed on each sample and background readings of < 2% were obtained.
Statistical analysis
Statistical analysis was performed using the non-parametric Mann–Whitney and Pearson correlation tests using spss software and differences between groups of P < 0·05 considered significant.
Results
Absolute blood CD4+ and CD8+ T cell counts
There was no significant difference between the absolute blood leucocyte count for patient and control groups (7·9 ± 4·3 and 6·5 ± 2·3 × 109/l, mean ± s.d. for patient and control groups, respectively, P > 0·05). There was no significant difference in the absolute lymphocyte counts for patient and control groups (1·3 ± 0·6 and 1·3 ± 0·7 × 109/l, mean ± s.d. for patient and control groups, respectively, P > 0·05).
There was no significant difference in the absolute T lymphocyte count for patient and control groups (1·4 ± 1·2 and 1·5 ± 1·3 × 109/l, mean ± s.d. for patient and control group, respectively, P > 0·05).
The percentage of CD4+ T cells was reduced significantly in the transplant compared to control groups (53 ± 11 and 69 ± 5, mean ± s.d. for patient and control groups, respectively, P = 0·033). The percentage of CD8+ T cells was significantly increased in the transplant compared to control groups (47 ± 11 and 31 ± 7, mean ± s.d. for patient and control groups, respectively, P = 0·047). The percentage of CD4−CD8− and CD4+ CD8+ T cells was < 2% for all patient and control subjects.
Absolute BAL CD4+ and CD8+ T cell and leucocyte counts
There was no significant difference between the absolute BAL leucocyte count for patient and control groups (0·60 ± 0·60 and 0·45 ± 0·43 × 109/l, mean ± s.d. for patient and control groups, respectively, P = 0·328). There was no significant difference in the absolute T cell counts for the groups (0·02 ± 0·05 and 0·02 ± 0·02 × 109/l, mean ± s.d. for patient and control groups, respectively). There was a significant increase in the absolute macrophage counts for the patient group (0·490 ± 0·108 and 0·284 ± 0·078 × 106/l, mean ± s.d. for patient and control groups, respectively, P = 0·005). There was a significant decrease in the absolute neutrophil counts for the patient group (0·072 ± 0·070 and 0·100 ± 0·032 × 106/l, mean ± s.d. for patient and control groups, respectively, P = 0·010).
There was a significant decrease in the percentage of CD4+ T cells in the BAL of transplant patients compared with controls (50 ± 16 and 71 ± 11, P = 0·004) and a significant increase in the percentage of CD8+ T cells in the BAL of transplant patients compared with controls (50 ± 16 and 29 ± 11 for transplant and control groups, respectively, P = 0·004).
Intracellular T cell cytokines from BB compared with blood and BAL from controls
There was a significant decrease in the percentage of CD4+ and a significant increase in the percentage of CD8+ T cells in BB compared to blood (Table 3). There was an increase in the percentage of CD8+ T cells producing IFN-γ and TNF-α, a decrease in the percentage of CD4+ and CD8+ T cells producing IL-2, and a decrease in the percentage of CD8+ T cells producing TGF-β in BB compared to blood.
Table 3.
Percentage of T cells producing intracellular cytokines in blood and bronchial brushing (BB) of the control group (mean ± s.d.). There was a significant decrease in the percentage of CD4+ and a significant increase in the percentage of CD8+ T cells in BB. There was a significant increase in the percentage of CD8+ T cells producing interferon (IFN)-γ and tumour necrosis factor (TNF)-α, a significant decrease in the percentage of CD4+ and CD8+ T cells producing interleukin (IL)-2 in BB compared to blood.
