Abstract
The complement regulatory (CR) proteins clusterin and vitronectin bind to the membrane attack complex (MAC) and thus prevent cytolysis. In this report, we demonstrate the presence of both of these CR proteins on MAC bound to circulating immune complexes (CIC). We measured the amount of clusterin and vitronectin on MAC in plasma, also referred to as soluble MAC (SMAC), as well as on MAC bound to CIC (MAC–CIC), using antibody directed to polymerized C9 in systemic lupus erythematosus (SLE) patients. We observed a strong correlation among the quantities of SMAC and MAC–CIC. The amount of both clusterin and vitronectin associated with MAC–CIC was two- to threefold higher in comparison to the SMAC. Patients with high levels of clusterin and vitronectin demonstrated renal involvement. We hypothesize that these complement regulatory proteins besides regulating the insertion of MAC play other critical roles, in disease pathogenesis.
Keywords: autoimmunity, circulating immune complex, clusterin, immune deposits, membrane attack complex, vitronectin
Introduction
The role of the membrane attack complex (MAC) also referred to as terminal complement complex (TCC) and serum complement C5b−9 (SC5b−9) in renal injury in systemic lupus erythematosus (SLE) patients has been documented [1]. In SLE patients, elevated plasma and urine levels of the MAC have been used as markers for disease flare [2]. MAC induces cell apoptosis or cell necrosis depending on the quantities deposited on the cell membrane [3]. In addition, MAC triggers a host of signalling events contributing to inflammatory responses [4]. The complement regulatory (CR) proteins such as clusterin (SP-40, 40, cytolysis inhibitor, or Apo J) and vitronectin (S protein) that reside in the plasma and CD59 present on the cell membrane regulates an efficient system of selective inhibition of MAC formation. Production of C5b upon complement activation leads to its binding to the other complement molecules C6 and C7, to form C5b−7. The complex C5b−7 thus assembled either inserts itself into the cell membrane (C5b−7 m) or binds to a single molecule of vitronectin and clusterin, thus forming soluble C5b−7 (SC5b−7), which prevents its insertion into the cell membrane. Subsequent to the formation of the C5b−7, complex complement proteins C8 and C9 bind to SC5b−7 to form soluble MAC (SMAC) in plasma and to C5b−7 m to form MAC (mMAC) on the cell membrane. The binding of clusterin and vitronectin to nascent amphiphillic C5b−9 complex renders it water-soluble and lytic inactive [5]. The lytic active mMAC also contains a small amount of vitronectin; however, its role is not clear [6]. Clusterin binds to C7 and a β-subunit of C8 and C9 [7]. Clusterin recognizes the C9b fragment containing the hydrophobic membrane interaction fragment. Binding of the clusterin to C9 is competed only by polymerized C9 and not by other components of the terminal pathway, suggesting that the conformational change occurring during the hydrophilic–amphiphilic transition of C9 exposes the interaction site for clusterin [7]. Complement proteins from the terminal pathway demonstrate the presence of highly conserved amphipathic helices that interact with the lipid layer in the target cell membrane [8]. The potential transmembrane domain of C9 with amino acid residues at 314–330 and 335–354 with a consensus sequence Y(n)6FGTHY is attributed as the domain responsible for membrane pore formation [8].
The N-terminus of vitronectin contains a sequence of somatomedin B, followed by a RGD (Arg–Gly–Asp) sequence and a collagen binding site [9]. The C-terminus contains the heparin binding domain, a proposed site for interaction with C9, and hence the RGD sequence is available to interact with cell surface integrin [10]. Although a clear role of mMAC in cell lysis and in proinflammatory events has been established, little is known about the biological significance of the so-called inactive SMAC. The presence of SMAC has been well documented in the renal tissue biopsies along with immune deposits containing circulating immune complexes (CIC) and C3 [11]. Thus, despite the presence of clusterin and vitronectin in SMAC, this molecular complex may be critical in the tissue injury in SLE and other diseases [11].
In our earlier studies, we have demonstrated the presence of late complement components C5 and MAC bound to CIC [12]. After capturing CIC in solid phase, we measured the amount of activated complement C1q, C3, C4, C5 and MAC bound to CIC in SLE patients. The correlation analysis of the complement proteins present on CIC validated the methods used in the present study. In order to understand the role of the MAC bound to CIC, we tested the presence of clusterin and vitronectin bound to the CIC–MAC complex. This is the first report demonstrating the presence of complement regulatory proteins clusterin and vitronectin bound to CIC.
The presence of the exposed RGD sequence in CIC–MAC or SMAC may be critical in the transfer of MAC to the cell surface. Granzyme B cleaves vitronectin to expose an RGD sequence and elevated levels of this enzyme have been reported at sites of tissue injury [13,14]. In addition, granzyme B plays a critical role in extracellular matrix reorganization. Thus, an exposed RGD sequence on vitronectin in CIC–MAC may interact with the integrin Vα-5 (vitronectin receptor) on a cell surface allowing the interaction of the CIC–MAC complex, which may facilitate the transfer of MAC from CIC to the cell surface [15]. Importantly, because MAC bound to clusterin and vitronectin can induce apoptosis, the question which remains unanswered is whether MAC transferred from CIC to cell membrane is cytolytically active or destined for endocytosis [16].
