Abstract
One of the most difficult laboratory challenges in the field of therapeutic cancer vaccines has been the development of uncomplicated/reproducible methods for the quantification of vaccine immunization efficacy in peripheral blood of cancer patients. Existing methods are limited by lack of functional information (tetramers), difficulties with standardization/reproducibility [enzyme-linked immunosorbent spot (ELISPOT)] and reliance on endogenous (sample-specific) antigen presentation (cytokine flow cytometry). Herein we present a reproducible method utilizing an artificial antigen-presenting cell platform for flow cytometry-based quantification of the frequency and activation status of peptide-specific cytotoxic T lymphocytes. The methodology [currently presented for cytomegalovirus human leucocyte antigen (HLA)-A2 cognant peptide antigens] allows simultaneous ex vivo quantification of activated (cytokine-producing) and inactive tetramer-positive T cells following HLA class I/peptide/CD28 stimulation independent of endogenous antigen presentation. The simplicity and reliability of the assay provide for high-throughput applications and automation. The utility and application of this method are discussed.
Keywords: artificial antigen-presenting cells, cytomegalovirus, immunity, T cells, tolerance
Introduction
Ongoing clinical efforts in the development of effective therapeutic cancer vaccines are significantly hampered by the lack of uncomplicated and reproducible methods for the quantification immunization efficacy. Current methods utilize three basic approaches of quantification of the frequency of antigen (peptide)-specific, peripheral blood CD8+ T cells: (1) tetramer staining, (2) enzyme-linked immunosorbent spot (ELISPOT) assay and (3) peptide-stimulated intracellular cytokine staining [cytokine flow cytometry, (CFC)] [1,2]. Tetramer-based methods are highly reproducible but offer no information regarding the activation status of the enumerated peptide-specific T cells. Functional assessment of the frequency of peptide-specific T cells assayed by ELISPOT or CFC require in vitro culture experiments in which peptide stimulation of T cells is driven by either peptide-loaded T2 antigen-presenting cells (ELISPOT) or peptides alone incubated with peripheral blood mononuclear cells (CFC). These assays are difficult to standardize (interassay reproducibility), require significant sample processing (ELISPOT) and may be challenging to interpret due to low signal-to-noise ratios in the setting of low-frequency CD8+ T cells and reliance on endogenous (peripheral blood sample) peptide processing/presentation (CFC). Importantly, recently summarized data from cancer vaccine clinical trials seem to suggest that such analyses do not correlate with clinical outcomes [3]. Thus, there is a need for improved methods for reliable quantification of the frequency of functional (and non-functional) peptide-specific cytotoxic T lymphocytes (CTLs).
We have developed a sensitive, reproducible, flow cytometry-based method for the quantification of the frequency of peptide-specific CTLs that synthesize interferon (IFN)-γ (or any cytokine) after stimulation with a cognate peptide/human leucocyte antigen (HLA)–A2 complex in the presence of CD28 co-stimulation. This assay utilizes a peptide/HLA–A2 complex to stimulate the T cell receptor and anti-CD28 co-stimulation coupled to a solid phase bead as a standardized synthetic artificial antigen-presenting cell (aAPC). Utilization of an aAPC for this purpose extends published data utilizing a similar platform for clonal T cell expansion in vitro [4–8]. The assay overcomes the need for endogenous antigen presentation of the CFC approach; is not reliant of variable T-2 cell peptide loading and prolonged in vitro culture of ELISPOT; and it offers a functional (cytokine production) dimension to tetramer staining. The aAPC is a synthetic reagent that directly activates peptide-specific CTLs. As such the assay read-out quantifies functional (IFN-γ-producing) and non-functional (non-IFN-γ-producing) peptide-specific CTL frequencies in peripheral blood.
Materials and methods
aAPCs
Strepavidin-coated, paramagnetic beads (Dynal Biotech, Oslo, Norway) were washed five times in phosphate-buffered saline (PBS) + 0·1% bovine serum albumin (BSA) prior to use. One mg of washed beads were suspended and incubated with either 5 µg biotinylated mouse anti-human CD3 (clone UCHT1; R&D Systems, Minneapolis, MN, USA); HLA–A2 monomer containing the cytomegalovirus (CMV) peptide (NLVPMVATV) or HLA–A2 monomer containing an irrelevant peptide (Beckman Coulter, San Diego, CA, USA); and/or 5 µg goat anti-human CD28 (BAF342; R&D Systems). After 30 min of incubation at room temperature, the beads were washed five times in PBS + 0·1% BSA and resuspended in the same buffer at a concentration of 1 × 106 aAPCs/µl and stored at 4°C for further use.