| CD3 | IFN-γ | IL-2 | IL-4 | TGF | TNF-α | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| Blood | 69 ± 4·4 | 31 ± 6·9 | 18 ± 7·8 | 20 ± 5 | 40 ± 17 | 6 ± 4 | 0·8 ± 0·4 | 0·5 ± 0·3 | 4 ± 2 | 3 ± 2 | 43 ± 12 | 20 ± 6 |
| BB | 36 ± 19 | 64 ± 19 | 15 ± 6 | 45 ± 17 | 10 ± 8 | 3 ± 0·2 | 0·5 ± 0·3 | 0·9 ± 0·7 | 3 ± 1 | 1 ± 0·3 | 20 ± 6 | 37 ± 17 |
| P | 0·003 | 0·003 | 0·065 | 0·019 | 0·001 | 0·038 | 0·560 | 0·189 | 0·185 | 0·064 | 0·017 | 0·025 |
There was a decrease in the percentage of CD4+ and an increase in the percentage of CD8+ T cells in BB compared to BAL (Table 4). There was an increase in the percentage of CD4+ and CD8+ T cells producing IFN-γ and IL-2 and CD8+ T cells producing TNF-α in BB. There was a decrease in the percentage of CD4+ and CD8+ T cells producing IL-4 in BB compared to BAL.
Table 4.
Percentage of T cells producing intracellular cytokines in bronchoalveolar lavage (BAL) and bronchial brushing (BB) of the control group (mean ± s.d.). There was a significant decrease in the percentage of CD4+ and a significant increase in the percentage of CD8+ T cells in BB. There was a significant increase in the percentage of CD4+ and CD8+ T cells producing and interleukin (IL)-2 and CD8+ T cells producing tumour necrosis factor (TNF)-α in BB. There was a significant decrease in the percentage of CD4+ and CD8+ T cells producing IL-4 in BB compared to BAL.
| CD3 | IFN-γ | IL-2 | IL-4 | TGF | TNF-α | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| BAL | 71 ± 11 | 29 ± 11 | 4 ± 2·7 | 6 ± 5 | 3 ± 0·8 | 0·8 ± 0·7 | 8·5 ± 2·4 | 5 ± 2 | 2 ± 2 | 0·5 ± 0·5 | 14 ± 8 | 7·5 ± 6 |
| BB | 36 ± 19 | 64 ± 19 | 15 ± 6 | 45 ± 17 | 10 ± 8 | 3 ± 0·2 | 0·5 ± 0·3 | 0·9 ± 0·7 | 3 ± 1 | 1 ± 0·3 | 20 ± 6 | 37 ± 17 |
| P | 0·002 | 0·003 | 0·041 | 0·000 | 0·028 | 0·042 | 0·001 | 0·002 | 0·198 | 0·358 | 0·530 | 0·000 |
Intracellular T cell cytokines from BB compared with blood and BAL from transplant patients
There was a decrease in the percentage of CD4+ and an increase in the percentage of CD8+ T cells in BB compared to blood (Table 5). There was an increase in the percentage of CD8+ T cells producing IFN-γ, IL-4 and TNF-α, a decrease in the percentage of CD4+ T cells producing IL-2 and CD4+ and CD8+ T cells producing TGF-β in BB compared to blood.
Table 5.
Percentage of T cells producing intracellular cytokines in blood and bronchial brushing (BB) of the lung transplant group (mean ± s.d.). There was a significant decrease in the percentage of CD4+ and a significant increase in the percentage of CD8+ T cells in BB. There was a significant increase in the percentage of CD8+ T cells producing interferon (IFN)-γ, interleukin (IL)-4 and tumour necrosis factor (TNF)-α, a significant decrease in the percentage of CD4+ T cells producing IL-2 and CD4+ and CD8+ T cells producing transforming growth factor (TGF)-β in BB compared to blood.
| CD3 | IFN-γ | IL-2 | IL-4 | TGF | TNF-α | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| Blood | 53 ± 11 | 47 ± 11 | 13 ± 7 | 26 ± 15 | 18 ± 13 | 4 ± 4 | 1 ± 0·8 | 0·9 ± 0·4 | 3·2 ± 2 | 3 ± 1·7 | 18 ± 14 | 16 ± 12 |
| BB | 37 ± 11 | 63 ± 11 | 17 ± 7 | 40 ± 13 | 8 ± 4 | 3 ± 1·6 | 1·2 ± 0·6 | 2·3 ± 0·8 | 2 ± 0·9 | 1·7 ± 1 | 18 ± 7 | 30 ± 11 |
| P | 0·002 | 0·002 | 0·085 | 0·026 | 0·042 | 0·300 | 0·179 | 0·043 | 0·039 | 0·028 | 0·738 | 0·002 |
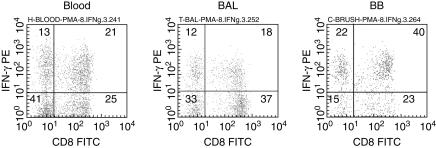
There was a significant decrease in the percentage of CD4+ and an increase in the percentage of CD8+ T cells in BB (Table 6). There was an increase in the percentage of CD8+ T cells producing IFN-γ and TNF-α, and a decrease in the percentage of CD4+ and CD8+ T cells producing IL-4 and TGF-β in BB compared to BAL. Representative dot plots showing IFN-γ production by CD8+ and CD8− (CD4+) T cells from blood, BAL and BB in a lung transplant patient are shown in Fig. 1.