Materials and methods
Human subjects
Thirty-five serum and plasma samples were collected from SLE patients attending the Rheumatology Clinic at the Saint Louis University Health Sciences Center. Informed written consent was taken from each subject and the study was approved by the Institutional Review Board of Saint Louis University. The blood was collected by venipuncture in red-topped tubes for serum and green-topped tubes (heparinized) for plasma. For serum separation, the samples were allowed to incubate at room temperature (RT) for 1 h, centrifuged at 1250 g for 20 min and the serum was then transferred to storage vials. The samples were frozen at − 70°C in small aliquots until analysis.
Establishing the capture of CIC by Proceptor™
The protein used for capture of CIC was isolated from Raji cells. The purified protein demonstrated an apparent molecular mass of 17–21 kDa on sodium dodecyl–polyacrylamide gel electrophoresis (SDS-PAGE) analysis supported by gel permeation on Superose resin (Amersham, Piscataway, NJ, USA). To establish the binding of CIC to the receptor preparation (Proceptor™, ProGen Biologics, Wildwood, MO, USA), we tested the binding of aggregated human γ-globulin (AHG), an in vitro formed CIC from tetanus toxoid (TT)-anti-TT, and observed a linear binding. The spiking and recovery experiments were also carried out to test the interference from plasma proteins. To establish the identity of CIC, the protein was used for affinity purification of CIC.
Measurement of clusterin and vitronectin bound to CIC and SMAC
The microtitre plates coated with Proceptor™ (ProGen Biologics) were used for the capture of MAC–CIC and monoclonal anti-C9 neoepitope clone aE 11 (DakoCytomation, Carpenteria, CA, USA) to capture the SMAC. Enzyme-linked immunosorbent assay (ELISA)-based measurements were used to quantify the amount of the CR proteins, clusterin and vitronectin, present on MAC–CIC complex and SMAC. The coated plates were blocked with 1% bovine serum albumin (BSA) at RT for 2 h. The plates were washed three times with phosphate-buffered saline (PBS) containing 0·5% Tween-20 (PBS-T). Thereafter, the serum samples were diluted 1 : 10 with PBS-T and 100 µl of sample was loaded into each well. The plates were then incubated at RT for 90 min. After incubation, plates were washed three times with PBS-T. After the washing step, diluted mouse monoclonal antibodies directed towards vitronectin or clusterin (Quidel Corp., San Diego, CA, USA) were added to each well and allowed to interact for 60 min at RT. After the incubation plates were washed three times with PBS-T, each well was filled with diluted anti-mouse horseradish peroxidase (HRP) conjugate (Jackson Immunoresearch, West Groove, PA, USA). Plates were incubated further for 60 min at RT. After the incubation, the plates were washed three times with PBS-T and the reaction was developed using the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma Chemicals, St Louis, MO, USA). For quantitating the vitronectin, a standard curve was generated using purified vitronectin (R&D systems, Minneapolis, MN, USA) from a concentration of 200 ng/ml to 3·12 ng/ml. The amount of vitronectin bound to MAC–CIC (vit–CIC) and SMAC (vit–SMAC) was calculated using a linear equation. The amount of clusterin bound to MAC–CIC (clust–CIC) and SMAC (clust–SMAC) is represented as optical density (OD450) measured at 450 nm.
Measurement of SMAC
The levels of SMAC were measured in a sandwich ELISA using a monoclonal antibody aE11 directed to neoepitope of C9 as a capture reagent and a polyclonal antibody towards neoepitope on C9 (CN BioSciences, San Diego, CA, USA) as a detection reagent. The monoclonal antibody aE11 at a concentration of 300 ng per well was used to capture SMAC. The results are represented as OD450 nm.
Measurement of MAC bound to CIC
To measure the amount of MAC bound to CIC, the CIC from the plasma were first captured by binding to receptors specific for complexed immunoglobulin (Proceptor™). Thereafter, the amounts of MAC present in these complexes were measured using the same polyclonal antibody that was used for measuring the SMAC. The results are presented as OD450.
Measurement of complement proteins C1q, C3, C4 and C5 bound to CIC
The amount of complement proteins bound to CIC was measured using an ELISA technique. The capture of CIC to plates was achieved by Proceptor™. Subsequent to the capture of CIC in the solid phase, the complement proteins bound in these complexes were measured using ELISA. The purified complement proteins C1q, C3, C4 (Quidel Corp.) and C5 (Calbiochem, San Diego, CA, USA) were used to generate the standard curve. The standard curves for each complement protein were generated using protein concentration from 200, 100, 50, 25, 12·5, 6·25, 3·12 and 1·625 ng/ml. A total amount of 100 µl sample at an appropriate dilution was used to measure the C1q, C3, C4, C5 and MAC. The C3 and C4 required higher dilutions compared to C1q, C5, and MAC. In the initial step, samples were allowed to interact with the receptor for 90 min at RT, whereas wells coated with complement standards did not receive any treatment. After the formation of complexes, each individual complement component was detected using a primary antibody specific for that particular complement protein. Each incubation step was followed by a washing step with PBS/T. A 100-µl of appropriately diluted primary antibody was added to each well of the microtitre plates. Plates were incubated for 60 min at RT. After washing, plates were incubated for 60 min at RT with a secondary antibody conjugated to HRP. The colour was developed using TMB substrate. Thereafter, the plates were read in an ELISA reader at 450 nm.
