Abstract
The purpose of this prospective study was to enumerate Toll-like receptor 9 (TLR9)+ cells and measure their function using synthetic oligonucleotides enriched in CG dinucleotide motifs (CpG)-induced proliferation within 48 h after trauma in severely injured patients prone to sepsis. Sixteen consecutive trauma patients with an injury severity score (ISS) > 21 and 16 blood donors (controls) were included in this study. Using two-colour flow cytometry, TLR9 expression was detectable intracellularly and also on the surface of B lymphocytes. The surface expression of TLR9 of B lymphocytes from whole blood and peripheral blood mononuclear cells (PBMC) stimulated with CpG was significantly increased in B cells of severely injured patients prone to sepsis compared to controls. No significant differences could be observed between CpG-induced proliferation of PBMC of severely injured patients prone to sepsis and controls. As a measure of immunosuppression, human leucocyte antigen (HLA)-DR expression of monocytes of the trauma patients was significantly diminished compared with controls in PBMC and in whole blood. Immunosuppression in the early phase after trauma seems not to be associated with a disturbed sensing of bacterial DNA.
Keywords: B cells, CpG, sepsis, TLR9, trauma
Introduction
Despite significant advances in the field of intensive care medicine, sepsis remains the most frequent cause of complications and death in severely injured patients [1,2].
It is well known that individuals suffering from severe injury have at least a transient immunodeficiency. This immunodeficiency is associated especially with a decrease of monocytic as well as lymphocytic human leucocyte antigen (HLA)-DR expression and an increase of the risk for developing Gram-positive or negative bacterial sepsis [3–6].
Among the microbial products sampled continuously by the mammalian immune system is DNA [7]. The ability to produce an immunostimulatory activity present in microbial DNA led to the elucidation of optimal stimulatory motifs and the identification of functional groups in nucleotides that influence inflammatory activity. Short segments of unmethylated, CG dinucleotide motif-rich DNA in a particular base context determine the immunostimulatory activity in a species-specific manner.
When microorganisms invade into the body, phagocytes such as macrophages, neutrophils and dendritic cells engulf and kill these organisms [8]. At the same time, these cells recognize conserved motifs in pathogens termed ‘pathogen-associated molecular patterns’ (PAMPS). The mammalian Toll-like receptor (TLR) is one of such examples of PAMPS recognition. Engagement of the TLR by their respective ligands initiates a multi-faceted response involving antigen presentation, cell surface expression of co-stimulatory molecules, as well as synthesis and release of cytokines, which in turn stimulates specific adoptive responses involving T and B lymphocytes.
Thirteen members of mammalian TLR identified to date are differently expressed on haematopoietic and non-haematopoietic cells. In general, mononuclear phagocytes and dendritic cells express the widest TLR repertoire. Several recent studies have suggested a role of TLR in the stimulatory effects on human B lymphocytes induced by microbial products. TLR9 is described as an intracellular receptor for unmethylated, CG dinucleotide motif-rich DNA released from bacteria during infection or endogenous cells during autoimmune diseases [9–12]. Correspondingly, high levels of mRNA for TLR9 are found in B lymphocytes [13]. Recently, the binding of TLR9 antibodies on the surface of B lymphocytes has also been described [14].
In the early phase after trauma, immunosuppression in terms of decreased HLA-DR expression as well as diminished cytokine response to endotoxin has been described in monocytes [5]. Therefore, it is hypothesized that the response to bacterial DNA, e.g. by change in TLR9 expression and function, may also be altered after trauma which leads to a diminished immune response against bacterial components and the consecutive development of sepsis.
For this purpose, we analysed flow cytometric TLR9 expression on the surface of B lymphocytes in whole blood and on the surface as well as intracellularly in isolated peripheral blood mononuclear cells (PBMC) before and after 2 days of stimulation with synthetic oligonucleotides enriched in CG dinucleotide motifs (CpG).
Materials and methods
Patients and controls
This was a prospective study of severely injured patients prone to sepsis. Criteria for inclusion of patients were age (>18 and <80 years), severity of injury of at least 21 points according to the injury severity score (ISS) [15] and primary admission to the surgical intensive care unit of the Department of Trauma Surgery, University Hospital of Essen within 8 h after accident. The specific threshold for the ISS was chosen because a significant increase of post-traumatic complications, including sepsis, has been reported [16]. All the patients' medical histories were free of pre-existing immunological disorders, systemic steroid medication or known cancer disease. Sepsis was defined according to the consensus conference criteria published by Bone et al. [17]. Sixteen consecutive patients were studied. The demographic and clinical profile including pattern of injury are given in Table 1. Fifteen patients developed sepsis later, caused predominantly by a pulmonary focus. The source of sepsis and relevant bacterial findings are summarized in Table 2.