Table 6.
The percentage of T cells producing intracellular cytokines in bronchoalveolar lavage (BAL) and bronchial brushing (BB) of the lung transplant group (mean ± s.d.). There was a significant decrease in the percentage of CD4+ and a significant increase in the percentage of CD8+ T cells in BB. There was a significant increase in the percentage of CD4 and CD8+ T cells producing interferon (IFN)-γ and CD8 T cells producing tumour necrosis factor (TNF)-α, and a significant decrease in the percentage of CD4+ and CD8+ T cells producing interleukin (IL)-4 and transforming growth factor (TGF)-β in BB compared to BAL.
| CD3 | IFN-γ | IL-2 | IL-4 | TGF | TNF-α | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| BAL | 50 ± 11 | 50 ± 11 | 10 ± 9 | 17 ± 15 | 5 ± 5 | 3 ± 3 | 5 ± 4 | 5 ± 4 | 4·6 ± 2 | 5 ± 2·1 | 19 ± 10 | 12 ± 11 |
| BB | 37 ± 11 | 63 ± 11 | 17 ± 7 | 40 ± 13 | 8 ± 4 | 3 ± 1·6 | 1·2 ± 1 | 2·3 ± 2 | 2 ± 0·9 | 1·7 ± 1 | 18 ± 7 | 30 ± 11 |
| P | 0·006 | 0·006 | 0·029 | 0·001 | 0·100 | 0·210 | 0·002 | 0·025 | 0·035 | 0·028 | 0·657 | 0·002 |
Fig 1.
Representative dot plots showing interferon (IFN)-γ production by CD8+ and CD8− (CD4+) T cells from blood, bronchoalveolar lavage (BAL) and bronchial brushing (BB) in a lung transplant patient. T cells were identified by CD3 PC5 versus side scatter characteristics. Transplant patients showed an increase in the percentage of CD8+ T cells producing IFN-γ in BB compared with blood. Transplant patients showed an increase in IFN-γ in both CD4+ and CD8 T cells in BB compared with BAL. Note the decrease CD4+ and increase in CD8+ T cells in BB compared with blood and BAL.
Intracellular T cell cytokines from blood from transplant patients and controls
There was a decrease in CD4+ T cells (P = 0·048) and an increase in CD8+ T cells (P = 0·048) in the blood of transplant patients compared with controls (data in Tables 3 and 4). The percentage of CD4+ T cells producing TNF-α, IFN-γ and IL-2 was decreased in the blood of transplant patients compared with controls (P = 0·001, P = 0·038 and P = 0·000, respectively). The percentage of CD8 + cells producing IFN-γ, IL-2 and IL-4 was unchanged between patients and controls (P > 0·05).
Intracellular T cell cytokines from BAL from transplant patients and controls
The percentage of CD8+ T cells producing IFN-γ, IL-2, TNF-α and TGF-β was increased in the BAL of transplant patients compared with controls (P < 0·05) (data in Tables 3 and 4). The percentage of CD4+ cells producing IFN-γ was increased in the BAL of transplant patients compared with controls (P = 0·025). The percentage of CD4+ cells producing IL-2, TNF-α, IL-4 and TGF-β was unchanged in the BAL of transplant patients compared with controls.
Intracellular T cell cytokines from BB from transplant patients and controls
The percentage of CD8+ T cells producing IL-4 was increased in BB of transplant patients compared with control (P = 0·042). There was no difference in the percentage of CD4+ T cells producing IL-4 or the percentage of CD4+ and CD8+ T cells producing IFN-γ, IL-2, TNF-α or TGF-β (P > 0·05 for all data) between transplant patients and controls.