Fluorochrome staining of aAPC-driven intracellular IFN-γ synthesis in CD8+ T cells
Previously frozen peripheral blood mononuclear cells (PBMCs) were thawed and washed in RPMI-1640 (Fisher Hampton, NH, USA). The cells were counted and resuspended in RPMI-1640 + 10% AB serum (Cambrex East Rutherford, NJ, USA) at a concentration of 5 × 106/ml and 100 µl were plated in a 96-well plate. Cells were incubated with aAPC coated with HLA–A2 CMV + anti-CD28, anti-CD3 + anti-CD28 (positive control) or HLA–A2/irrelevant peptide + anti-CD28 (negative control). The aAPCs were added to thawed PBMCs in ratios from 10 : 1 (bead : cell) to 50 : 1 and incubated with 1 µg of Brefeldin A (Sigma, St Louis, MO, USA) at 37°C for 6 h. After the 6-h stimulation, the cells were fixed in 1% paraformaldehyde (Sigma) for 10 min at 4°C followed by centrifugation and washing with 0·1% saponin (Sigma) and 0·5% sodium azide (Sigma). After one wash with saponin, the cells were resuspended in 50 µl 0·1% saponin and 5 µl of each phycoerythrin (PE)-conjugated anti-human IFN-γ (25723) (R&D Systems) and FITC conjugated anti-human-CD8 (B9·11) (Beckman Coulter, San Diego, CA, USA). After a 30-min incubation with the antibodies, the cells were washed twice in fluorescence activated cell sorter (FACS) buffer (PBS + 0·5% BSA and 0·1% NaN3) and analysed by flow cytometry (FACScan cytometer, BD Biosciences, San Jose, CA, USA). Data analysis was performed using Cellquest software (BD Biosciences).
Cell culture and restimulation conditions (ELISPOT)
PBMC were isolated from blood using a Ficoll-Histopaque gradient (Amersham, Piscataway, NJ, USA). CD8+ cells were separated using the Dynal CD8 isolation kit (Dynal, Oslo, Norway) following the manufacturer's instructions. Briefly, 2 × 107 cells were incubated with anti-human-CD8 magnetic beads for 20 min at 4°C with constant agitation. The bead bound cells were then incubated 45 min at room temperature with Detachabead. After the CD8+ cells were detached from the magnetic beads they were counted and plated in 96-well plates at 5 × 105/well in RPMI-1640 + 10% human AB serum. The CD8-negative cells were saved and pulsed with CMV peptide for 1 h at 37°C and irradiated. The peptide-pulsed, irradiated CD8-negative cells were added to the wells at 5 × 105/well. The cells were incubated at 37°C, 5% CO2 for 10 days. The cells were fed on days 1 and 4 with RPMI-1640 + 10% human antibody serum. Recombinant IL-2 (Chiron, Emeryville, CA, USA) and interleukin (IL)-7 (R&D Systems) were added at a final concentration of 10 U/ml and 10 ng/ml, respectively.
After CD8+ cell expansion the cells were harvested and plated at 50 000 and 10 000 cells/well in Millipore filter plates that had been coated with anti-IFN-γ (1-D1K at 2 µg/ml) antibody (Mabtech, Mariemart, OH, USA). The CD8+ cells were restimulated with either CMV peptide-pulsed T2 cells (ATCC, Rockville, MD, USA [9]), naive T2 cells, beads coated with HLA–A2 CMV + anti-human CD28, or beads coated with HLA–A2-negative peptide + anti-human CD28 and phytohaemagglutinin (PHA) (Sigma). The cells were incubated overnight at 37°C and 5% CO2. After incubation, the wells were washed six times with PBS 0·1% Tween-20 (Sigma) and twice with PBS. After washing, the detection antibody anti-human IFN-γ 7-B6-1 (Mabtech) was added to the wells at a concentration of 0·2 µg/ml and incubated at 37°C for 2 h. The washes were repeated as above and streptavidin-ALP 1000 : 1 (Mabtech) was added to the wells and incubated at 37°C for 1 h. The washes were repeated and spots were developed with NBT-BCIP (Sigma). After spot development, the wells were rinsed several times with distilled water and plates were left to dry at room temperature protected from light. Once dry the spots were counted on an immunospot plate reader (Cellular Technologies Ltd, Cleveland, OH, USA).