Table 1.
Demographic and clinical profile of severely injured patients prone to sepsis.
| Number of patients | 16 |
| Age (years) | 37 ± 4a |
| Sex (male/female) | 13/3 |
| ISSb | 42 ± 2 |
| AISc brain/neck | 2·7 ± 0·5 |
| AIS face | 0·9 ± 0·3 |
| AIS thorax | 3·4 ± 0·3 |
| AIS abdomen | 2·1 ± 0·4 |
| AIS extremities | 3·1 ± 0·3 |
| AIS general | 0·5 ± 0·2 |
| Outcome (survived/died) | 13/3 |
| ICUd days | 33 ± 6 |
| Ventilator days | 25 ± 5 |
| Sepsis days | 13 ± 3 |
Mean ± standard error of the mean
ISS: injury severity score
AIS: abbreviated injury scale
ICU: intensive care unit.
Table 2.
Onset of sepsis and characteristics of infection.
| Patient no. | Onset of sepsis (first day) | Bacteria | Focus |
|---|---|---|---|
| 1 | 8 | Escherichia coli | Lung |
| 2 | 4 | Staphylococcus aureus | Lung |
| 3 | 8 | Pseudomonas aeruginosa | Catheter, lung |
| 4 | 26 | S. epidermidis | Catheter, lung |
| 5 | 7 | S. epidermidis | Catheter, lung |
| 6 | 18 | Enterobacter | Catheter |
| 7 | No sepsis | ||
| 8 | 4 | S. aureus | Lung |
| 9 | 6 | Enterobacter | Catheter |
| 10 | 3 | P. aeruginosa | Lung |
| Haemophilus parainfluenzae | |||
| 11 | 3 | Streptococcus group G | Lung |
| 12 | 4 | H. influenzae | Lung |
| 13 | 3 | S. aureus | Lung |
| 14 | 11 | Klebsiella pneumoniae | Lung |
| 15 | 8 | Enterococcus faecalis | Lung |
| 16 | 10 | H. influenzae | Catheter, lung |
Sixteen blood volunteers (14 men and two women) served as controls and gave informed consent to deliver blood for this study. Their ages [mean 45 years, standard error of the mean (s.e.m.) 3 years] were not significantly different from the appropriate values of the patients.
The study and the blood sampling were approved by the ethic committee of the University Hospital of Essen. Informed consent of all subjects was obtained.
Blood sampling
Fifty ml heparinized venous blood was collected from the patients as well as controls in the morning. Samples from the patients were obtained on days 1 or 2 after hospital admission.
Cell counts
Leucocytes were counted using an electronic cell counter (Sysmex, Hamburg, Germany). Whole blood leucocytes were differentiated into granulocytes, monocytes and lymphocytes by flow cytometry using monoclonal antibodies (MoAb) fluorescein isothiocyanate (FITC)-conjugated CD45 (all leucocytes) and phycoerythrin (PE)-conjugated CD14 (monocytes). Expression of TLR9 and HLA-DR was measured using whole blood as well as PBMC separated by standard density gradient centrifugation and stimulated for 2 days with CpG (see B cell proliferation). TLR9 expression of granulocytes, monocytes and T lymphocytes as well as CD19+ B lymphocytes was estimated by an indirect two-colour labelling technique. In brief, 10 μl TLR9-specific MoAb from BioCarta Europe (Hamburg, Germany) was given to 250 μl phosphate-buffered saline (PBS). The BioCarta antibody was developed against synthetic peptides corresponding to the amino acids 268–284 of human TLR9. After addition of 100 µl whole blood or at least 1 × 105 PBMC the cells were incubated for 15 min in the dark at room temperature. After a washing step, 200 μl of 1 : 200 PBS-diluted second antibody anti-human IgG H + L [F(ab)2, FITC-labelled] from Coulter-Immunotech (Krefeld, Germany) was added and incubated as mentioned above. Cells were again incubated as described above following addition of 200 μl of a 1 : 20 dilution in PBS of CD19 or CD3 (T lymphocytes) or CD14 (monocytes) PE-conjugated MoAb (Becton Dickinson, Heidelberg, Germany). After lysis of erythrocytes (Becton Dickinson lysing solution) and washing the cells were measured using a FACSCalibur (Becton Dickinson) and CELLQuest software. HLA-DR+ CD14+ monocytes were stained by two colours using the MoAb combination HLA-DR FITC/CD14 PE from Becton Dickinson, with the exception of the second antibody, used as described previously [18].
