Abstract
Accumulating evidence suggests that intestinal epithelial cells (IECs) constitutively express the immunoregulatory cytokine interleukin (IL)-18. IECs also serve as the host cell for the intracellular parasitic protozoan Cryptosporidium parvum. In the present study, C. parvum infection of a human enterocyte cell-line HCT-8 resulted in increased expression of IL-18 mRNA as measured by quantitative reverse transcription–polymerase chain reaction (RT-PCR). IL-18 protein was detected in control uninfected cells and following infection there was increased expression as measured by enzyme-linked immunosorbent assay (ELISA). Gene expression revealed the presence of the IL-18 receptor subunits not only in cell-lines but also in freshly isolated IECs, suggesting that IL-18-mediated signalling events may contribute to epithelial host defence during infection. Recombinant IL-18 inhibited intracellular development of the parasite in HCT-8 and HT-29 cells. Increased expression of bactericidal antibiotic peptides LL-37 and α-defensin 2 by IL-18 in HCT-8 and HT-29 cells may represent one mode of action by which this pluripotent cytokine aids in limiting the development of intracellular pathogens such as C. parvum in the gastrointestinal tract.
Keywords: Cryptosporidium, enterocytes, epithelial anti-microbial peptides, IL-18, IL-18R
Introduction
Cryptosporidium parvum is a monoxenous mucosal parasite associated with severe diarrhoeal disease, particularly affecting infants and the immunocompromised [1]. Infection is transmitted faecal–orally by oocysts and the entire endogenous development comprising asexual reproduction, gametogony and oocyst formation occurs within intestinal epithelial cells (IECs) [2]. The development of a Th1 immune response has been shown previously to be critical in the control of C. parvum infection [3,4]. We have suggested previously that interferon (IFN)-γ and other proinflammatory cytokines such as interleukin (IL)-1β and tumour necrosis factor (TNF)-α may act directly on IECs to control parasite development [5,6].
It is now widely accepted that IECs function as a critical sensor of infection through the production of a variety of proinflammatory cytokines, chemokines and anti-microbial peptides which in turn may have autocrine actions on the epithelium [7,8]. Several studies suggest that epithelial cells, including IECs, may also produce IL-18 [9–13], which can play an important role in the establishment of a protective Th1 response to a wide range of pathogens [14–22]. Studies also suggest that IL-18 may have a distinct proinflammatory action [23–27]; macrophages and neutrophils treated with IL-18 show enhanced production of IL-1β, TNF-α and IL-8 [28]. Previous studies have also shown increased IL-18 production by macrophages in response to direct infection [16,29,30] and expression of the cytokine by epithelial cells may also be altered following infection [10,12].
Upon appropriate activation the inactive precursor pro-IL-18 (24 kDa) molecule undergoes cleavage by caspase-1 to form mature bioactive IL-18 (18 kDa) [31]. IL-18-mediated signals are transduced via the IL-18 receptor (R), a member of the IL-1 receptor/Toll-like receptor superfamily. IL-18R is composed of two subunits IL-1Rrp (also known as IL-1Rrp1 or IL-1R5 or IL-18R-α) [32] and AcPL (also known as IL1–1RacPL or IL-1R7 or IL-18R-β) [33]. Both IL-18 subunits have been identified previously on T, B and natural killer (NK) cells [34–36] and recently on intestinal epithelial lymphocytes [13] and primary keratinocytes [37].
To date, studies investigating the role and contribution of IEC IL-18 to gastrointestinal mucosal immunity remain limited; in particular, the protective role of IEC-derived IL-18 during C. parvum infection is unknown. In the present study we utilized an in vitro model of C. parvum infection to investigate its effect on enterocyte IL-18 gene and protein expression. We observed increased IL-18 mRNA and protein expression in the human enterocyte cell line HCT-8 24 h post-infection. Both intestinal cell-lines and freshly isolated paediatric small intestinal epithelia expressed the IL-18 receptor as judged by reverse transcription–polymerase chain reaction (RT-PCR). Further, HCT-8 and HT-29 cells responded to exogenous IL-18 by inhibiting C. parvum reproduction, establishing functionality of the IL-18 receptor on IECs. Induction of epithelial anti-microbial peptides LL-37 and α-defensin 2 by IL-18 in HCT-8 and HT-29 cells identifies one molecular mechanism that IL-18 may utilize not only in inhibiting C. parvum development but in enhancing mucosal immunity in general against enteropathogens during infections of the gastrointestinal tract.
Materials and methods
Parasites
Purified oocysts of the C. parvum MD isolate (Moredun Scientific, Penicuik, UK) were stored at 4°C in phosphate buffered saline (PBS; pH 7·3). To purify sporozoites, oocysts were incubated in RPMI-1640 medium at 37°C for 60 min to excyst the parasites. The excystation mixture was then passed through a 5·0-µm pore filter that trapped oocyst debris and allowed sporozoites to pass through.