Results
Structure of aAPC
In an effort to avoid interassay variability attributable to the use of peptide-loaded live APC (either cultured or PBMC-derived) for the purpose of activation of peptide-specific CTLs, and attempting to increase the signal-to-noise ratio for the quantification of low-frequency CTLs, we constructed an artificial (synthetic) reagent that could function as an APC capable of stimulating IFN-γ (or other cytokine) synthesis in peptide-specific human CTLs. A synthetic APC avoids variations associated with peptide loading live APC and introduces a ‘standardized’ reagent that can be used over an extended period of time. Our aAPC consists of a solid-phase 2 µm paramagnetic bead coated with avidin to which we added class I HLA-A*0201 molecules containing an A2-restricted CMV peptide (NLVPMVATV) to activate antigen receptors on CMV-reactive T cells plus biotinylated anti-CD28. We elected to develop this assay using the HLA–A2 CMV complex due to the high frequency of CMV immune HLA–A2 patients in our community. The juxtaposed co-stimulatory signal increases the efficiency of T cell activation over tetramer/peptide alone (see below). Preliminary experiments indicated optimal activation responses could be obtained with aAPC containing equal molar concentrations of HLA–A2/CMV and anti-CD28 molecules.
Peptide-loaded aAPC induced intracellular IFN-γ production in peptide-specific CTLs
In an effort to test the ability of aAPC to stimulate peptide-specific CTLs in vitro and functionally ascertain the frequency of peptide-specific CTLs beyond their ‘tetramer’ enumeration, we conducted a series of experiments where varying concentrations of aAPC were incubated for different times with human PBMCs. Optimal results were obtained following 6 h incubation at 37°C using an aAPC expressing both peptide-loaded HLA–A2 complex and anti-CD28 (Fig. 1). PBMCs were incubated with aAPCs loaded with anti-CD28 and either HLA–A2 cognate CMV peptide or irrelevant peptide-loaded complexes. At the completion of incubation, PBMCs were stained for CD8 and anti-IFN-γ. In repeated experiments the 6-h incubation time appeared to generate the best signal-to-noise ratio (Fig. 1). Background activity was too high following 12 h incubation. Stimulation with aAPC containing anti-CD3 and anti-CD28 (maximum antigen receptor-initiated IFN-γ response) resulted in CD8+/IFN-γ+ lymphocyte frequencies of 5·48%, while aAPCs containing only the HLA–A2/peptide complex or only anti-CD28 resulted in CD8+/IFN-γ+ frequencies of 0·5–0·56% (Fig. 1). Co-stimulation with anti-CD28 was necessary, as aAPCs containing only the HLA–A2/peptide complex were unable to induce intracellular IFN-γ synthesis. Incubation of PBMCs with aAPCs containing HLA–A2/peptide (CMV) complexes and anti-CD28 demonstrated a CD8+/IFN-γ+ lymphocyte frequency of 2·12%. In this example, the CTL frequency of CMV peptide-specific CTLs determined by tetramer assay was 2·52%.
Fig 1.
(a) Human peripheral blood mononuclear cells were incubated for 6 h with artificial antigen-presenting cells (aAPC) carrying anti-CD3 [aAPC(CD3)], anti-CD28 [aAPC(CD28)], human leucocyte antigen (HLA)-A2/CMV complex [aAPC(A2CMV)], anti-CD3 and anti-CD28 [aAPC(CD3/CD28)] or HLA–A2/CMV complex and anti-CD28 [aAPC(A2CMV/CD28)]. Following incubation, cells were immunophenotyped for intracellular interferon (IFN)-γ [anti-IFN-γ phycoerythrin (PE)] and CD8 [anti-CD8 fluorescein isothiocyanate (FITC)]. Confocal microscopy of the cells/aAPCs suspension prior to analysis (bottom right) demonstrates intracellular staining of IFN-γ (PE) in a cytotoxic T lymphocyte (CTL) that is surface labelled by an anti-CD8-FITC antibody. The frequency of CMV tetramer-positive CTLs in the illustrated experiment was 2·52%. (b) aAPCs containing anti-CD3 and anti-CD28 (CD3/CD28) or anti-CD28 and HLA–A2 containing an irrelevant tetramer (A2ir/CD28) or anti-CD28 and HLA–A2 complex containing a cognant cytomegalovirus (CMV) peptide (A2CMV/CD28) were incubated with peripheral blood mononuclear cells (PBMCs) for 3, 6 and 12 h prior to intracellular cytokine staining for IFN-γ. PBMCs were gated on lymphocytes and analysed for CD8 and IFN-γ immunostaining. Similar results were observed in at least three different experiments.