Intracellular staining of PBMC was performed as described previously [18]. In brief, the CpG-stimulated cells were first incubated with CD19 MoAb and then, after rendering them permeable with Perm/Wash solution (Becton Dickinson), with TLR9 MoAb for 30 min in the dark at 4°C. The following procedure was the same as described above.
Fluorescence values for monocytes and lymphocytes were collected after gating on a combination of forward scatter (FSC) and side-scatter (SSC) characteristics. A total of 20 000 cells per sample were acquired. The signals were acquired in a linear mode for FSC and SSC characteristics, and in a logarithmic mode for fluorescence intensities. Measurement included percentages of populations as well as mean channel fluorescence intensity (MFI) in energy channels. The second antibody was used for determination of unspecific binding.
PBMC proliferation
For 48 h at 37°C in a humidified atmosphere with 5% CO2, 1 × 106 PBMC/ml RPMI-1640 medium containing 10% of heat-inactivated pooled human serum (Institute of Transfusion Medicine, University Hospital of Essen) were cultured either in the presence of 1 nmol/ml of the activating CpG 2006, the control CpG 2006GC (TibMolBiol, Berlin, Germany) or without any stimulus (autologous value), as described previously [19]. After incubation the cells were centrifuged (5 min, 250 g). After a washing step, 2 × 105 CpG-stimulated PBMC were placed in a flat-bottomed 96-well microtitre plate (Greiner, Solingen, Germany), labelled with 1 μCi of [3H]-thymidine (Amersham Pharmacia, Little Chalfont, UK) and incubated for a further 16 h at 37°C in a humidified atmosphere with 5% CO2. Using a multiple automatic sample harvester (Mach III Harvester96; Tomtec, Hamden, UK) the cells were harvested on glass fibre paper and counted as triplicates in scintillation fluid (Betaplate-Scint, Wallac, Finland). The results were given as counts per minute (cpm). The evaluation of proliferation was performed using the difference (mean cpm of stimulated cells − mean autologous values) or the stimulation index (SI; mean cpm of stimulated cells/mean autologous values).
Statistics
If not mentioned otherwise, data are given in mean ± s.e.m. Comparison of values between the groups of severely injured patients and controls was performed using the Mann–Whitney U-test. The signed-rank Wilcoxon test was performed for the comparison of TLR9 MFI values with the appropriate unspecific binding, activating CpG 2006-induced proliferation (difference or SI) with control CpG 2006GC-induced proliferation and CpG2006-induced HLA-DR expression with control CpG 2006GC-induced HLA-DR expression on monocytes within one group. The level of significance was designated as P < 0·05 (two-tailed tests). sas software (SAS, Cary, NC, USA) was used.
Results
Cell counts
In comparison to controls, severely injured patients prone to sepsis showed significant granulocytosis (controls 65 ± 2%, 3867 ± 215 cells/μl; patients 81 ± 2%, 7137 ± 1065 cells/μl) and lymphopenia (controls 27 ± 2%, 1544 ± 137 cells/μl; patients 12 ± 1%, 1016 ± 164 cells/μl) during the first 2 days after trauma. In contrast to relative B cell counts (controls 8·6 ± 0·8%; patients 9·8 ± 1·5%), absolute B cell numbers were significantly diminished in severely injured patients prone to sepsis compared with controls (controls 133 ± 17 cells/μl; patients 123 ± 45 cells/μl).