Purification of CIC using Proceptor™ affinity chromatography
The CIC were isolated from the 1·5 ml of patient plasma using affinity chromatography. The affinity resin was prepared by coupling 10 µg of protein to 1 ml of activated Sepharose 4B resin (GE Amersham, Piscataway, NJ, USA). A total of 1 ml resin was used to purify the CIC from 1·5 ml of plasma. The patient plasma was applied to 1 ml of resin under gravity flow. Thereafter, the resin was washed with 20 column volumes of PBS with 0·5 M NaCl. After washing, the bound complexes were eluted with low pH buffer. The eluates were concentrated to a final volume of 300 µl.
2D SDS-PAGE analysis of CIC purified by affinity chromatography
Twenty µl of the purified CIC protein were mixed with isoelectric focusing (IEF) renaturing solution consisting of 8 M urea, CHAPS, NP40 and 2 mercaptoethanol (2ME) before applying to 7 mm immobilized pH gradient (IPG) strips, pH 3·5–10 (Bio-Rad, Hercules, CA, USA). A total amount of 10 000 volt-hours were applied for IEF. After IEF, strips were removed and incubated with buffer containing 8 M urea, 0·375 M of Tris-HCl buffer, pH 8·8, 20% glycerol and 100 mM dithiothreitol (DTT) for 15 min with constant shaking. After the first incubation, the IEF strips were incubated for another 15 min at RT in the same buffer, replacing DTT with 125 mg of iodoacetamide per 10 ml of buffer. Thereafter, IPG strips were overlaid for a second dimension run on 4–12% SDS-PAGE, NuPAGE ZOOM gel (Invitrogen, Carlsbad, CA, USA). The electrophoresis was carried out using 3-(N-morpholino) propane-sulphonic acid (MOPS) buffer at 170 V for 2 h. The protein spots were visualized with silver stain. The comparative analysis of CIC from different patients was performed utilizing the 2D protein database from EMBO to establish the identity of globulin heavy and light chains. The immunoglobulins were identified in Western blotting using isotype-specific anti-sera and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-Mass) analysis.
Statistical analysis
Data were analysed using GraphPad Prism. Parametric analysis was carried out as we considered the distribution to be normal. Pearson's correlation coefficient r was calculated for the amount of complement proteins and CR proteins bound to CIC. The mean and standard deviation for each group were calculated using the column statistics program in GraphPad Prism.
Results
Presence of MAC and CR proteins vitronectin and clusterin on CIC
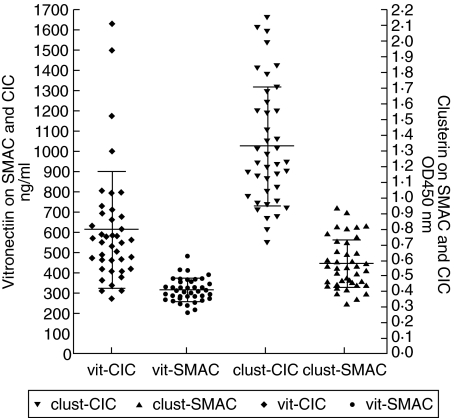
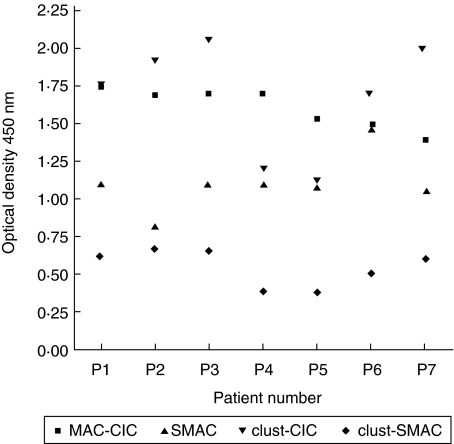
SLE patients were analysed for the presence of vitronectin and clusterin bound to CIC, as well as to SMAC. The quantity of vitronectin present on CIC in these patients ranged from 269 ng/ml to 1630 ng/ml with a mean ± s.d. of 611 ± 288 ng/ml. The amount of vitronectin present on SMAC ranged from 202 ng/ml to 483 ng/ml with a mean ± s.d. of 315 ± 57. The vitronectin on MAC–CIC and SMAC demonstrated a high correlation with a coefficient value of r = 0·72. The amount of clusterin present on MAC–CIC varied from a low of 0·70 to a high of 2·14 with a mean ± s.d. of 1·32 ± 0·383. The amount of clusterin present on SMAC varied from a low of 0·323 to a high of 0·931 with a mean ± s.d. of 0·573 ± 0·157. A strong correlation among clusterin present on MAC–CIC and SMAC was observed with a coefficient value of r = 0·935. As with vitronectin, the amount of clusterin present on the MAC–CIC was two- to threefold higher than present on SMAC in most of the patients analysed (Fig. 1). In the normal control group, the levels of vitronectin on MAC–CIC were 403 ± 178 ng/ml and on SMAC were 331 ± 205 ng/ml. The levels of clusterin on MAC–CIC were 0·456 ± 0·178 and on SMAC were 0·313 ± 0·09. In the normal group (n = 10) the amount of SMAC was 0·476 ± 0·110 and CIC–MAC was 0·890 ± 0·329. For validating the measurement of MAC–CIC, we also measured SMAC in a limited number of patients. We observed the presence of MAC–CIC in all the patients that also demonstrated the presence of SMAC. There was no apparent correlation among these parameters. However, the quantities of clusterin present on CIC–MAC and SMAC demonstrated a very significant correlation coefficient r = 0·95 (Fig. 2).