Interassay reproducibility
For this assay to be useful for analysis of clinical samples, it must be able to quantify reproducibly the number of functional, peptide-specific CTLs in archived (frozen) peripheral blood samples. Thus, we analysed the interassay variability of the 6-h assay using aliquots of the same clinical sample (frozen PBMCs) performed in assays on different days. In the illustrated example (Fig. 2) the assay was highly reproducible when the same PBMC sample was analysed in five different experiments conducted on different days. The assay was most reliable [least coefficient of variation (CV)] at the aAPC/PBMC ratio of 50 : 1. There was a clear dose–response relationship between aAPC numbers in the assay and the frequency of CMV-reactive CTLs. Further increases in the aAPC/PBMC ratio did not increase the frequency of CD8+/IFN-γ+ lymphocytes (data not shown). Using aAPC : T cell ratios of 10 : 1–50 : 1, the coefficient of variation (CV) ranged from 15·2% to 4·6% (Fig. 2). Thus, at optimal conditions (6 h incubation at aAPC : PBMC ratio of 50 : 1), the assay was highly reproducible (CV = 4·6%). All further experiments were conducted using these experimental conditions.
Fig 2.
Five different experiments performed with a single patient sample human leucocyte antigen (HLA)-A2+ patient with tetramer-positive cytotoxic T lymphocytes (CTLs) recognizing cytomegalovirus (CMV) peptide on five different days. Data are expressed as mean percentage CD8+/interferon (IFN)-γ+ lymphocytes (± s.d.) analysed following 6 h incubation with: media alone (unstimulated); anti-CD3 coated artificial antigen-presenting cells (aAPCs) [aAPC(CD3)]; anti-CD28 coated aAPCs [aAPC(CD28)]; HLA–A2/CMV peptide coated aAPCs [aAPC(A2CMV)]; anti-CD3/anti-CD28 [aAPC(CD3/CD28)] and a series of HLA–A2CMV and anti-CD28 coated aAPCs [aAPC(A2CMV/CD28)] incubated with PBMCs at ratios ranging from 10 : 1 to 50 : 1. IFN-γ and CD8 immunostaining on gated live lymphocytes were performed in each experiment. The table depicts the coefficients of variation among repeat experiments for the given assays.
In lieu of a true positive control we decided to compare the results of our CTL estimates to those of the tetramer assay. The results of the estimated CTL frequencies using the aAPC method of intracellular IFN-γ synthesis were highly correlated with the results of tetramer staining for the same peptide (Fig. 3). In a series of 21 different experiments using PBMC samples from seven different donors for the quantification of CMV peptide-specific CTLs, the tetramer and the aAPC methods demonstrated excellent correlation (r = 0·89). Similar testing using the same PBMC samples comparing CTL frequency estimates obtained by conventional ELISPOT compared with tetramer frequencies for the same peptides did not demonstrate the same level of correlation (Fig. 3, r = 0·06).
Fig 3.
Peripheral blood lymphocytes were analysed for the frequency of cytomegalovirus (CMV) peptide-specific cytotoxic T lymphocytes (CTLs) using commercially available CMV tetramers (Beckman Coulter, Fullerton, CA, USA) or intracellular interferon (IFN)-γ staining following incubation of lymphocytes with artificial antigen-presenting cells (aAPCs) containing anti-CD28 and the same CMV peptide associated with human leucocyte antigen (HLA)-A2 analysed intracellular IFN-γ synthesis flow cytometry (left). CMV peptide-specific CTL frequencies were also analysed using conventional enzyme-linked immunospot assay (ELISPOT) using CMV peptide-loaded T2 cells (right). CTL frequencies using the tetramer method are presented as percentage positive lymphocytes that stain for both tetramer and CD8 (% tetramer-positive). CTL frequencies obtained using the aAPC method are presented as the percentage of lymphocytes that stain positive for intracellular IFN-γ and CD8 (% CD8/IFN-positive). The data represent 21 different human samples (from seven different donors) analysed in both methods. The correlation coefficient is between tetramer staining and aAPC-driven intracellular IFN-γ staining is 0·89. The correlation between tetramer staining and conventional ELISPOT for IFN-γ is 0·06.
Tetramer and aAPC restimulation of lymphocytes previously in vitro expanded with CMV peptide-loaded CD8− cells
The rapidity with which the 6-h assay can be used to quantify antigen-reactive CTL is an asset for analysing large numbers of patient samples in a clinical laboratory. However, the magnitude of the CMV-reactive CTL response is relatively small, and it could be anticipated that the number of tumour antigen-reactive CTL in PBMC from cancer patients could be even smaller. To determine if the fraction of responding cells could be increased after in vitro expansion, CD8+ cells were cultured with CMV peptide-loaded CD8− cells for 10 days and then analysed for the frequency of CMV peptide-reactive CTLs using tetramers, ELISPOT (T2 and aAPC) and aAPC restimulation followed by intracellular IFN-γ staining. The data presented (Fig. 4) represent five different experiments in which aliquots of the same patient sample were used on different days for the analysis of the frequency of CMV-reactive CTLs using different methods. The number of CMV-reactive CTLs obtained by the aAPC assay and tetramer staining were not significantly different. Conventional ELISPOT performed with either T2 or aAPC, as the antigen-presenting cells demonstrated significantly lower frequencies of IFN-γ-producing CTLs than determined with the aAPC or tetramer assays. In the ELISPOT assays, the aAPC appeared to be at least as effective as peptide-loaded T2 cells in stimulating CD8+ CTLs to produce IFN-γ (Fig. 4, photograph). Antigen specificity was confirmed using aAPCs containing non-CMV (irrelevant) peptides (A2ir).