Discussion
This is the first report of the use of flow cytometry to measure intracellular pro- and anti-inflammatory cytokines in BB-derived intraepithelial T cells and provides important new information regarding compartmentalization of inflammatory cytokines between blood, BAL and BB in stable lung transplant patients. Although there was a decrease in T cell proinflammatory cytokine production in blood of transplant patients, this was not found in BAL or bronchial intraepithelial CD8 T cell subsets, suggesting that the same level of immunosuppression may not occur in the lung of transplant recipients. We now show that there is no difference in the percentage of bronchial intraepithelial CD8 T cells that produce proinflammatory cytokines between stable lung transplant patients and control subjects, indicating that current immunosuppression protocols are ineffective at reducing proinflammatory T cell cytokines in transplant grafts. In contrast, we have previously shown a decrease in CD4+ T cell proinflammatory cytokine production in blood of stable transplant patients compared with control subjects consistent with immunosuppression protocol strategy [4] and our current study confirms these findings. We have also previously shown non-compartmentalization of inflammatory T cells cytokines between blood and BAL in stable lung transplant patients compared with control group [5], results also consistent with our current findings. However, in both studies we found failure to suppress CD8+ T cell proinflammatory cytokine production, results consistent with our current findings in BB T cells from stable transplant patients. Transplant patients showed a CD4 : CD8 inversion in BAL and BB, consistent with a previous report [9], in contrast to control compared with control subjects who showed a CD4 : CD8 inversion only in the BB compartment. The relative increase in CD8 T cells in BB may be due to an increase in proliferation of these cytotoxic cells and/or an increase in migration of these cells via specific Th1 chemokine receptors [10]. The percentage of CD8+ T cells producing IFN-γ and TNF-α in BB was increased compared with blood and BAL. As IL-4 and TGF-β are negative regulators of Th1 inflammatory cytokines [11,12], our findings of decreased IL-4 and TGF-β in some bronchial intraepithelial T cell subsets compared with blood and BAL may help to explain these latter findings. Conversely, chronic rejection or OB is associated with an increase in TGF-β production [3], indicating that current immunosuppression protocols may be effective in reducing OB in lung transplant patients via this pathway.
Others have suggested that studies of intragraft immune cells may be more relevant in terms of effecting graft injury than analysis of peripheral circulating cells [6]. Intragraft cytokine expression has been assessed previously by assay of mRNA by in situ hybridization, secondly by protein expression and thirdly by markers of indirect cytokine expression. In situ hybridization is often limited by the transient nature and low level of cytokine mRNA [13] and cytokine expression is also transient and often bound to receptors, while indirect cytokine detection within allograft does not quantify individual cytokine levels [14]. We believe intracellular cytokine analysis using flow cytometry is superior to these techniques as it allows rapid quantification of multiple pro- and anti-inflammatory cytokines in thousands of bronchial intraepithelial T cells.
All transplant patients in this study had plasma levels of CsA or Tac within their therapeutic range. Our findings therefore suggest that analysis of T cell cytokine production may provide a more accurate assessment of immunosuppression in the various compartments than systemic drug levels and show that these patients are inadequately immunosuppressed especially in the lung compartment.
A longitudinal surveillance of cell phenotypes in individuals has been suggested to identify a preclinical state of rejection [15]. Monitoring intracellular T cell cytokine profiles may be more appropriate indicator of patient immunosuppression and transplant status than cell phenotypes and we are currently undertaking such a study to investigate this. In conclusion, this study demonstrates that we can monitor intracellular cytokines in bronchial intraepithelial T cells using flow cytometry. We have shown compartmentalization of pro-and anti-inflammatory CD8+ T cell cytokine production in BB compared with blood and BAL. Current immunosuppression protocols have a limited effect on proinflammatory cytokine production by bronchial intraepithelial CD8+ T cells. Drugs that effectively reduce bronchial intraepithelial CD8+ T cell proinflammatory cytokines may improve current protocols for prolonging graft survival in these patients. The clinical relevance of this work is being pursued further with longitudinal follow-up of this patient group, as comparison of T cell cytokine levels between the three compartments may show important changes during rejection episodes.