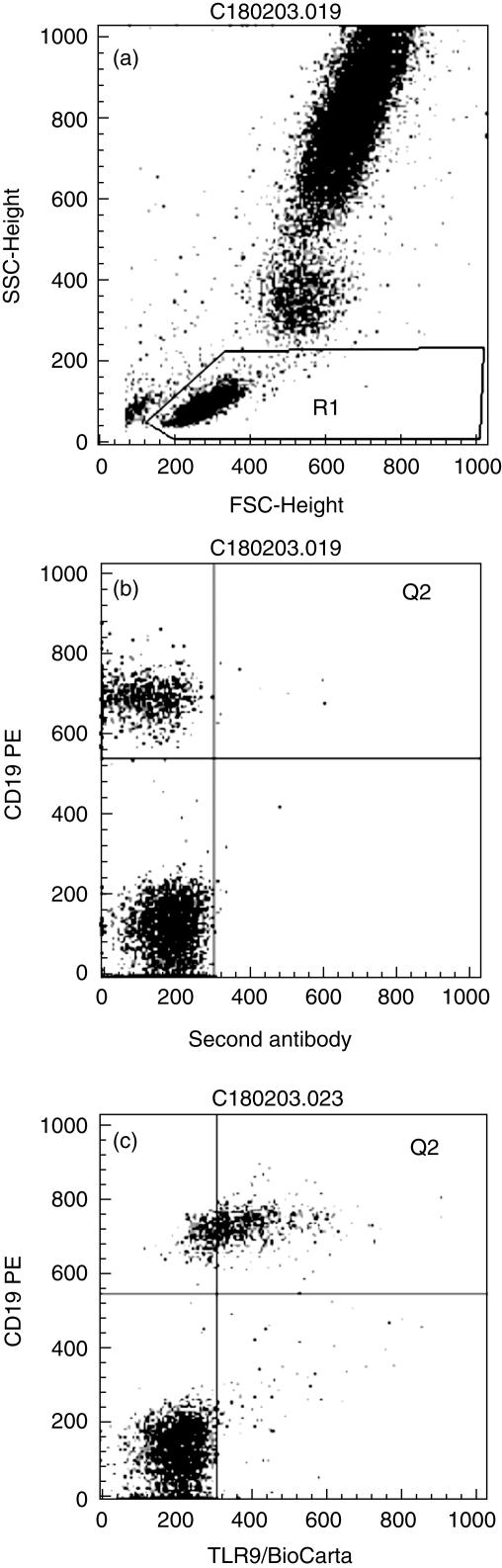
TLR9 expression was detectable on the surface of B lymphocytes (Fig. 1) and also intracellularly (not shown). Further analyses were restricted to the high TLR9-expressing B cell population shown in the upper right quadrant (Q2).
Fig 1.
Surface Toll-like receptor 9 (TLR9) staining of B lymphocytes of a severely injured patient prone to sepsis. (a) Scatter diagram of leucocytes in lysed whole blood. R1: lymphocyte region; (b) B lymphocyte (CD19+) staining with the second antibody using R1. Q2 labels the interesting quadrant. (c) B lymphocyte (CD19+) staining with a TLR9 antibody of the BioCarta company using R1. Q2 quadrant shows TLR9+ B lymphocytes.
The portion of TLR9+ B cells (located in Q2) from all B lymphocytes was not significantly different between healthy controls and patients (controls 41 ± 7%; patients 37 ± 10%).
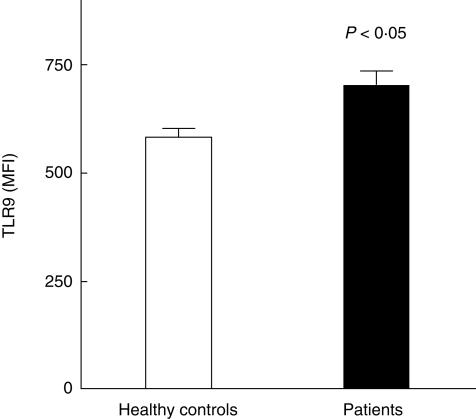
However, surface expression of TLR9 was significantly increased on B lymphocytes in whole blood of severely injured patients prone to sepsis compared with healthy controls (Fig. 2). There was no significant difference of the binding of the secondary FITC-labelled antibody (unspecific binding) on B cells between healthy controls (332 ± 35 channels) and patients (366 ± 46 channels).
Fig 2.
Surface Toll-like receptor 9 (TLR9) expression of B lymphocytes in whole blood from healthy controls and severely injured patients. MFI: mean fluorescence intensity in channels; values are expressed as mean ± s.e.m.