Fig 4.
CD8+ cytotoxic T lymphocytes (CTLs) were co-cultured with cytomegalovirus (CMV) peptide-loaded CD8− cells for 10 days. Following culture, the frequency of CMV peptide-reactive CTLs was determined using: (1) tetramer staining (CMV tetramer); (2) interferon (IFN)-γ-enzyme-linked immunospot assay (ELISPOT) assay using either CMV-loaded T2 cells (T2-CMV) or CMV-loaded artificial antigen-presenting cells (aAPCs) [aAPC(A2CMV/CD28)] stimulation of interferon (IFN)-γ production (photographs developed ELISPOT wells of unstimulated and aAPC restimulated CTLs); or (3) flow cytometry-based intracellular staining for IFN-γ in CD8+ T cells following in vitro restimulation with aAPCs containing anti-CD28+ human leucocyte antigen (HLA)-A2/CMV peptide [aAPC(A2CMV/CD28), or anti-CD28+ HLA–A2/non-CMV peptide [aAPC(A2ir/CD28)], and anti-CD28+ anti-CD3 [aAPC(CD3/CD28)] (positive control). Negative controls of in vitro expanded, unstimulated (no aAPC stimulation post-expansion) cells were also analysed for IFN-γ production (unstimulated). The data represents the mean (± s.e.) relative frequencies of CMV reactive CTLs from a single specimen analysed in five different experiments performed on five different days. Similar results were observed in at least three other experiments with peripheral blood mononuclear cell (PBMC) samples from other donors.
Simultaneous quantification of cytokine-producing (IFN-γ) versus non-producing peptide-specific CTLs
Despite the ability to stimulate and quantitatively measure peptide-specific CTLs and induce IFN-γ synthesis, we were unable to ascertain directly the relative numbers of active (IFN-γ-producing) versus inactive (IFN-γ-non-producing) peptide-specific CTLs. In vitro expansion methods (e.g. ELISPOT) favour proliferation of peptide-specific CTLs but do not identify non-reactive, peptide-specific CTLs. Tetramer-based analyses reveal only the total number of peptide-specific CTLs but do not discriminate active from inactive cells. The CFC assay is dependent on endogenous APCs to present the peptide of interest that may/may not remain constant in serial blood samples quantifying changes of peptide-specific CTL frequencies. Identification of the relative ratios of peptide-specific active (cytokine producing) versus inactive (non-producing) CTLs may correlate with clinical outcomes in cancer vaccine studies [3]. The ability to do so may be particularly relevant, considering the poor functional (quiescent) status of many tumour antigen peptide-specific CTLs in patients with cancer undergoing peptide immunizations [9,10].
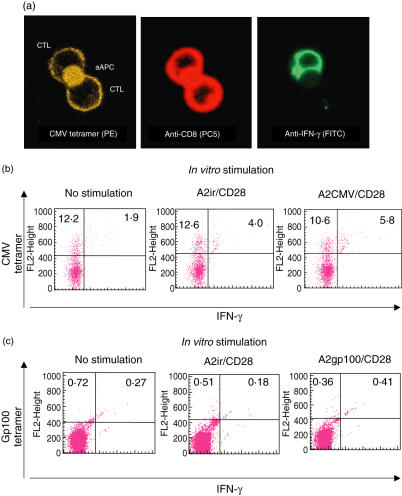
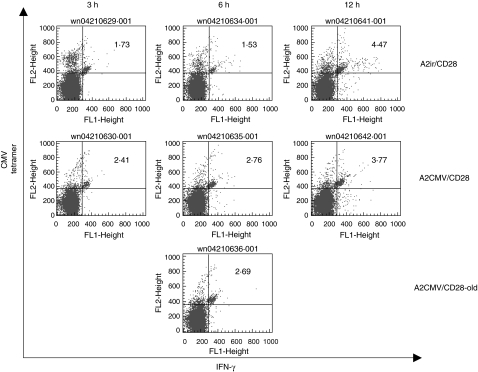
To address these issues we modified our assay and added a tetramer stain along with intracellular IFN-γ immunostaining at the end of the 6- h aAPC incubation. Similar to the CFC assay kit (Beckman-Coulter, Fullerton, CA, USA), gating on CD8+ lymphocytes allowed us to directly visualize the ratio of IFN-γ-positive versus IFN-γ-negative, tetramer-positive CTLs (Fig. 5). The confocal photomicrograph demonstrates an aAPC generating endogenous IFN-γ synthesis in one, but not the other tetramer-positive CTL (Fig. 5a). After CMV-specific aAPC stimulation we were able to detect a reciprocal ‘shift’ of a fraction of tetramer-positive CTLs from IFN-γ− to IFN-γ+. In the example illustrated (Fig. 5b) the total number of CMV tetramer-positive CTLs in the unstimulated (no aAPC) controls is 14·8% (12·2 ± 1·9). Upon stimulation with aAPCs containing a non-cognate peptide and anti-CD28 (A2ir/CD28) the fraction of IFN-γ negative, CMV tetramer-positive CTLs does not change relative to unstimulated controls (12·2% versus 12·6%). The number of CMV tetramer-positive/IFN-γ-positive CTLs increases to 4·08%, probably reflecting non-specific tetramer staining of anti-CD28-stimulated cells. However, upon cognate stimulation with aAPCs carrying CMV peptide and anti-CD28 (A2CMV/CD28), the increase of CMV tetramer-positive/IFN-γ-producing CTLs changes from a control level of 4·08–5·84% with a reciprocal decrement of CMV tetramer-positive/IFN-γ-non-producing CTLs (change from 12·61% to 10·6%). Thus, it would appear that roughly 14% of the CMV tetramer-positive CTLs became IFN-γ producers upon cognate stimulation. Similar ratios of active (IFN-γ-producing) versus inactive peptide-specific CTLs to CMV peptide (recall) antigens were observed in several different healthy volunteers (data not shown). This method was also able to ascertain low-frequency CTLs in HLA–A2+ patients with metastatic melanoma, demonstrating spontaneous anti-melanoma differentiation antigen (gp100) CTL immune responses (Fig. 5c). Even though the frequency of gp100-reactive CTLs is much lower, the assay is still able to detect intracellular IFN-γ production relative to controls (irrelevant peptide/HLA–A2 complex-stimulated CTLs). For this analysis, varying the incubation time of aAPCs and PBMCs between 3 and 12 h confirmed the optimal incubation time of 6 h (Fig. 6). The results were also reproducible using different batches of aAPCs (up to 3 months old).
Fig 5.
(a) Confocal fluorescent photomicrographs of two CD8+/tetramer+ cytotoxic T lymphocytes (CTLs) binding a single artificial antigen-presenting cell (aAPC) (A2CMV/CD28). However, only one (right panel) demonstrates intracellular interferon (IFN)-γ synthesis. (b) Flow cytometry analysis of CD8+ CTLs stimulated in vitro (6 h) with aAPCs carrying anti-CD28 and either: (a) human leucocyte antigen (HLA)-A2 molecules loaded with cognate cytomegalovirus (CMV) peptide (A2CMV/CD28); or (b) irrelevant negative control peptide (A2ir/CD28), and stained with CMV tetramer and intracellular IFN-γ. Similar results were obtained in three other experiments. (c) Flow cytometry analysis of CD8+ CTLs stimulated in vitro (6 h) with aAPCs carrying anti-CD28 and either: (a) HLA–A2 molecules loaded with cognate gp100 peptide (A2CMV/CD28); or (b) irrelevant negative control peptide (A2ir/CD28), and stained with gp100 tetramer and intracellular IFN-γ. Similar results were obtained in five other experiments.
Fig 6.
Different incubation times (3, 6 or 12 h) of peripheral blood mononuclear cells (PBMCs) with artificial antigen-presenting cells (aAPCs) containing anti-CD28 and either human leucocyte antigen (HLA)-A2/cytomegalovirus (CMV) peptide (A2CMV/CD28) or HLA/A2-irrelevant peptide complexes (A2ir/CD28), prior to immunostaining for interferon (IFN)-γ and CMV tetramer. The same clinical sample was also incubated with a 3-month-old aAPC containing anti-CD28 and HLA–A2/CMV peptide complex. (A2CMV/CD28-old). Represented are flow cytometry results of CMV tetramer/IFN-γ positive cytotoxic T lymphocytes.