Cell culture and infection of intestinal cell lines with C. parvum
Monolayers of the human enterocyte cell lines HCT-8, HT-29 and Caco-2 were maintained in humidified incubators at 37°C with 5% CO2 + 95% air and cells were passaged every 7 days as described previously [5]. In infection studies, HCT-8 and HT-29 cells were seeded onto glass coverslips in 24-well tissue culture plates, grown to confluence at 37°C and normally infected using 2 × 105 oocysts per well. Parasite development was quantified 24 h later by immunofluorescence microscopy using a rabbit anti-sporozoite polyclonal antibody serum [5]. For IL-18 mRNA and protein expression studies, 1 × 105 or 1 × 106 live or 1 × 106 heat-inactivated sporozoites (70°C for 15 min) were added to each well for 24 h prior to analysis. Each treatment was performed in quadruplicate and experiment repeated three times. The action of exogenous IL-18 on parasite development was determined by treating cells with increasing concentrations of recombinant cytokine (Peprotech, London, UK) for 24 h prior to infection.
Isolation of primary human IECs
This study was approved by the Research Ethics Committee of the City and Hackney Health Authority. All reagents for primary cell culture were from Life Technologies, Paisley, UK. Primary epithelial cells were isolated from human small bowel and colon surgical specimens, as described previously [38]. Fetal gut explants (13–16 weeks' gestation) were cultured in Autrups media (1% CMRL 1066, gentamicin 50 mg/l, retinyl acetate 1 mg/l, penicillin 200 µ/ml, streptomycin 200 µg/ml, glutamine 3 mm, d-glucose 5 g/l, methionine 10 mg/l, 20 mm tricine pH 7·4, sodium bicarbonate 2 g/l) for 48 h. Epithelial cells were obtained by incubation in Hanks's buffered saline solution with 1 mm ethylenediamine tetraacetic acid (EDTA) for 1 h followed by centrifugation.
Preparation of synthetic RNA for quantitative RT-PCR
IL-18 mRNA transcripts were quantified by competitive RT-PCR using a plasmid encoding a standard RNA molecule constructed by cloning sequence specific intron-spanning primers into plasmid pMBEK, as described in detail previously [39]. To generate standard RNA, the plasmid was linearized with HindIII and transcribed in vitro using T7 RNA polymerase under conditions recommended by the supplier (Promega, Southampton, UK). Using a single primer set, RT-PCR for IL-18 yielded a standard PCR product of 478 base pairs (bp), whereas the cellular target size was 384 bp [39]. To exclude the amplification of genomic DNA in RNA preparations, experiments were also performed using target RNA as substrate for PCR assay in the absence of synthetic competitor.
RNA extraction and competitive RT-PCR
All molecular biological reagents were from Life Technologies, Paisley, UK unless otherwise stated. Total cellular RNA was extracted from samples using the TRIZOL® reagent according to the manufacturer's instructions. Tissue RNA (2 µg) and serial 10-fold dilutions of synthetic RNA (100 pg to 10 fg) were co-reverse transcribed in a 20-µl reaction mixture containing 50 mm Tris pH 8·3, 75 mm KCl, 3 mm MgCl2, 500 µm each deoxy adenosine (dATP), 2′-deoxycytidine-5′-triphosphate (dCTP), feoxythymidine triphosphate (dTTP) and 2′-deoxyguanosine 5′-triphosphate (dGTP), 10 mm dithiothreitol (DTT), 0·5 µg oligo(dT)12–18 and 100 units Moloney murine leukemia virus (MMLV)-RT at 42°C for 1 h followed by heat inactivation.
PCR amplification was carried out routinely in 50 µl of reaction volume containing 10 mm Tris pH 8·3, 50 mm KCl, 1·5 mm MgCl2, 200 µm deoxyribonucleoside triphosphate (dNTPs), 20 pm 5′ and 3′ primers and 0·5 U Taq polymerase. PCR conditions were: denaturation for 6 s at 94°C, annealing for 60 s at 58°C and extension for 75 s at 72°C for 35 cycles. Products were visualized on a 2% agarose gel using ethidium bromide. Densitometric analysis of fluorescent bands was carried out using image analysis software (1-D image analysis software, Kodak, Rochester, NY, USA). The ratio of band intensities for the PCR products of standard RNA to target RNA were plotted against the starting number of standard RNA molecules for each reaction on a double logarithmic scale using GraphPad Prism software (San Diego, CA, USA) [40].