According to whole blood staining of B cells in PBMC of patients cultured with control CpG 2006GC revealed significantly higher surface TLR9 MFI values than healthy controls (Table 3), but culturing of PBMC with control CpG 2006GC or activating CpG 2006 also significantly increased unspecific binding of B lymphocytes from patients compared with healthy controls. In contrast, intracellular staining of B lymphocytes for TLR9 expression of severely injured patients prone to sepsis showed at least a trend to decreased MFI values compared with controls (Fig. 3). Unspecific binding of B cells was not significantly different between patients and healthy controls (data not shown).
Table 3.
Surface Toll-like receptor 9 (TLR9) expression of B lymphocytes in peripheral blood mononuclear cells from healthy controls and severely injured patients prone to sepsis stimulated with control CpG 2006GC or activating CpG 2006.
| Controls | Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Stimulation with | Antibody | n | MFIa | s.e.m.b | n | MFI | s.e.m. | P-value |
| 2006GC | TLR9 | 13 | 694c | 42 | 9 | 888c | 42 | 0·009 |
| 2006GC | Unspecific | 11 | 305 | 23 | 10 | 415 | 18 | 0·002 |
| 2006 | TLR9 | 13 | 713c | 45 | 9 | 816c | 43 | 0·142 (n.s.d) |
| 2006 | Unspecific | 11 | 299 | 22 | 11 | 438 | 23 | 0·002 |
MFI: mean fluorescence intensity (channels)
s.e.m.: standard error of the mean
P < 0·05 versus unspecific antibody
n.s.: not significant; P > 0·05.
Fig 3.
Intracellular Toll-like receptor 9 (TLR9) expression of B lymphocytes in peripheral blood mononuclear cells from healthy controls and severely injured patients stimulated with control CpG 2006GC or activating CpG 2006. MFI: mean fluorescence intensity in channels, values are expressed as mean ± s.e.m. PBMC: peripheral blood mononuclear cells. P-values are given for the comparison with the appropriate values of healthy controls.
TLR9 staining was specific, because all MFI values were significantly higher than the appropriate MFI values for unspecific binding.
We obtained a later blood sample from five patients (mean day 9) under septic conditions. The MFI values for TLR9 surface expression on B lymphocytes collected from septic patients were 702 ± 46 channels. These values were even higher than the corresponding values obtained on days 1 or 2 after trauma (665 ± 84 channels), but the difference did not reach statistical significance.
Surface expression of TLR9 comparable with B lymphocytes could not be detected either on granulocytes or on T lymphocytes (data not shown). Because monocytes may show weak TLR9 expression, 14 patients were analysed for TLR9 expression in comparison with the second antibody. There was no statistical difference between binding of the second antibody (MFI 308 ± 42 channels) and TLR9 antibody (MFI 360 ± 18 channels).
HLA-DR expression of monocytes in whole blood of severely injured patients prone to sepsis (480 ± 21 channels) was significantly diminished compared with the appropriate values of controls (642 ± 13 channels). Stimulation with CpG 2006 significantly increased HLA-DR expression of monocytes in PBMC of both patients (630 ± 25 channels) and controls (861 ± 24 channels) compared with the appropriate control CpG 2006GC values (patients 597 ± 26, controls 795 ± 21 channels).
Cell proliferation
No significant difference could be observed between CpG 2006-induced proliferation of severely injured patients prone to sepsis (11 848 ± 3398 cpm) and controls (11 537 ± 2207 cpm), neither calculating the differences between stimulated and unstimulated (autologous) cultures nor the appropriate quotients. CpG 2006 increased significantly PBMC proliferation of patients (11 848 ± 3398 cpm) and controls (11 537 ± 2207 cpm) compared with control CpG 2006GC (patients 5605 ± 1554 cpm; controls 4675 ± 845 cpm). However, both control CpG 2006GC and CpG 2006 also significantly increased proliferation of PBMC from both trauma patients and controls compared with the appropriate unstimulated proliferation (data not shown).
Discussion
In this study, expression of TLR9 was observed intracellularly and on the surface of B lymphocytes using whole blood and isolated PBMC. After severe injury TLR9 expression was up-regulated while TLR9-dependent responses were unchanged.
Members of the TLR9 subfamily are generally regarded to be expressed intracellularly in the endosomal–lysosomal compartment [20]. However, recently TLR9 was detected not only at the surface of B cells in tonsils [21] but could also be observed on B lymphocytes in cultured [22] or uncultured PBMC [14]. Now we can corroborate these findings for PBMC and can clearly extend TLR9 expression on the surface of whole blood B lymphocytes. In agreement with the findings of Dasari et al. [14], T cells showed no surface TLR9 expression.