Fig 1.
Concentration of the complement regulatory proteins clusterin and vitronectin bound to circulating immune complexes (CIC) and the fluid phase soluble membrane attack complex (SMAC), measured in systemic lupus erythematosus (SLE) patients. The amount of vitronectin is presented on the left y axis (ng/ml). Clusterin is represented on the right y axis, measured as optical density at 450 nm. The amount of clusterin and vitronectin bound to CIC is two- to threefold higher in every patient, when compared to the vitronectin and clusterin bound to fluid phase SMAC.
Fig 2.
Scatter-plot of the amount of membrane attack complex (MAC)-circulating immune complexes (CIC), soluble MAC (SMAC), clusterin (clust)–CIC and clust–SMAC. Monoclonal antibody aE11 directed to a neoepitope on polymerized C9 was used as capture reagent in an enzyme-linked immunosorbent assay (ELISA)-based assay to measure SMAC. A strong correlation was observed between the levels of the clusterin present on SMAC and MAC–CIC.
Establishing the presence of complement proteins on CIC in SLE patients
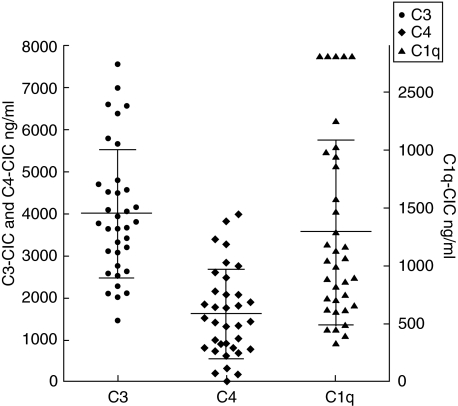
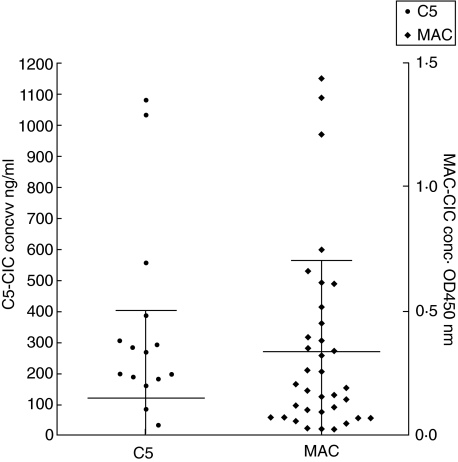
The CIC from 35 SLE patients were analysed for the presence of C1q, C3, C4, C5 and MAC (Figs 3 and 4). The amount of C1q–CIC (C1q bound to CIC) ranged from 314 ng/ml to 2825 ng/ml and the C3–CIC (C3 bound to CIC) ranged from 1457 ng/ml to 7568 ng/ml. The C4–CIC (C4 bound to CIC) levels ranged from a low of 1·74 ng/ml to a high of 3981 ng/ml (Fig. 3). We also detected C5–CIC (C5 bound to CIC). The amounts varied from 1·7 ng/ml to 1087 ng/ml. Levels of C5–CIC in 16 samples were undetectable. The optical density for MAC–CIC (MAC bound to CIC) varied from 0·02 to a high of 1·36 (Fig. 4). A correlation analysis revealed a very strong correlation among C3 : C4 and C5–CIC : MAC–CIC (Table 1). The second group that demonstrated the strong correlation was between C1q–CIC : C5–CIC and C1q–CIC : MAC–CIC. The least strong correlation was seen between C3–CIC : C5–CIC, C4–CIC: C5–CIC, C4–CIC : MAC–CIC, C1q–CIC : C4-CIC and C1q–CIC : C3–CIC (Table 1). The analysis demonstrated a strong correlation among all the complement proteins present on CIC in SLE. Analysis of the data is suggestive of the participation of complements proteins from the classical complement pathway in the studied group of SLE patients. A strong correlation among C3 and C4 bound to CIC is also suggestive of the fact that all the C3 activation in these patients is due classical pathway without significant contribution from the alternative pathway. The conclusion from the correlation r-value of 0·79 between C1q, the first component in the classical pathway to C3 on CIC points to 80% of the complement activation may be the result of activation of the classical pathway resulting from binding of CIC to C1q.
Fig 3.
Complement proteins bound to circulating immune complexes (CIC) were measured in samples from 35 systemic lupus erythematosus (SLE) patients. Levels of C1q, C3, and C4 bound to CIC were measured using enzyme-linked immunosorbent assay (ELISA). The concentration of complement proteins present on CIC is represented as ng/ml. The C3 and C4 bound to CIC are represented on the left y axis while C1q bound to CIC are represented on the right y axis. The C3 levels were the highest on CIC followed by the C1q and C4 amounts.
Fig 4.
Quantities of the C5 and membrane attack complex (MAC) present on circulating immune complexes (CIC) as measured using enzyme-linked immunosorbent assay (ELISA)-based assays. A strong correlation was observed between these late complement components. C5 levels were lower than the detection limit of the assay in 16 patients. The amount of C5 is represented on the left axis in ng/ml, while the amount of MAC on CIC is represented on the right axis as OD at 450 nm.