Semi-quantitative RT-PCR
The expression of IL-1 Rrp and AcPL mRNA was determined by semiquantitative RT-PCR. Total RNA was extracted from samples (intestinal cell lines, whole intestinal mucosa or freshly isolated human epithelial cells) and reverse-transcribed as described above. Primer sequences for IL1Rrp, AcPL and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as reported previously [39]. The PCR conditions were: denaturation for 60 s at 94°C, annealing for 60 s (58°C for IL-1Rrp and 53°C for AcPL) and extension for 75 s at 72°C. Expression of IL-18 subunits was normalized to that of the housekeeping gene GAPDH. In preliminary experiments the optimal number of PCR cycles was determined to terminate the reaction within the exponential phase of amplification. To exclude the amplification of genomic DNA contaminating the samples, experiments were also performed using RNA as substrate for PCR assay.
Inflamed colonic mucosa obtained from a patient with active Crohn's disease served as a positive control [41]. The identity of the IL-18R subunits, IL1rp and AcPL, was confirmed by specific restriction enzyme analysis of the PCR products. IL1-Rrp PCR product was digested with HindIII and Bcl enzymes and AcPL PCR product was digested with MboI and BstOI. All digests were performed over 2 h at the appropriate temperature in enzyme buffer supplied by the manufacturer (Promega). Digested fragments were electrophoresed on a 2% agarose gel and visualized.
Determination of IL-18 protein by Western blotting and enzyme-linked innumosorbent assay (ELISA)
Cell lysates were prepared by the addition of lysis buffer (25 mm Tris/HCl pH 8·0, 50 mm NaCl, 10 µg/ml leupeptin, 2 µg/ml benzamidine, 20 µg/ml aprotonin, 50 µg/ml soybean trypsin inhibitor, 1 mm ethylene glycol-bis (b-aminoethyl ether) tetra-acetic acid (EGTA), 100 µg/ml phenylmethylsulphonyl fluoride (PMSF) (Sigma, Poole, UK). For the detection of IL-18 by Western blotting, 50 µg of total protein [as determined by the bicinchoninic acid (BCA) assay; Pierce, Nottingham, UK] was resolved by 15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Recombinant IL-18 (R&D Systems, Abingdon, UK) was used as a positive control. Gels were electrophoretically transferred onto nitrocellulose membrane (Amersham Biosciences, Bucks, UK) for 1·5 h at 400 mA. Non-specific binding was blocked by incubating the membrane in 5% powdered milk in PBS/0·1% Tween 20 for 1 h at room temperature. IL-18 was detected after incubation with a goat anti-human IL-18 antibody (1 : 500, Santa Cruz Biotechnology, CA, USA) overnight at room temperature and subsequent incubation with horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG monoclonal antibody (1 : 2000, Dako, Birmingham, UK) for 2 h at room temperature. The antibody reaction was detected by chemiluminescence (ECL PLUS detection kit, Amersham Biosciences). More sensitive quantification of IL-18 protein from infected supernatants and cell lysates was performed by utilizing a standard commercial ELISA kit (R&D Systems) according to the manufacturer's instructions.
Epithelial α-defensin and LL-37 gene expression in response to IL-18
Control and IL-18-stimulated HCT-8 and HT-29 cells were subjected to RNA extraction followed by RT-PCR for the detection of α-defensins (1, 2 and 3) and LL-37. Primer sequences and PCR conditions for α-defensin gene expression has been described previously [42]. The primers utilized for LL-37 expression were: sense, 5′-GCGGTGGTCACTG GTGCTCCTGCTGCT-3′ and anti-sense, 5′-GAAGAAAT CACCCAGCAGGGCAAATC-3′.
PCR conditions were essentially as described for the α-defensin family.
Statistical analysis
Results are presented as means ± s.e.m. of three experiments each performed in triplicate. Statistical analyses were performed using GraphPad Instat statistical software, variables were compared using a t-test or anova and a probability value of less than 0·05 was regarded as significant.
Results
C. parvum increases IL-18 mRNA and protein expression in HCT-8 cells
The effect of C. parvum infection on IL-18 mRNA was investigated initially in a series of intestinal epithelial cell-lines. We confirmed high constitutive expression of IL-18 in uninfected control IECs, as has been reported previously [13]. Upon C. parvum infection, only a modest induction of IL-18 mRNA was observed in HT-29 and Caco-2 cells (data not shown). In contrast, HCT-8 cells responded with a substantial increase in expression during infection that was dependent on the initial parasite inoculum. Infection in the presence of 105 and 106 sporozoites/well induced a three- and eightfold increase in IL-18 mRNA expression, respectively (Table 1). Expression of IL-18 transcripts by HCT-8 cells was also quantified following treatment with a panel of proinflammatory cytokines (IFN-γ, TNF-α, IL-6 or IL-1α) but none increased expression of IL-18 mRNA under the conditions tested (data not shown).
Table 1.