TLRs play an essential role in the innate recognition of ‘pathogen-associated molecular patterns’ and bridge innate and adoptive immunity leading to protective immune responses [20]. Because trauma is associated with a disturbance of the innate and adoptive immunity, the TLR9 sensing of bacterial DNA may also be influenced.
Indeed, during 48 h after accident, B lymphocytic surface TLR9 expression showed a significantly higher intensity in severely injured patients prone to sepsis compared with healthy controls in whole blood as well as in PBMC incubated with control CpG 2006GC. We obtained a later blood sample from five patients under septic conditions. These values were higher than the five values obtained on days 1 or 2 after trauma but reached no statistical significance.
Because the portion of TLR9+ B cells was not significantly different between healthy controls and patients, intensified TLR9 expression of B lymphocytes from patients is explained by TLR9 up-regulation rather than through the appearance of new TLR9 expressing B cells. Although TLR9 surface expression is more intensive in severely injured patients prone to sepsis, TLR9-mediated function as measured by CpG-induced proliferation of B lymphocytes was not different between patients and controls. In contrast to the last-mentioned result, Adib-Conquy et al. [23] found that patients with trauma had a significantly impaired response to CpG oligonucleotides compared with healthy volunteers within 48 h after arrival in the intensive care unit. This difference may be due to different patient characteristics and different measurements for TLR9 function. Adib-Conquy et al. [23] reported a higher rate of non-survivors than in this study; in contrast to their study our patients, with one exception, developed sepsis later. Moreover, they measured tumour necrosis factor (TNF)-α production as a response to CpG stimulation. The TNF-α response of PBMC is not only influenced by TLR9 stimulation but also by a variety of conditions and factors. First, the main source of TNF-α in PBMC is probably monocytes and not B cells, so an indirect effect of CpG stimulation due to secondary mediators may be assumed. Secondly, reduced TNF-α response of whole blood or isolated PBMC after trauma has also been reported upon lipopolysaccharide stimulation [24,25], so a disturbance in leucocyte cytokine response generally may be present after trauma. Therefore, we chose the CpG-dependent cell proliferation as a measure for TLR9-dependent responses.
Another aspect of TLR9 function is that TLR9 recognizes not only bacterial but also viral DNA [26]. This may be important, because B lymphocytes can function as antigen-presenting cells and TLR9 expression may influence the immune response against viruses; but early viral infections after trauma seem to have no influence for the later development of sepsis. We found no data regarding the incidence of viral infections for severely injured patients in the literature.
Moreover, also self-DNA is recognized by TLR9 in an experimental model [12]. Significant increases in circulating DNA in the plasma of trauma patients have been reported [27], and DNA drives autoimmunity [11]. Therefore, we analysed anti-DNA antibodies in trauma patients with and without sepsis during 14 days after trauma and found no difference between these two groups; moreover, all values were in the normal range (unpublished results). In our opinion, these results support the conclusion that TLR9 function in trauma patients seems not to be disturbed in the early phase.
In contrast to intensified surface expression, intracellularly localized TLR9 showed lower MFI values in patients compared with controls. This might be explained by a TLR9 shift from inside to the surface of B lymphocytes during trauma. One could speculate that this phenomenon is caused by bacterial products or induced by an autoimmune process following tissue destruction.
The effects of B cell stimulatory CpG 2006 regarding TLR9 expression and function were the same in severely injured patients prone to sepsis and healthy individuals. This underlines our suggestion that at least up to 2 days after injury bacterial DNA is also effectively sensitized in severely injured patients prone to sepsis.
In contrast to TLR9 on B lymphocytes, HLA-DR expression on monocytes is inducible under these experimental conditions through activating CpG 2006 in healthy controls as well as in patients in the early phase after trauma. This may be a direct effect, as low levels of TLR9 have been found on the cell surface of human monocytes [22], although we were not able to confirm these data or be mediated secondarily by CpG-induced cytokines such as interferon (IFN)-γ.
Up-regulation of HLA-DR on monocytes underlines the immunostimulatory effect of CpG application. In fact, CpG have been introduced into vaccines as an immunomodulating drug [28]. Therefore, CpG treatment may also represent a perspective as an enhancer of immune functions in severely injured patients.