Table 1.
The values of correlation coefficient (r) among the various complement proteins bound to circulating immune complexes (CIC) in SLE patients. The strongest correlation was observed among the late complement components C5 and MAC as well as C3 and C4. The rest of the complement present on CIC also demonstrated a strong correlation.
| Parameter | r-value |
|---|---|
| C1q: C3 | 0·791 |
| C1q: C4 | 0·791 |
| C1q: C5 | 0·828 |
| C1q: MAC | 0·825 |
| C3: C4 | 0·918 |
| C3: C5 | 0·778 |
| C4: C5 | 0·788 |
| C4: MAC | 0·793 |
| C5: MAC | 0·965 |
Establishing the CIC binding to Proceptor™
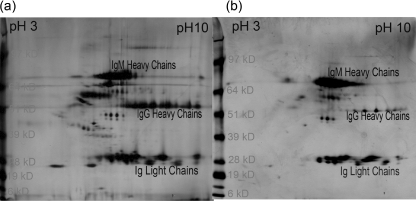
Utilizing multiple approaches, we have established that the receptor used in the present study only captured CIC without binding monomeric immunoglobulin. Monomeric immunoglobulin was prepared from commercially purchased IgG (Sigma Chemicals, St Louis, MO, USA). To prepare a monomeric fraction, immunoglobulin at a concentration of 10 mg/ml was dissolved in PBS at 10 mg/ml. This preparation was then subjected to ultracentrifugation; thereafter, followed by gel permeation to remove the aggregated globulin. This preparation was used at a concentration of 25 µg/ml to 200 µg/ml in spiking experiments using 5% normal human serum (NHS) to test the binding of the monomeric IgG. We did not observe any binding of this preparation. The other support of non-binding of monomeric immunoglobulin also comes from serum analysis of CIC. The CIC were composed of all three immunoglobulin isotypes. Because the normal concentration of monomeric IgG in samples is about 10–13 mg/ml, we only observed a maximum capture of 370 µg/ml AHG equivalents. To obtain additional support for the binding of CIC to receptor, we coupled the receptor to Sepharose 4B (Amersham). This preparation was used to purify CIC from 30 different samples. The analysis of protein profile in these samples on 2D SDS-PAGE revealed different patterns of immunoglobulin γ chains, µ chains and light chains. In some samples, despite the presence of very high concentrations of monomeric IgG, we only captured complexed IgM, without capturing any significant IgG (Fig. 5).
Fig 5.
2D sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the circulating immune complexes (CIC) purified from systemic lupus erythematosus (SLE) patients. The first dimension isoelectric focusing (IEF) was carried out using immobilized pH gradient (IPG) immobiline strip pH 3–10 linear gradient. The second-dimension run was performed on 4–12% gradient SDS-PAGE gel. The spots for both heavy and light chains of human globulins are marked. Both the γ chain and µ chain, in addition to ι and κ light chains, were identified by comparison of gel images with National Biological Research Foundation (NBRF) 2D database. The identity of these spots was also confirmed using Western blot analysis and MASS analysis. 2D SDS-PAGE profile of heavy chains in one patient (a) demonstrated the presence of both IgG and IgM containing CIC, while in the other patient CIC were composed mainly of IgM isotype (b). The patient with CIC composed of both immunoglobulin isotype also demonstrated a broader light chain profile in comparison to CIC composed of only IgM isotype.
Affinity purification and 2D SDS-PAGE analysis of CIC from SLE patients
The purified CIC were analysed by 2D SDS-PAGE for establishing that the captured and analysed entities were CIC. Using the comparative analysis of results from the National Biological Research Foundation (NBRF) protein database, we established the presence of IgM and IgG heavy chains as well as kappa and lambda light chains in the captured CIC. The identity of the IgG and IgM heavy chains were also established by MALDI-TOFF analysis of protein spots from 2D SDS-PAGE gels. The presence of complement proteins in purified CIC were also established using Western blotting (data not shown).