Interleukin (IL)-18 mRNA expression in Cryptosporidium parvum-infected HCT-8 cells.
| Uninfected cells | 1 × 106 inactive spz | 1 × 105 Live spz | 1 × 106 Live spz | |
|---|---|---|---|---|
| Transcripts/of RNAµg | 1·1 × 105 | 1·5 × 105 | 3·2 × 105 | 8·9 × 105 |
| Ratio to uninfected | – | 1·4 | 2·9 | 8·1 |
Spzs = sporozoites.
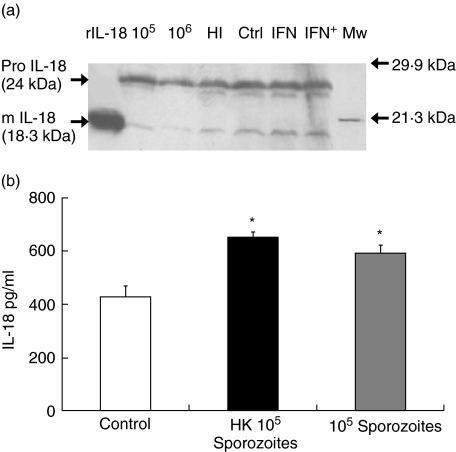
IL-18 protein expression in C. parvum-infected HCT-8 cells was determined initially by Western blotting (Fig. 1a). There were detectable quantities of both pro- and mature IL-18 in control uninfected cells and any changes during infection were difficult to quantify by this technique. Due to the greater sensitivity of ELISA, we next proceeded to estimate IL-18 protein levels in both supernatants and cell lysates of infected cells. No IL-18 protein was detectable in infected supernatants (data not shown). High constitutive expression was observed in control cell lysates, with a further increase in both the presence of heat-killed and live sporozoites (Fig. 1b). This increase reached statistical significance for both heat-killed and live sporozoites (P < 0·007 and 0·02), respectively.
Fig 1.
Interleukin (IL)-18 protein expression in HCT-8 cells following infection with Cryptosporidium parvum. (a) 24 h post-infection or 48 h after interferon (IFN)-γ treatment cell homogenates were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and Western blotting. rIL-18 = 1 ng recombinant mature IL-18; 105 = 1 × 105 live sporozoites; 106 = 1 × 106 live sporozoites; HI = 1 × 106 heat-inactivated sporozoites; ctrl = uninfected cells, interferon (IFN) = 200 units/ml IFN-γ, IFN+ = IFN-γ; and 24 h later 1 × 106 sporozoites; MW = molecular weight markers. (b) Quantification of IL-18 protein by enzyme-linked immunosorbent assay. 24 h post-infected HCT-8 cell lysates were analysed for IL-18. Significant IL-18 induction was noted both with heat-inactivated (HK) and live 105 sporozoites (P < 0·007 and P < 0·02).
Intestinal cell-lines and primary small intestinal epithelia express IL-18 receptor
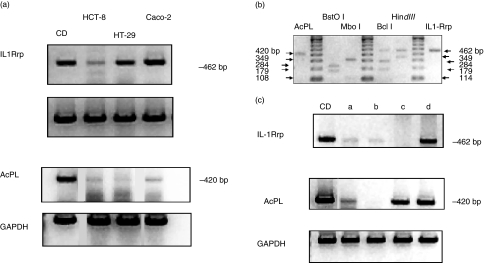
To further our understanding of the potential role of IEC IL-18 signalling in mucosal immunity we investigated the expression of the IL-18 receptor (IL-18R) subunits, IL1-Rrp and AcPL by semiquantitative RT-PCR. Each component was detected readily in a positive control sample, an intestinal biopsy from a Crohn's disease patient. Both components were also demonstrable in intestinal cell-lines HCT-8, HT-29 and Caco-2 (Fig. 2a). IL-1Rrp required a minimum of 25 thermocycles for its detection. In contrast, expression of AcPL mRNA was relatively weak, requiring at least 35 cycles (Fig. 2a). Restriction endonuclease fragment analysis of both IL-18R subunits confirmed the specificity of the PCR reaction. Digestion of AcPL PCR product produced the predicted fragment pairs of 312/108 bp and 241/179 bp, respectively. Similarly, IL1Rrp PCR product yielded the predicted fragment pairs of 349/114 bp and 284/179 bp, respectively (Fig. 2b). This series of experiments confirmed the authenticity of IL18R mRNA in IECs. Neither treatment with a panel of proinflammatory cytokines (IFN-γ, IL-6, Il-1α or TNF-α) nor infection of HCT-8 cells with C. parvum altered IL1-Rrp mRNA expression as determined by semiquantitative RT-PCR (data not shown).
Fig 2.
