Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by an excessive inflammatory response to inhaled particles, mainly tobacco smoking. T lymphocytes are important regulatory cells that secrete several cytokines and participate actively in this inflammatory response. According to the pattern of cytokines secreted, the immune response is classified as cytotoxic or type 1 [interferon (IFN)-γ-, interleukin (IL)-2-dependent] and humoral or type 2 (IL-4-, IL-5-, IL-10- and IL-13-dependent). This paper sought to compare the intracellular profile of cytokine expression determined by flow cytometry in T lymphocytes harvested from bronchoalveolar lavage (BAL) and peripheral blood in patients with COPD, smokers with normal lung function and never smokers. We found that BAL T lymphocytes from COPD patients had a higher percentage of positive stained cells for most of the cytokines analysed when compared to never smokers or smokers with normal lung function. Differences reached statistical significance for IL-4, IL-10 and IL-13, particularly in CD8+ T cells. Furthermore, the expression of most of these cytokines was related inversely to the degree of airflow obstruction present suggesting local activation and/or selective homing of T lymphocytes to the lungs in COPD patients. These observations were not reproduced in circulating T lymphocytes. These results suggest that BAL T lymphocytes in patients with COPD produce more cytokines than in controls and tend to show a type 2 pattern of intracellular cytokine expression, particularly a Tc-2 profile. This is related inversely to the degree of airflow obstruction present.
Keywords: bronchitis, emphysema, flow cytometry, lung inflammation
Introduction
Tobacco smoking induces an inflammatory response of the airways and lung parenchyma in all smokers. However, for reasons that are not fully understood, only some of them develop chronic airflow obstruction [1]. It is therefore likely that the nature, intensity and/or localization of this inflammatory response may differ in those smokers who manage to preserve lung function despite their habit, compared to those who develop chronic obstructive pulmonary disease (COPD) [2].
Several inflammatory cells, including macrophages, neutrophils and lymphocytes, participate actively in the inflammatory response that characterizes COPD [3]. Among them T lymphocytes, and the array of cytokines they produce, play a key role in orchestrating the type of immune response mounted, which can be classified as cytotoxic or humoral. The former involves the production of mainly interferon (IFN)-γ and interleukin (IL)-2 by either CD4+ T helper (Th1) or CD8+ T cytotoxic (Tc1) lymphocytes; the latter is associated with the production of IL-4, IL-5, IL-10 and IL-13 (Th2 or Tc2, respectively) [4].
Several previous studies have already attempted to characterize the pattern of lymphocyte cytokine production in COPD, but results are conflicting. Some reported that patients with COPD show a predominant Th2/Tc2 phenotype [5–7], whereas others found a Th1/Tc1 pattern [8–10]. Differences are probably related to the different tissues sampled (blood, bronchi, alveoli), the different T cell subpopulations studied (CD4+, CD8+) and/or the fact that these studies focused on a limited number of cytokines. We sought to overcome these limitations and to define the pattern of intracellular cytokine expression of T lymphocytes more precisely in patients with COPD by analysing both CD4+ and CD8+ T lymphocytes isolated simultaneously from peripheral blood and bronchoalveolar lavage (BAL) fluid, and by determining the pattern of expression of a wide variety of Th1/Tc1 and Th2/Tc2 cytokines. Although this is a descriptive study, a detailed characterization of the pattern of lymphocyte cytokine expression in COPD is essential for a better understanding of the molecular mechanisms that drive the abnormal inflammatory response that characterizes this devastating disease.
Materials and methods
Population and ethics
We studied smokers (with and without COPD) and never smokers (Table 1). All participants were recruited consecutively from the endoscopy unit of our institution from those requiring a bronchoscopy for clinical reasons (solitary pulmonary nodule or haemoptysis). All patients gave their written consent after being fully informed of the nature, characteristics, risks and potential benefits of the study, which was approved previously by the Ethics Committee of our institution. COPD patients were clinically stable and had not had an episode of exacerbation during the last 3 months. Six patients were being treated with inhaled steroids, but none was receiving oral steroid therapy. We excluded individuals with other chronic lung diseases (asthma, bronchiectasis and interstitial lung diseases), atopy, cardiac, hepatic or renal failure. To avoid any potential effect of acute smoking, active smokers refrained from smoking 12 h before bronchoscopy. Exhaled carbon monoxide concentration determined before sampling was lower than 10 parts per million (p.p.m.) in all subjects.
Table 1.
Clinical and functional data of the subjects included in the study.
| Never smokers (n = 7) | Smokers with normal lung function (n = 16) | COPD patients (n = 14) | |
|---|---|---|---|
| Age (years) | 57 ± 5 | 61 ± 2 | 64 ± 2 |
| Smoking history (pack- years) | 0 | 38 ± 4 | 45 ± 4 |
| FEV1 (% ref) | 97 ± 6 | 93 ± 3 | 57 ± 2† |
| FEV1/FVC (%) | 77 ± 2 | 74 ± 1 | 55 ± 2† |
P < 0·01 [chronic obstructive pulmonary disease (COPD) versus with normal lung function and never smokers]. FEV1 = forced expiratory volume in 1 s.