References
- 1.Sundaresan S, Alevy YG, Steward N, et al. Cytokine gene transcripts for tumor necrosis factor-alpha, interleukin-2, and interferon-gamma in human pulmonary allografts. J Heart Lung Transplant. 1995;14:512–18. [PubMed] [Google Scholar]
- 2.Neuringer IP, Walsh SP, Mannon RB, Gabriel S, Aris RM. Enhanced T cell cytokine gene expression in mouse airway obliterative bronchiolitis. Transplantation. 2000;69:399–405. doi: 10.1097/00007890-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 3.El-Gamel A, Sim E, Hasleton P, et al. Transforming growth factor beta (TGF-beta) and obliterative bronchiolitis following pulmonary transplantation. J Heart Lung Transplant. 1999;18:828–37. doi: 10.1016/s1053-2498(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 4.Hodge G, Hodge S, Reynolds P, Holmes M. Intracellular cytokines in blood T cells in lung transplant patients − a more relevant indicator of immunosuppression than drug levels. Clin Exp Immunol. 2004;139:159–64. doi: 10.1111/j.1365-2249.2005.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge G, Hodge S, Reynolds P, Holmes M. Increased intracellular pro- and anti-inflammatory cytokines in bronchoalveolar lavage T cells of stable lung transplant patients. Transplantation. 2005;80:1040–5. doi: 10.1097/01.tp.0000173997.92753.25. [DOI] [PubMed] [Google Scholar]
- 6.Corris PA, Kirby JA. A role for cytokine measurement in therapeutic monitoring of immunosuppressive drugs following lung transplantation. Cin Exp Immunol. 2004;139:176–8. doi: 10.1111/j.1365-2249.2005.02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge S, Hodge G, Holmes M, Reynolds P. Flow-cytometric characterisation of cell populations in bronchoalveolar lavage (BAL) and bronchial brushings from patients with chronic obstructive pulmonary disease (COPD) Clin Cytometry. 2004;61:27–34. doi: 10.1002/cyto.b.20020. [DOI] [PubMed] [Google Scholar]
- 8.Hodge S, Hodge G, Reynolds P, Holmes M. Differential rates of apoptosis in bronchoalveolar lavage and blood of lung transplant patients. J Heart Lung Transpl. 2005;24:1305–14. doi: 10.1016/j.healun.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Erle DJ, Brown T, Christian D, Aris R. Lung epithelial lining fluid T cell subsets defined by distinct patterns of beta 7 and beta 1 integrin expression. Am J Respir Cell Mol Biol. 1994;10:237–44. doi: 10.1165/ajrcmb.10.3.7509610. [DOI] [PubMed] [Google Scholar]
- 10.Neuringer IP, Chalermskulrat W, Aris R. Obliterative bronchiolitis or chronic lung allograft rejection: a basic science review. J Heart Lung Transplant. 2005;24:3–19. doi: 10.1016/j.healun.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 12.Kehrl JH. Transforming growth factor-β: an important mediator of immunoregulation. Int J Cell Clon. 1991;9:438–50. doi: 10.1002/stem.1991.5530090502. [DOI] [PubMed] [Google Scholar]
- 13.Dallman MJ, Montgomery RA, Larsen CP, Wanders A, Wells AF. Cytokine gene expression: analysis using Northern blotting, polymerase chain reaction and in situ hybridisation. Immunol Rev. 1991;119:163–79. doi: 10.1111/j.1600-065x.1991.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 14.Robertson H, Kirby JA. Post-transplant renal tubulitis: the recruitment, differentiation and persistence of intra-epithelial T cells. Am J Transplant. 2003;3:3–10. doi: 10.1034/j.1600-6143.2003.30102.x. [DOI] [PubMed] [Google Scholar]
- 15.Slebos DJ, Schloma J, Boezen HM, et al. Longitudinal profile of bronchoalveolar lavage cell characteristics in patients with good outcome after lung transplantation. Am J Crit Care Med. 2002;165:501–7. doi: 10.1164/ajrccm.165.4.2107035. [DOI] [PubMed] [Google Scholar]