(a) Interleukin (IL)-18R subunit gene expression in intestinal cell-lines. IL-1Rrp and AcPL mRNA expression was detected by semiquantitative reverse transcription–polymerase chain reaction. glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was followed in parallel as control. CD = inflamed colonic mucosa from a patient with Crohn's disease (positive control). (b) Specificity of IL-18R subunits as determined by restriction endonuclease analyses. AcPL and IL1Rrp PCR products were subjected to two specific enzyme digests to confirm their identity. The size of expected polymerase chain reaction product and digested fragments for each enzyme are indicated by arrows. (c) IL-18R subunit mRNA expression in human intestinal mucosa and freshly isolated human intestinal epithelial cells (IECs). CD = inflamed colonic mucosa from an adult patient with Crohn's disease (control); lane a, whole fetal gut; lane b, colonic epithelium from a paediatric patient with active ulcerative colitis; lane c, freshly isolated fetal gut epithelium; lane d, normal paediatric freshly isolated small bowel epithelium.
We further extended our findings by examining IL-18 receptor expression in freshly isolated IECs of fetal, paediatric and adult origin (Fig. 2c). Expression of transcripts of both IL-18R subunits was observed in inflamed adult whole colonic mucosa (patient with Crohn's disease); whole fetal gut (lane a) and normal freshly isolated paediatric small bowel epithelium (lane d). Variation in subunit expression was noted in paediatric and fetal colonic epithelia (lanes b and c).
IL-18 inhibits C. parvum development in IECs
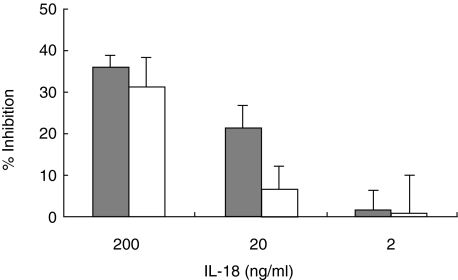
To determine if IL-18 modulated epithelial innate immune responses with subsequent effects on infection, HCT-8 and HT-29 cells were pretreated with exogenous IL-18 24 h prior to infection and development of parasite followed as described above. Parasite reproduction in HCT-8 cells was significantly reduced by IL-18 at concentrations of 20 and 200 ng/ml with 21·5% ± 5·3 (P < 0·01) and 36·0% ± 3·0 (P < 0·0001) inhibition of parasite development, respectively (Fig. 3). In HT-29 cells, significant inhibition (31·2% ± 3·0; P < 0·0001) of parasite development was also observed at IL-18 concentration of 200 ng/ml (Fig. 3).
Fig 3.
The effect of exogenous recombinant interleukin (IL)-18 on Cryptosporidium parvum development in the enterocyte cell lines HCT-8 and HT-29. Cells were pretreated with IL-18 (2–200 ng/ml). Grey histogram = HCT-8 cells; white histogram = HT-29 cells.
IL-18 modulates epithelial anti-microbial responses
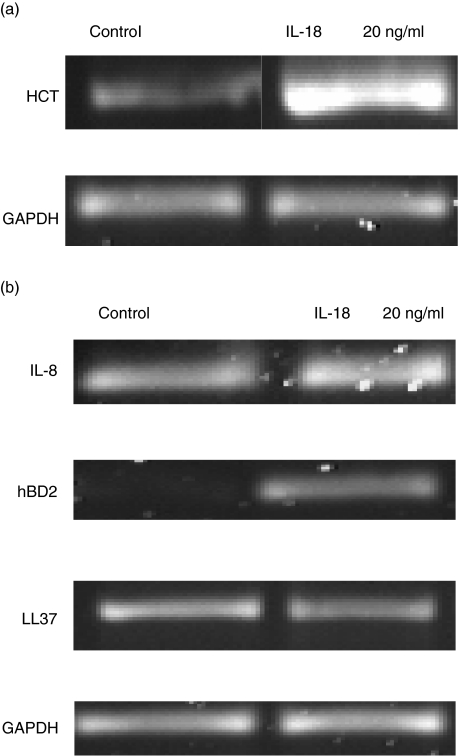
To elucidate if the proinflammatory properties of IL-18 could be responsible for the observed inhibitory effect on parasite development, we pretreated HCT-8 and HT-29 cells with recombinant IL-18 for 24 h prior to investigating the expression of epithelial innate defense genes. This included the expression of IL-8, members of the α-defensin family (1, 2 and 3) and LL-37, an anti-microbial peptide of the cathelicidin family. IL-18 led to increased LL-37 gene expression in HCT-8 cells (Fig. 4a) but not in HT-29 cells (Fig. 4b). However, IL-18 enhanced the constitutive expression of IL-8 and induced α-defensin 2 in HT-29 cells (Fig. 4b). α-Defensin 3 expression was undetectable following stimulation with IL-18 (data not shown).
Fig 4.
Interleukin (IL)-18 modulates anti-microbial peptide expression in intestinal cell-lines. (a) LL-37 expression in HCT-8 cells 24 h post-IL-18 stimulation measured by reverse transcription–polymerase chain reaction. (b) Innate defence gene (IL-8, α-defensin 2 and LL-37) expression in IL-18-stimulated HT-29 cells.