Discussion
The role of complement and CIC in the pathophysiology of SLE and renal disease has been well established [11,17]. The opsonization of CIC and apoptotic debris with complement proteins is critical for the clearance of CIC. Activation of terminal complement components is associated with exacerbations of disease and damage to organs in SLE patients. Elevated levels of MAC from plasma and urine samples have been reported in SLE patients during a disease flare and with renal disease [2,18]. The role of clusterin and vitronectin in the renal pathology has not been explored. In the kidney, tubular cells lack presence of CR proteins on the luminal side; thus complement-mediated injury may be crucial in causing the tubulointestinal damage [19]. The deposition of the lytic MAC on the cell membrane initiates a signalling pathway contributing to inflammatory responses and tissue necrosis [4]. The deposition of MAC on leucocytes leads to cell activation. In SLE nephritis the damage to the kidney is mediated by immune deposits, and a role of C1q containing CIC has been implicated [20]. The activation of C1q is essential in activation of the terminal complement pathway. Our data demonstrate a strong correlation among C1q and C5 as well as MAC bound to the CIC. Microscopic localization of CIC, C3 and MAC has been demonstrated within dense deposits in the subepithelial basement membrane. Smaller granular deposits of immune complexes, C3 and MAC has also been reported in the subendothelial region of the lamina rara interna and the lamina densa in experimental immune complex glomerulonephritis [21]. In a previous study, examination of 90 consecutive percutaneous renal biopsies by immunofluorescence demonstrated the deposition of the SC5b−9 components C6, C9, vitronectin and clusterin. All components of SC5b−9 were found in arteries and arterioles, along the tubular basement membrane, and in areas of glomerulosclerosis. In biopsies with glomerular deposition of immunoglobulin and C3, the SC5b−9 components co-localized with the immune deposits. However, glomeruli without immune deposits or glomerulosclerosis contained none of the SC5b−9 components. Furthermore, the incidence and pattern of distribution of clusterin was similar to that of vitronectin, C6 and C9 in all of cases. The deposition of C6 and C9 were never found in the absence of vitronectin or clusterin. The study confirmed that the MAC in the kidney is at least partly is in the SC5b−9 form. It is found in the specific immune glomerular deposition and in the ‘non-specific’ deposition in areas of renal injury. SP-40,40 is also found in the SC5b−9 complex in all forms of renal disease [11]. The association of both CR proteins with MAC was observed mainly in glomeruli containing immunoglobulin deposits, but not when immunoglobulins were absent [22]. A later study also found increased co-localization of MAC, vitronectin and vitronectin receptor (alpha V beta 3 integrin) both within and around the subepithelial deposits in membranous nephropathy [23]. Although CIC-associated complement proteins have been studied using techniques such as polyethylene glycol precipitation (PEG) and analysis of higher molecular weight fractions from plasma, the CR proteins have never been identified from such preparations.
In the present study, we have analysed for the first time the composition of the CIC for the presence of the MAC along with the CR proteins, clusterin and vitronectin. We observed high levels of these proteins associated with the CIC in the SLE patients. The presence of a higher amount of MAC, clusterin and vitronectin on CIC leaves an open question about their role in the complement pathway. Vitronectin is a major cell attachment promoting factor that binds to integrins via its RGD sequence [24]. Vitronectin and clusterin have been shown to be associated with SMAC [5,25]. High levels of vitronectin associated with thrombin–anti-thrombin complexes have been reported in sepsis patients [26]. These studies support our finding of the presence of vitronectin and clusterin in CIC–MAC complexes. Based on these findings, we speculate that the interaction between the RGD sequences from vitronectin–CIC–MAC complex with integrins on the cell surface may provide initial tethering to hold the vitronectin–CIC–MAC complex. This allows the transfer of MAC to the cell membrane [24]. Granzyme B cleaves vitronectin at the RGD sequences and is shown to be elevated in the inflammatory responses and is a key player in apoptosis [14,15,27]. In soluble plasma, the RGD site in vitronectin is buried and unable to bind integrin [28]. It is only after binding of the vitronectin to the surface that this site becomes accessible for cleavage by granzyme B. It is also known that vitronectin bound to SMAC exposes a cryptic heparin binding site, thus making it available for cleavage by granzyme B [29]. Data from a previous study indicate that such conformational changes in vitronectin are likely to occur in areas of tissue injury and thrombosis [28]. In another study, using L8 myoblast cells it was demonstrated that these cells adhere to the MAC through interaction of the vitronectin with the integrin vitronectin receptor [30]. This binding was dependent on divalent cations and was shown to be inhibited by ethylenediamine tetraacetic acid (EDTA) and was blocked by RGD containing peptides and by antibody to the integrin vitronectin receptor. Furthermore, antibodies to C6, C7 or C9 only slightly reduced cell attachment [30].