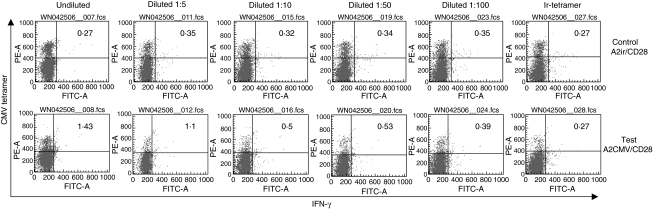
In an effort to address the lower limits of reliable detection of cytokine-producing peptide-specific CTLs we conducted an experiment where a patient's PBMC sample was separated into CD8+ and CD8− fractions. The CD8+ cells were serially diluted with CD8− cells at ratios ranging from 1 : 5 to 1 : 100 before incubation with the aAPCs (Fig. 7). The cellular mixtures were incubated with aAPCs containing anti-CD28 and HLA–A2 complexes carrying either a CMV cognant peptide (test) or an irrelevant control (control). The frequency of tetramer positive/IFN-γ-producing CTLs was calculated as the difference between the test and control values. At a dilution of 1 : 100, the measured frequency of IFN-γ-producing tetramer-positive CTLs was 0·04% (0·39–0·35) and the predicted frequency based on the dilutions was 0·01%. Further dilutions did not demonstrate any measurable differences among test and control samples. Thus, it would appear that the assay is capable of detecting frequencies of IFN-γ-producing CTLs as low as 0·05%. Irrelevant tetramer-stained CMV or irrelevant peptide-stimulated CTLs did not detect any IFN-γ production (0·27–0·27, last column). Similar observations were made in at least five other experiments.
Fig 7.
Dilution of known cytomegalovirus (CMV) tetramer-reactive cytotoxic T lymphocytes (CTLs) with syngeneic CD8 negative lymphocytes followed by in vitro aAPC stimulation with A2CMV/CD28 (‘test’ bottom row) or A2ir/CD28 (‘control’ top row). Undiluted samples were stained with irrelevant tetramer (Ir-tetramer column). Dilutions were created by diluting CD8+ T cells with CD8− cells from the same donor prior to assay. Similar results were obtained in at least three other experiments.
Discussion
The absence of reliable and broadly applicable methods for the quantification of ‘functional’ T cell immune responses in cancer vaccine clinical trials has presented a significant obstacle to efforts in improving vaccine immunization/clinical efficacy. Without effective immunization, it is unlikely to expect significant clinical efficacy. Ongoing difficulties in correlating immune responses to cancer vaccines with anti-tumour clinical activity may be due, at least in part, to existing methods of immune response monitoring [10,11]. Even in established laboratories, results of ‘standard’ methods are difficult to correlate with clinical outcomes in cancer vaccine trials [3]. Combined with the possibility of generating immune tolerance (or quiescence) as a consequence of immunization, improved methods for quantification of active anti-peptide vaccine immune responses becomes critical [9–14]. Therefore, having the ability to distinguish reproducibly between active (cytokine-producing) versus quiescent (non-producing) CTL responses in serially collected peripheral blood specimens would be very useful in the effort to develop clinically effective cancer vaccines.
The basic principle of the presented method using aAPCs for CTL stimulation is not new. The first reports using aAPCs for in vitro expansion of antigen-specific T cells were the basis of our hypothesis that similar reagents could be used for functional peptide-specific CTL quantification [4,7]. A similar concept of using peptide-loaded chimeric HLA–A2-Ig fusion proteins combined with anti-CD28 antibodies coating magnetic beads to stimulate T cell proliferation and cytokine production (IFN-γ and IL-4) in vitro has been suggested [7]. In our experiments, we elected to use biotinylated, soluble HLA–A2 molecules loaded with nano-peptides of interest because of their similarity to highly standardized tetramer reagents. The minimal interassay variability of tetramer staining suggested that any approach attempting to improve upon this assay by adding a functional component would probably be successful and there would be a relevant/reliable ‘gold standard’ for the new assay. Both our aAPC and those described by Oelke et al. were able to stimulate intracellular cytokine production in T cells as well as expand them in vitro. Additionally, we were able to directly visualize the relative ratios of cytokine-producing (IFN-γ+) versus cytokine non-producing (IFN-γ−), tetramer-specific CTLs in human peripheral blood. To the extent that T cell receptor-driven stimulation of intracellular IFN-γ synthesis is reflective of an activated T cell, this method displays the relative ratio of activated (IFN-γ+) versus inactive (IFN-γ−) peptide-specific CTLs. In general, our method allows for direct comparisons of any cytokine-producing versus non-producing tetramer-positive CTLs. The choice of intracellular cytokine staining will depend upon particular experimental requirements. This may be particularly relevant in peptide vaccine clinical trial in patients with advanced cancers, where issues of tolerance may play a greater role in the observed increase in tetramer-specific CTLs of peripheral blood [12,13]. Moreover, data obtained with ex vivo clinical samples suggest the possibility that interpretation of peptide-specific CTL frequencies using in vitro CTL expansion may be misleading, as reactive CTLs (IFN-γ+) may have a proliferative advantage over their tolerized/quiescent counterparts. Application of a CFC-based method of CTL quantification by adding peptides of interest to endogenous, patient-derived PBMC may be difficult to interpret in serial samples in view of the increasing realization of dysfunctional antigen presentation in cancer patients [14–16]. Sample-to-sample changes in APC numbers/function in the PBMC preparation may affect CTL functional enumeration results. This may explain, in part, some of the difficulties in correlating laboratory data of effective immunization in the context of poor clinical outcomes of ‘immunized’ cancer patients. Although poor clinical outcomes in advanced cancer patients undergoing vaccine therapy could be the result of a number of events, including loss of tumour antigens, the possibility of induction of ineffective immunity (e.g. aAPC-driven stimulation of intracellular down-regulatory cytokines: IL-5 or IL-10) is particularly concerning, as more and more cancer vaccine clinical studies are used for patients that are tumour-free and at high risk of tumour recurrence. Generating tumour-specific tolerance rather than active immunity in that setting may be permissive rather than protective of tumour relapse. Additional studies are needed to evaluate which cytokines (or combination of cytokines) are associated with specific effector T cell activity in different disease settings. Our flow cytometric method has the potential to assay multiple cytokines at once, thus increasing the potential value of the assay.