Discussion
In the past decade it has become increasingly evident that the gastrointestinal epithelium participates actively in host innate immunity by producing soluble mediators, including the immunomodulatory cytokine IL-18 [9–13].The pathogenesis of cryptosporidiosis is not understood completely. However, it is quite probable that initial host–parasite interactions of attachment and invasion are important in determining the magnitude of infection. In the present study we aimed to elucidate a potential role for IL-18 in these early host–parasite interactions.
We confirmed high constitutive expression of IL-18 mRNA and protein in a range of intestinal cell-lines, as was noted previously by Okazawa and colleagues [13]. The exact function of constitutive IL-18 expression remains unclear, but one may speculate that it may contribute to epithelial surface homeostasis even in the absence of infection and inflammation. We observed modest induction in IL-18 mRNA and protein but this varied between the cell-lines tested, with HCT-8 cells being the most responsive. As with IL-1α, regulation of IL-18 is complex, occurring both at transcriptional level and in processing of the precursor to its bioactive form [43,44]. In the present study, no IL-18 protein was detected in the supernatant of infected cells (even 48 h post-infection; data not shown), suggesting that additional signals may be required for the release of active cytokine.
Increased production of IL-18 at the transcriptional level has been observed during infection by diverse pathogens [10,26,30]. For example, infection of the intracellular epithelial pathogen Chlamydia trachomatis led to enhanced IL-18 protein mediated at the post-transcriptional level through the action of caspase-1 [10]. Oral epithelium infected with Candida albicans showed no increase in IL-18 transcripts, but pro-IL-18 protein was decreased with a resultant increase of mature IL-18 in supernatant, due probably to increased caspase-1 activity [12]. The absence of secretory IL-18 protein during C. parvum infection may relate to multiple factors, including pathogen-derived virulence factors, cell type used and differences in pathogen-induced nuclear factor (NF)-κB and caspase-1 activation.
Although IECs produce IL-18, it has not been clear if these cells themselves are a target of IL-18. In the present study we observed expression of the IL-18 receptor components in intestinal cell-lines (Fig. 2a) and the specificity of expression was confirmed by restriction enzyme analysis (Fig. 2b). Importantly, we were able to detect the expression of the two subunits in freshly isolated small-intestinal epithelia (Fig. 2c), lending support to the notion that the IL-18/IL-18 receptor complex may participate actively in host defence at the gastrointestinal mucosal surface. In the present study we investigated the effect of a panel of proinflammatory cytokines (IL-1α, TNFα, IFN-γ) on IL-18 and IL-18 receptor gene regulation and found no significant effect on either of the two molecules (data not shown). A recent report by Wittmann and colleagues also suggests a minimal effect of IFN-γ and TNF-α on IL-18 receptor expression in primary keratinocytes [37], but the authors observed potent induction when two cytokines were combined in the experimental system, suggesting that synergy with activation of multiple signalling pathways is a probable requirement for IL-18 receptor gene regulation. Similar observations have also been noted in immune cells. Expression of the receptor components increased on T and NK cells following exposure to IFN-α and IL-12, resulting in enhanced response to IL-18 [35]. Up-regulation of IL-1Rrp expression on CD4+ T cells by antigen stimulation requires the presence of both IL-12 and IFN-γ, whereas substitution of IL-12 by IL-4 down-regulated IL-1Rrp expression [36]. Further studies are required to elucidate IL-18/IL-18 receptor regulation in IECs.
We observed significant inhibition in parasite development in the presence of exogenous IL-18 (Fig. 3), implying that the IEC itself is a potential target of IL-18. To delineate mechanism(s) involved in IL-18-mediated inhibition of parasite development, we hypothesized that NF-κB-mediated proinflammatory actions of IL-18 may play a role in inducing resistance to parasite infection. Surprisingly, no changes in NF-κB-driven IL-8 or α-defensin 2 expression were observed in the HCT-8 cell-line (data not shown); however, a marked increase in the expression of a related anti-microbial peptide LL-37 was observed (Fig. 4a). In contrast, IL-18 enhanced basal IL-8 and hBD2 expression in HT-29 cells (Fig. 4b) with minimal effects on LL-37 expression. Induction of epithelial anti-microbial responses highlights a novel role for IL-18 in eliciting host innate immunity.
We have shown previously that IFN-γ inhibits C. parvum development in a human enterocyte cell line by at least two mechanisms. Invasion of the host cell is reduced and intracellular parasite development declines due to the decreased availability of cellular Fe [5] and further, synergistic effects of IL-4 and IFN-γ in parasite killing were found to be independent of the activation status of STAT1 [6]. In the present study we have identified a novel mechanism, i.e. potential IL-18 mediated increase in epithelial bactericidal agents such as LL-37 and α-defensin 2, thus enhancing host immunity during enteric infection and inflammation. The present data, along with our previous study describing potent effects of α-defensins on C. parvum viability [42], adds further weight to the role of local immunity in modulating early host–microbial interactions.