Acknowledgments
The authors thank Mrs B. Nyadu for excellent technical assistance. They also thank Dr N. Lehmann for his statistical advice. Financial support for this study was obtained by the Deutsche Forschungsgemeinschaft (DFG FL 353/2–1).
References
- 1.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Regel G, Lobenhoffer P, Grotz M, Pape HC, Lehmann U, Tscherne H. Treatment results of patients with multiple trauma: an analysis of 3406 cases treated between 1972 and 1991 as a German level I trauma center. J Trauma. 1995;38:70–8. doi: 10.1097/00005373-199501000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Hershman MJ, Cheadle WG, Kuftinee D, Polk HC., Jr An outcome predictive score for sepsis and death following injury. Injury. 1988;19:263–6. doi: 10.1016/0020-1383(88)90042-3. [DOI] [PubMed] [Google Scholar]
- 4.Döcke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat Med. 1997;3:678–81. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 5.Ditschkowski M, Kreuzfelder E, Rebmann V, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229:246–54. doi: 10.1097/00000658-199902000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ditschkowski M, Kreuzfelder E, Majetschak M, Obertacke U, Schade UF, Grosse-Wilde H. Reduced B cell HLA-DR expression and natural killer cell counts in patients prone to sepsis after injury. Eur J Surg. 1999;165:1129–33. doi: 10.1080/110241599750007630. [DOI] [PubMed] [Google Scholar]
- 7.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 8.Akira S. Toll receptor families: structure and function. Semin Immunol. 2004;16:1–2. doi: 10.1016/j.smim.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 10.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinuesa CG, Goodnow CC. Immunology: DNA drives autoimmunity. Nature. 2002;416:595–8. doi: 10.1038/416595a. [DOI] [PubMed] [Google Scholar]
- 12.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MI, Marshak-Rothstein A. Chromatin-IgG comoplexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 13.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes. inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 14.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of toll-like receptors on B lymphocytes. Cell Immunol. 2005;236:140–5. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Baker SP, O'Neill B, Haddon W, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 16.Trupka A, Waydhas C, Nast-Kolb D, Schweiberer L. Early intubation in severely injured patients. Eur J Em Med. 1994;1:1–8. doi: 10.1097/00063110-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Am College Chest Physicians/Soc Crit Care Med Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Mueller A, Kreuzfelder E, Nyadu B, et al. Human leucocyte antigen-DR expression in peripheral blood mononuclear cells from healthy donors influenced by the sera of injured patients prone to severe sepsis. Int Care Med. 2003;29:2285–90. doi: 10.1007/s00134-003-1992-8. [DOI] [PubMed] [Google Scholar]
- 19.Bauer M, Heeg K, Wagner H, Lipford GB. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology. 1999;97:699–705. doi: 10.1046/j.1365-2567.1999.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–5. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Eaton-Bassiri A, Dillon SB, Cunningham M, et al. Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect Immun. 2004;72:7202–11. doi: 10.1128/IAI.72.12.7202-7211.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saikh KU, Kissner TL, Sultana A, Ruthel G, Ulrich RG. Human monocytes infected with Yersinia pestis express surface TLR9 and differentiate into dendritic cells. J Immunol. 2004;173:7426–34. doi: 10.4049/jimmunol.173.12.7426. [DOI] [PubMed] [Google Scholar]
- 23.Adib-Conquy M, Moine P, Asehnoune K, et al. Toll-like receptor-mediated tumor necrosis factor and interleukin-10 production differ during systemic inflammation. Am J Respir Crit Care Med. 2003;168:158–64. doi: 10.1164/rccm.200209-1077OC. [DOI] [PubMed] [Google Scholar]
- 24.Flohe S, Lendemans S, Selbach C, et al. Effect of granulocyte-macrophage colony-stimulating factor on the immune response of circulating monocytes after severe trauma. Crit Care Med. 2003;31:2462–9. doi: 10.1097/01.CCM.0000089640.17523.57. [DOI] [PubMed] [Google Scholar]
- 25.Majetschak M, Flach R, Heukamp T, et al. Regulation of whole blood tumor necrosis factor production upon endotoxin stimulation after severe blunt trauma. J Trauma. 1997;43:880–7. doi: 10.1097/00005373-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 27.Lo YMD, Rainer TH, Chan LYS, Hjelm NM, Cocks RA. Plasma DNA as a prognostic marker in trauma patients. Clin Chem. 2000;46:319–23. [PubMed] [Google Scholar]
- 28.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]