Lung function
Forced spirometry (GS; Warren E. Collins, Braintree, MA, USA) was obtained in all participants [11]. Spirometric reference values were those of a Mediterranean population [12].
BAL and blood sampling
Bronchoscopy was performed with a flexible fibreoptic bronchoscope (Pentax 15v, Tokyo, Japan). Under topical lidocaine, BAL was performed by instilling eight 25 ml aliquots of sterile saline solution in one pulmonary segment of the middle lobe or lingula not containing any nodule [13]. The liquid recovered was filtered, washed twice in phosphate-buffered saline (PBS) and resuspended in RPMI-1640 medium at 4°C. Heparinized blood samples were collected before bronchoscopy by peripheral venipuncture. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Ficoll Hypaque). PBMC cells were washed twice and re-suspended at 106 per millilitre in RPMI-1640 medium.
We excluded the presence of airway bacterial infection by using a sterile protected specimen brush (PSB) (Mill-Rose, Lab Inn, OH, USA) performed before BAL [14]. The culture of PBS samples yielded less than 103 colony-forming units (CFU)/ml in all participants.
Stimulation, staining and flow cytometry analysis of T lymphocytes
PBMC and BAL cells were stimulated with or without (negative controls) phorbol myristate acetate (PMA; 25 ng/ml) and ionomycin (1 μg/ml) in the presence of Brefeldin A (10 μg/ml) for 5 h at 37°C in a 5% CO2 humidified atmosphere.
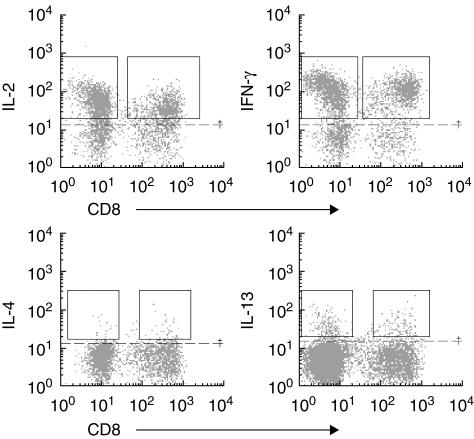
After stimulation, cells were harvested, stained with anti-CD8 and anti-CD3 monoclonal antibodies (Coulter Immunotech, Isaza, Madrid, Spain), fixed, permeabilized and intracellularly stained with anti-IL-2, IL-4, IL-10, IL-13, tumour necrosis factor (TNF)-α (all from Pharmingen Becton Dickinson, Madrid, Spain) and IFN-γ (Coulter Immunotech) phycoerythrin (PE)-conjugated monoclonal antibodies. The analysis was carried out in an Epics XL flow cytometer (Coulter Immunotech) using Expo32 software (Coulter Immunotech). T lymphocytes were gated on a side versus CD3+ dot plot. We analysed CD8+ T lymphocytes as CD3+CD8+ and CD4+ T lymphocytes as CD3+CD8− T cells. Positive staining was established above the background level of cells stained with isotype-matched PE-conjugated monoclonal antibodies and non-stimulated samples (Fig. 1).
Fig 1.
Flow cytometry analysis of bronchoalveolar lavage fluid (BALF) T lymphocytes staining positive for intracellular interleukin (IL)-2, interferon (IFN)-γ, Il-4 and IL-13 cytokines. CD4+ T (CD3+CD8−) and CD8+ (CD3+CD8+) lymphocytes were previously gated on a side versus CD3 positive dot plot. Positive staining was established above the background level (dotted line) of cells stained with isotype-matched phycoerythrin-conjugated monoclonal antibodies and non-stimulated samples.
Statistical analysis
Results are shown as mean ± standard error of the mean (s.e.m.). Mann–Whitney, Kruskal–Wallis and Dunn's multiple comparison tests were used to compare groups as appropriate. Correlations between variables of interest were explored by the Pearson correlation test. A P-value lower than 0·05 was considered significant.
Results
Clinical and functional findings
Table 1 shows the main clinical and functional characteristics of the three groups studied. COPD patients were slightly older than the other two groups, but differences were not statistically significant. The smoking history of patients with COPD and smokers with normal lung function was similar. Patients with COPD showed moderate airflow obstruction (GOLD stage II) whereas, by definition, spirometry was normal in the other two groups.
Pattern of intracellular cytokine expression in BAL T lymphocytes
Table 2 shows the absolute and differential cell counts in BAL. As reported previously [15], BAL recovery was reduced in COPD; in contrast, total cell count was higher in COPD and in smokers with normal lung function. The distribution of CD4+ and CD8+ T cells in BAL samples was not different between groups (Table 3), as described previously [16].
Table 2.
Absolute and differential cell counts in bronchoalveolar lavage fluid.
| Never smokers (n = 7) | Smokers with normal lung function (n = 16) | COPD (n = 14) | |
|---|---|---|---|
| Total cell count ( × 103/ml) | 65·6 ± 17·6 | 271 ± 58* | 182 ± 33 |
| Macrophages (%) | 92 ± 1 | 92 ± 2 | 85 ± 3 |
| Lymphocytes (%) | 7 ± 1 | 7 ± 2 | 9 ± 2 |
| Neutrophils (%) | 1 ± 0·4 | 1 ± 0·2 | 3 ± 2 |
| BAL recovery (ml) | 105 ± 7 | 94 ± 4† | 70 ± 7** |
P < 0·05 versus never smokers
P < 0·01 versus never smokers
P < 0·01 versus chronic obstructive pulmonary disease (COPD).