A large body of evidence supports a role for IL-18 in the acquired immune response to a variety of infections, primarily by the induction of IFN-γ production by T cells and NK cells [14,16,18,19]. In certain intracellular microbial infections the role of IL-18 is conflicting. For example, Toxoplasma gondii infection of severe combined immunodeficiency (SCID) mice was not enhanced when mice were treated with anti-IL-18; however, infection was ameliorated by administration of exogenous IL-18 and this response was found to be dependent on IL-12 and IFN-γ production by NK cells [17]. Sansonetti et al., using cytokine reconstituted Caspase-1 deficient (Casp-1−/−) mice, demonstrated a role for IL-18 in the inflammatory response to Shigella flexneri [27]. The rapid kinetics of this IL-18-dependent inflammatory response to the bacterium favoured an additional, IFN-γ independent, proinflammatory action of IL-18. Another study indicates an IFN-γ independent role for IL-18 in the early immune response to Listeria monocytogenes that is dependent, in part, on the induction of TNF-α from macrophages by IL-18 [20]. Although we implicate a role for anti-microbial peptides in IL-18-mediated responses during C. parvum infection, the possibility that additional, as yet unidentified epithelial-derived mediators may further contribute to the inhibitory action of IL-18 on C. parvum development cannot be excluded. Differences in intracellular signalling pathways between IL-1β and IL-18 may also be relevant in this respect [44].
In conclusion, we confirmed the expression of functional IL-18R by IECs, which explains why these cells were susceptible to IL-18-mediated inhibition of C. parvum reproduction. Increased expression of bactericidal peptides in response to IL-18 may provide at least one arm of resistance to C. parvum. Further work in animal models of infection is required to substantiate the multi-factorial role of this cytokine in host defence.
Acknowledgments
Funding for this work was from The Wellcome Trust.
References
- 1.Blanshard C, Jackson AM, Shanson DC, Francis N, Gazzard BG. Cryptosporidiosis in HIV-seropositive patients. Q J Med. 1992;85:813–23. [PubMed] [Google Scholar]
- 2.Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culshaw RJ, Bancroft GJ, McDonald V. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving gamma interferon production. Infect Immun. 1997;65:3074–9. doi: 10.1128/iai.65.8.3074-3079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald V, Bancroft GJ. Immunological control of Cryptosporidium infection. Chem Immunol. 1998;70:103–23. doi: 10.1159/000058702. [DOI] [PubMed] [Google Scholar]
- 5.Pollok RCG, Farthing MJG, Bajaj-Elliott M, Sanderson IR, McDonald V. Interferon gamma induces enterocyte resistance against infection by the intracellular pathogen Cryptosporidium parvum. Gastroenterology. 2001;120:99–107. doi: 10.1053/gast.2001.20907. [DOI] [PubMed] [Google Scholar]
- 6.Lean IS, McDonald SA, Bajaj-Elliott M, Pollok RC, Fathing MJG, McDonald V. Interleukin 4 and transforming growth factor beta have opposing regulatory effects on gamma-interferon mediated inhibition of Cryptosporidium parvum reproduction. Infect Immun. 2003;8:4580–5. doi: 10.1128/IAI.71.8.4580-4585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human α-defensins (1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175:1776–84. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi M, Nishizaki Y, Sano O, Ohta T, Ikeda M, Kurimoto M. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (‘interleukin-18’) in mouse intestinal epithelial cells. Cell Tissue Res. 1997;289:499–503. doi: 10.1007/s004410050895. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Shen C, Brunham RC. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J Immunol. 2000;165:1463–9. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- 11.Muneta Y, Goji N, Tsuji NM, et al. Expression of interleukin-18 by porcine airway and intestinal epithelium. J Interferon Cytokine Res. 2002;8:883–9. doi: 10.1089/107999002760274908. [DOI] [PubMed] [Google Scholar]
- 12.Rouabhia M, Ross G, Page N, Chakir J. Interleukin-18 and gamma interferon production by oral epithelial cells in response to exposure to Candida albicans or lipopolysaccharide stimulation. Infect Immun. 2002;70:7073–80. doi: 10.1128/IAI.70.12.7073-7080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okazawa A, Kanai T, Nakamaru K, et al. Human intestinal epithelial cell-derived interleukin (IL)-18, along with IL-2, IL-7 and IL-15, is a potent synergistic factor for the proliferation of intraepithelial lymphocytes. Clin Exp Immunol. 2004;136:269–76. doi: 10.1111/j.1365-2249.2004.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohn E, Sing A, Zumbihl R, et al. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 15.Wei XQ, Leung BP, Niedbala W, et al. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J Immunol. 1999;163:2821–8. [PubMed] [Google Scholar]