In lieu of crucial role of terminal complement in diseases, it is of significant importance to delineate the steps involved in the deposition of MAC on tissue sites. It is known that polymerization of C9 upon its attachment with C5b−8 results in the generation of a transmembrane channel resulting in cell lysis. The amino acid residues 266–345 in C9 forms the membrane-spanning region (MSR) that is homologous to 135–223 perforin MSR [8].The amino acid sequence in this region allows the formation of two anti-parallel alpha helices orientated such that it ensures the formation of an amphipathic structure. Two widely spaced peptide sequences within the primary structure of the C9b domain, residues 292–295 and residues 527–531, are aligned in the disulphide folded C9 polypeptide into a membrane-intercalating conformational epitope, thus forming a possible binding site for the binding of CD59 in the C9b domain of C9 [31]. It has been suggested that vitronectin and clusterin blocks the formation of cytolytic active MAC by interacting at the formation of C5b−7 and later at the polymerization of C9. Studies have shown that clusterin interacts with C9 through multiple interaction sites [7]. There are at least four predicted amphipathic structures distributed evenly in two clusterin subunits. In the case of vitronectin, the binding interaction occurs via the heparin binding domain of the molecule with the cluster of negatively charged amino acids of low density lipoprotein receptor domain near the N-terminus in C9a. As clusterin binds to C9b, both molecules can simultaneously bind to the C9. It is generally accepted that binding of these CR proteins to MAC makes it cytolytically inactive and hence prevents any participation in the pathophysiology of disease. Contrary to this belief, a number of earlier studies [26] and our finding of the presence of both CR proteins on CIC in SLE patients provide a convincing argument to clearly look back at the role of these CR proteins in disease pathogenesis and physiology of CIC. The evidence of such an argument can be made on the basis of the finding in tissue pathology as well as physiological mechanisms associated with the CR proteins. The data on inhibition of C5b−7 by vitronectin are somewhat controversial. It has been shown that while vitronectin is a potent inhibitor of C5b−7 membrane attachment at low concentration, it was ineffective at higher concentration [10]. We observed a significantly higher amount of MAC bound to CIC than present in the plasma (SMAC). In addition, we also observed the presence of both the CR proteins on CIC importantly in four SLE patients. High levels of vitronectin were associated with significant damage to the kidney. Ten patients were diagnosed with lupus nephritis. The patient with highest levels of vit–CIC and clust–CIC showed class III and IV glomerulonephritis (GN). The patient with the second-highest level of vit–CIC and clust–CIC presented with class V GN. The patient was diagnosed with end-stage renal disease (ESRD) and was on haemodialysis. The other two patients demonstrated class IV GN. The levels of clust–CIC in all four patients were above 1·99 OD450. Another patient with type V GN demonstrated 1·3 OD450 for clusterin bound to MAC–CIC and 662 ng/ml of vitronectin bound to MAC–CIC. Five patients that demonstrated type II GN, the values for vitronectin on MAC–CIC, ranged from 406 to 583 ng/ml; vitronectin on SMAC ranged from 253 to 294 ng/ml. The amount of clusterin bound to MAC–CIC ranged from 1·13 to 1·84 OD450 and clusterin on SMAC ranged from 0·411 to 0·768 OD450. In lupus nephritis group values for both clusterin and vitronectin present on MAC–CIC and SMAC were higher in comparison to the normal subjects. The question thus can be raised as to whether MAC present on the CIC is active for cytolysis or is simply destined for endocytosis. Even if the MAC on CIC is ineffective in cytolysis, it still can play a critical role with antigen presentation by assisting in the antigen transfer, which is a part of the CIC–MAC complex. The MAC bound to CIC can also play a crucial role in apoptosis, as SMAC alone can activate the caspase pathway and apoptosis [16]. Also, the cytolytically inactive MAC (SMAC) can cause transendothelial migration of polymorphonuclear leucocytes [32]. Thus, the presence of MAC on CIC associated with the high levels of CR can not be ignored as a non-pathogenic event.
The presence of the high levels of vitronectin within CIC in SLE patients with renal involvement may be indicative of active disease process. Further expanded studies are required to establish the value of vitronectin bound to CIC as a marker of disease activity. The presence of another CR protein clusterin in this complex may be necessary to keep these complexes in soluble phase, thus preventing their precipitation [33].
This study demonstrates for the first time the presence of the CR proteins clusterin and vitronectin associated with the CIC–MAC complex. The analysis demonstrates higher quantities of MAC and CR proteins on CIC when compared to the fluid phase SMAC. Further studies are necessary to clearly evaluate the role of the CR proteins on CIC–MAC in disease pathogenesis.
Acknowledgments
Funding for this study was provided by the Campbell-Avery Charitable Trust, the Dorr Family Charitable Trust and Lupus/Juvenile Arthritis Research Group of St Louis, MO, USA.
References
- 1.Rother RP, Mojcik CF, McCroskery EW. Inhibition of terminal complement. a novel therapeutic approach for the treatment of systemic lupus erythematosus. Lupus. 2004;13:328–34. doi: 10.1191/0961203303lu1021oa. [DOI] [PubMed] [Google Scholar]
- 2.Chiu YY, Nisihara RM, Wurzner R, Kirschfink M, Messias-Reason IJ. SC5b-9 is the most sensitive marker in assessing disease activity in Brazilian SLE patients. J Invest Allergol Clin Immunol. 1998;8:239–44. [PubMed] [Google Scholar]
- 3.Fishelson Z, Attali G, Mevorach D. Complement and apoptosis. Mol Immunol. 2001;38:207–19. doi: 10.1016/s0161-5890(01)00055-4. [DOI] [PubMed] [Google Scholar]
- 4.Bohana-Kashtan O, Ziporen L, Donin N, Kraus S, Fishelson Z. Cell signals transduced by complement. Mol Immunol. 2004;41:583–97. doi: 10.1016/j.molimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Choi NH, Nakano Y, Tobe T, Mazda T, Tomita M. Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b−9) of complement. Int Immunol. 1990;2:413–7. doi: 10.1093/intimm/2.5.413. [DOI] [PubMed] [Google Scholar]
- 6.Bhakdi S, Kaflein R, Halstensen TS, Hugo F, Preissner KT, Mollnes TE. Complement S-protein (vitronectin) is associated with cytolytic membrane-bound C5b−9 complexes. Clin Exp Immunol. 1988;74:459–64. [PMC free article] [PubMed] [Google Scholar]