In summary, the method described builds on existing technology, furthering the effort in developing more reliable means for the quantification of CTL peptide-specific immunity in humans. The assay's simplicity and reproducibility allow consideration for high-throughput applications (multiple beads coated with multiple peptides) and automation (standardized aAPC reagents). Studies evaluating the clinical utility (validity) of this method in cancer patients undergoing vaccine therapy are under way.
Acknowledgments
This work was supported by a grant from the Cancer Vaccine Collaborative/Cancer Research Institute/Ludwig Institute for Cancer Research.
References
- 1.Nagorsen D, Marincola FM. How to analyze ex vivo T-cell responses in cancer patients. In Vivo. 2002;16:519–25. [PubMed] [Google Scholar]
- 2.Nagorsen D, Scheibenbogen C, Thiel E, Keilholz U. Immunological monitoring of cancer vaccine therapy. Expert Opin Biol Ther. 2004;4:1677–84. doi: 10.1517/14712598.4.10.1677. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 4.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–24. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 5.Thomas AK, Maus MV, Shalaby WS, June CH, Riley JL. A cell-based artificial antigen-presenting cell coated with anti-CD3 and CD28 antibodies enables rapid expansion and long-term growth of CD4 T lymphocytes. Clin Immunol. 2002;105:259–72. doi: 10.1006/clim.2002.5277. [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME. To bead or not to bead. J Immunother. 2003;26:187–9. doi: 10.1097/00002371-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Oelke M, Schneck JP. HLA-Ig-based artificial antigen-presenting cells: setting the terms of engagement. Clin Immunol. 2004;110:243–51. doi: 10.1016/j.clim.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Yan X, Johnson BD, Orentas RJ. Murine CD8 lymphocyte expansion in vitro by artificial antigen-presenting cells expressing CD137L (4–1BBL) is superior to CD28, and CD137L expressed on neuroblastoma expands CD8 tumour-reactive effector cells in vivo. Immunology. 2004;112:105–16. doi: 10.1111/j.1365-2567.2004.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–46. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 10.Monsurro V, Wang E, Yamano Y, et al. Quiescent phenotype of tumor-specific CD8+ T cells following immunization. Blood. 2004;104:1970–8. doi: 10.1182/blood-2004-02-0525. [DOI] [PubMed] [Google Scholar]
- 11.Walker EB, Disis ML. Monitoring immune responses in cancer patients receiving tumor vaccines. Int Rev Immunol. 2003;22:283–319. doi: 10.1080/08830180305226. [DOI] [PubMed] [Google Scholar]
- 12.Toes RE, van der Voort EI, Schoenberger SP, et al. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol. 1998;160:4449–56. [PubMed] [Google Scholar]
- 13.Toes RE, Blom RJ, Offringa R, Kast WM, Melief CJ. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. J Immunol. 1996;156:3911–8. [PubMed] [Google Scholar]
- 14.Toes RE, Offringa R, Blom RJ, Melief CJ, Kast WM. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci USA. 1996;93:7855–60. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinzon-Charry A, Maxwell T, Lopez JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–61. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinzon-Charry A, Maxwell T, McGuckin MA, Schmidt C, Furnival C, Lopez JA. Spontaneous apoptosis of blood dendritic cells in patients with breast cancer. Breast Cancer Res. 2005;8:R5. doi: 10.1186/bcr1361. [DOI] [PMC free article] [PubMed] [Google Scholar]