- 16.Mastroeni P, Clare S, Khan S, et al. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–83. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai G, Kastelein R, Hunter CA. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect Immun. 2000;68:6932–8. doi: 10.1128/iai.68.12.6932-6938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami K, Koguchi Y, Qureshi MH, Yara S, Kinjo Y, Uezu K, Saito A. NK cells eliminate Cryptococcus neoformans by potentiating the fungicidal activity of macrophages rather than by directly killing them upon stimulation with IL-12 and IL-18. Microbiol Immunol. 2000;44:1043–50. doi: 10.1111/j.1348-0421.2000.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohkusu K, Yoshimoto T, Takeda K, et al. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect Immun. 2000;68:2449–56. doi: 10.1128/iai.68.5.2449-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neighbors M, Xu X, Barrat FJ, et al. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J Exp Med. 2001;194:343–54. doi: 10.1084/jem.194.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vankayalapati R, Wizel B, Lakey DL, et al. T cells enhance production of IL-18 by monocytes in response to an intracellular pathogen. J Immunol. 2001;166:6749–53. doi: 10.4049/jimmunol.166.11.6749. [DOI] [PubMed] [Google Scholar]
- 22.Lauw FN, Branger J, Florquin S, et al. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J Immunol. 2002;168:372–8. doi: 10.4049/jimmunol.168.1.372. [DOI] [PubMed] [Google Scholar]
- 23.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFN gamma-inducing factor) induces IL-8 and IL-1beta via TNF alpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–21. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gracie JA, Forsey RJ, Chan WL, et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joosten LA, van De Loo FA, Lubberts E, et al. An IFN-gamma-independent proinflammatory role of IL-18 in murine streptococcal cell wall arthritis. J Immunol. 2000;165:6553–8. doi: 10.4049/jimmunol.165.11.6553. [DOI] [PubMed] [Google Scholar]
- 26.McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260–9. doi: 10.1136/gut.46.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansonetti PJ, Phalipon A, Arondel J, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–90. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 28.Leung BP, Culshaw S, Gracie JA, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001;167:2879–86. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- 29.Elhofy A, Bost KL. Limited interleukin-18 response in Salmonella-infected murine macrophages and in Salmonella-infected mice. Infect Immun. 1999;67:5021–6. doi: 10.1128/iai.67.10.5021-5026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–9. [PubMed] [Google Scholar]
- 31.Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–23. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 32.Torigoe K, Ushio S, Okura T, et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–42. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 33.Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273:29445. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 34.Airoldi I, Gri G, Marshall JD, et al. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J Immunol. 2000;165:6880–8. doi: 10.4049/jimmunol.165.12.6880. [DOI] [PubMed] [Google Scholar]
- 35.Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol. 2000;165:1933–8. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- 36.Smeltz RB, Chen J, Hu-Li J, Shevach EM. Regulation of Interleukin (IL)-18 receptor alpha chain expression on CD4(+) T Cells during T helper (Th) 1/Th2 differentiation. Critical downregulatory role of IL-4. J Exp Med. 2001;194:143–54. doi: 10.1084/jem.194.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittmann M, Purwar R, Hartmann C, Gutzmer R, Werfel T. Human keratinocytes respond to interleukin-18. Implication for the course of chronic inflammatory skin diseases. J Invest Dermatol. 2005;124:1225–33. doi: 10.1111/j.0022-202X.2005.23715.x. [DOI] [PubMed] [Google Scholar]
- 38.Naik S, Kelly EJ, Meijer L, Pettersson S, Sanderson IR. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J Pediatr Gastroenterol Nutr. 2001;32:449–53. doi: 10.1097/00005176-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Salvati VM, MacDonald TT, Bajaj-Elliott M, et al. Interleukin 18 and associated markers of T helper cell type 1 activity in coeliac disease. Gut. 2002;50:186–90. doi: 10.1136/gut.50.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajaj-Elliott M, Breese E, Poulsom R, Fairclough PD, MacDonald TT. Keratinocyte growth factor in inflammatory bowel disease. Increased mRNA transcripts in ulcerative colitis compared with Crohn's disease in biopsies and isolated mucosal myofibroblasts. Am J Pathol. 1997;151:1469–76. [PMC free article] [PubMed] [Google Scholar]
- 41.Monteleone G, Trapasso F, Parrello T, et al. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–7. [PubMed] [Google Scholar]
- 42.Zaalouk TK, Bajaj-Elliott M, George JT, McDonald V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect Immun. 2004;5:2772–9. doi: 10.1128/IAI.72.5.2772-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1 and IL-18 mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 44.Lee JK, Kim SH, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1beta and IL-18. Proc Natl Acad Sci USA. 2004;101:8815–20. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]