- 7.Tschopp J, Chonn A, Hertig S, French LE. Clusterin, the human apolipoprotein and complement inhibitor, binds to complement C7, C8 beta, and the b domain of C9. J Immunol. 1993;151:2159–65. [PubMed] [Google Scholar]
- 8.Peitsch MC, Amiguet P, Guy R, Brunner J, Maizel JV, Jr, Tschopp J. Localization and molecular modelling of the membrane-inserted domain of the ninth component of human complement and perforin. Mol Immunol. 1990;27:589–602. doi: 10.1016/0161-5890(90)90001-g. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Oldberg A, Hayman EG, Pierschbacher MD, Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985;4:2519–24. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milis L, Morris CA, Sheehan MC, Charlesworth JA, Pussell BA. Vitronectin-mediated inhibition of complement: evidence for different binding sites for C5b−7 and C9. Clin Exp Immunol. 1993;92:114–9. doi: 10.1111/j.1365-2249.1993.tb05956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy BF, Davies DJ, Morrow W, d’Apice AJ. Localization of terminal complement components S-protein and SP-40,40 in renal biopsies. Pathology. 1989;21:275–8. doi: 10.3109/00313028909061073. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan AK, Moore TL. Membrane attack complex (MAC) in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) Arthritis Rheum. 2004;50:S518. [Google Scholar]
- 13.Tak PP, Spaeny-Dekking L, Kraan MC, Breedveld FC, Froelich CJ, Hack CE. The levels of soluble granzyme A and B are elevated in plasma and synovial fluid of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1999;116:366–70. doi: 10.1046/j.1365-2249.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeets TJ, Kraan MC, Galjaard S, Youssef PP, Smith MD, Tak PP. Analysis of the cell infiltrate and expression of matrix metalloproteinases and granzyme B in paired synovial biopsy specimens from the cartilage–pannus junction in patients with RA. Ann Rheum Dis. 2001;60:561–5. doi: 10.1136/ard.60.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzza MS, Zamurs L, Sun J, et al. Extracellular matrix remodeling by human granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol Chem. 2005;280:23549–58. doi: 10.1074/jbc.M412001200. [DOI] [PubMed] [Google Scholar]
- 16.Nauta AJ, Daha MR, van de Tijsma OWB, Tedesco F, Roos A. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol. 2002;32:783–92. doi: 10.1002/1521-4141(200203)32:3<783::AID-IMMU783>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Murphy BF, Kirszbaum L, Walker ID, d’Apice AJ. SP-40,40, a newly identified normal human serum protein found in the SC5b−9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988;81:1858–64. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda K. [Terminal complement complex (TCC) levels in urine in patients with renal diseases] Hokkaido Igaku Zasshi. 2001;76:71–84. [PubMed] [Google Scholar]
- 19.Ichida S, Yuzawa Y, Okada H, Yoshioka K, Matsuo S. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 1994;46:89–96. doi: 10.1038/ki.1994.247. [DOI] [PubMed] [Google Scholar]
- 20.Trouw LA, Groeneveld TW, Seelen MA, et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J Clin Invest. 2004;114:679–88. doi: 10.1172/JCI21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koffler D, Biesecker G, Noble B, Andres GA, Martinez-Hernandez A. Localization of the membrane attack complex (MAC) in experimental immune complex glomerulonephritis. J Exp Med. 1983;157:1885–905. doi: 10.1084/jem.157.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French LE, Tschopp J, Schifferli JA. Clusterin in renal tissue: preferential localization with the terminal complement complex and immunoglobulin deposits in glomeruli. Clin Exp Immunol. 1992;88:389–93. doi: 10.1111/j.1365-2249.1992.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa T, Yorioka N, Yamakido M. Immunohistochemical studies of vitronectin, C5b-9, and vitronectin receptor in membranous nephropathy. Nephron. 1994;68:87–96. doi: 10.1159/000188225. [DOI] [PubMed] [Google Scholar]
- 24.Ruoslahti E, Pierschbacher MD. Arg–Gly–Asp: a versatile cell recognition signal. Cell. 1986;44:517–8. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- 25.Declerck PJ, De Mol M, Alessi MC, et al. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin) J Biol Chem. 1988;263:15454–61. [PubMed] [Google Scholar]
- 26.Hogasen K, Mollnes TE, Brandtzaeg P. Low levels of vitronectin and clusterin in acute meningococcal disease are closely associated with formation of the terminal-complement complex and the vitronectin–thrombin–antithrombin complex. Infect Immun. 1994;62:4874–80. doi: 10.1128/iai.62.11.4874-4880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spaeny-Dekking EH, Hanna WL, Wolbink AM, et al. Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J Immunol. 1998;160:3610–16. [PubMed] [Google Scholar]
- 28.Seiffert D, Smith JW. The cell adhesion domain in plasma vitronectin is cryptic. J Biol Chem. 1997;272:13705–10. doi: 10.1074/jbc.272.21.13705. [DOI] [PubMed] [Google Scholar]
- 29.Hogasen K, Mollnes TE, Harboe M. Heparin-binding properties of vitronectin are linked to complex formation as illustrated by in vitro polymerization and binding to the terminal complement complex. J Biol Chem. 1992;267:23076–82. [PubMed] [Google Scholar]
- 30.Biesecker G. The complement SC5b−9 complex mediates cell adhesion through a vitronectin receptor. J Immunol. 1990;145:209–14. [PubMed] [Google Scholar]
- 31.Laine RO, Morgan BP, Esser AF. Comparison between complement and melittin hemolysis: anti-melittin antibodies inhibit complement lysis. Biochemistry. 1988;27:5308–14. doi: 10.1021/bi00414a054. [DOI] [PubMed] [Google Scholar]
- 32.Dobrina A, Pausa M, Fischetti F, et al. Cytolytically inactive terminal complement complex causes transendothelial migration of polymorphonuclear leukocytes in vitro and in vivo. Blood. 2002;99:185–92. doi: 10.1182/blood.v99.1.185. [DOI] [PubMed] [Google Scholar]
- 33.Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP-independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding-competent state. Biochemistry. 2000;39:15953–60. doi: 10.1021/bi002189x. [DOI] [PubMed] [Google Scholar]