Table 3.
Percentage of CD4+ and CD8+ T lymphocytes in blood and BAL samples.
| Never smokers (n = 7) | Smokers with normal lung function (n = 16) | COPD (n = 14) | ||||
|---|---|---|---|---|---|---|
| CD4+ (%) | ||||||
| Blood | 71 ± 3·7 | 72 ± 2·2 | 64 ± 3·6 | |||
| BAL | 62 ± 5·4 | 59 ± 3·3 | 51 ± 3·7 | |||
| CD8+ (%) | ||||||
| Blood | 28 ± 3·7 | 27 ± 2·1 | 34 ± 3·6 | |||
| BAL | 36 ± 5·3 | 38 ± 3·2 | 45 ± 3·8 | |||
COPD: chronic obstructive pulmonary disease.
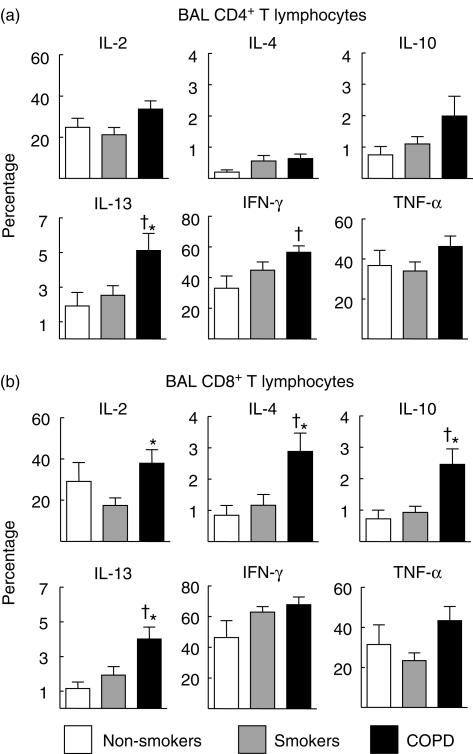
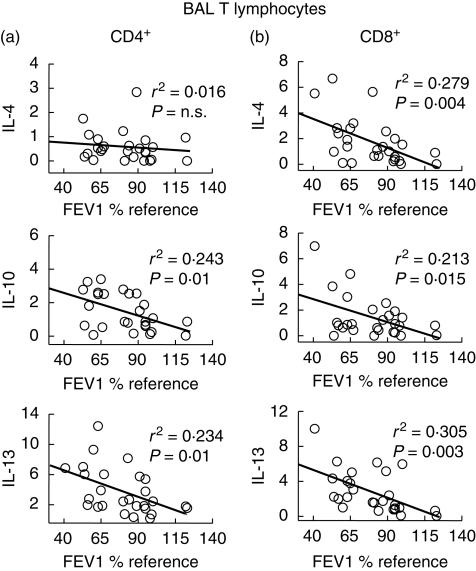
Figure 2 presents the percentage of BAL CD4+ and CD8+ T lymphocytes (panels a and b, respectively) with positive intracellular staining for the different cytokines studied in patients with COPD (dark columns), smokers with normal lung function (grey columns) and never smokers (white columns). Patients with COPD showed a tendency towards higher positive staining for basically all cytokines studied. In CD4+ T lymphocytes (a), differences reached statistical significance for IL-13 and IFN-γ, whereas in CD8+ T lymphocytes (b) differences were statistically significant for IL-2, IL-4, IL-10 and IL-13. Results were not different in patients receiving (or not) treatment with inhaled steroids (data not shown). Interestingly, the percentage of CD4+ T cells staining positive for IL-10 and IL-13, and CD8+ T cells staining positive for IL-4, IL-10 and IL-13 showed a significant negative correlation with forced expiratory volume in 1 s (FEV1) values (Fig. 3). In contrast, type I cytokines (IL-2 and IFN-γ) were not correlated with lung function (data not shown).
Fig 2.
Percentage (mean ± s.e.m.) of bronchoalveolar lavage (BAL) CD4+ (a) and CD8+ T lymphocytes (b) with positive intracellular staining for the different cytokines studied in patients with chronic obstructive pulmonary disease (COPD) (dark columns), smokers with normal lung function (grey columns) and never smokers (white columns). †P < 0·05 COPD versus non-smokers; *P < 0·05 COPD versus smokers with normal lung function.
Fig 3.
Correlation analysis between the percentage of CD4+ (a) and CD8+ (b) bronchoalveolar lavage (BAL) T lymphocytes with positive intracellular staining for type 2 cytokines [interleukin (IL)-4, IL-10 and IL-13] and the degree of airflow obstruction [forced expiratory volume in 1 s (FEV1), % reference] in smokers [with and without chronic obstructive pulmonary disease (COPD)].
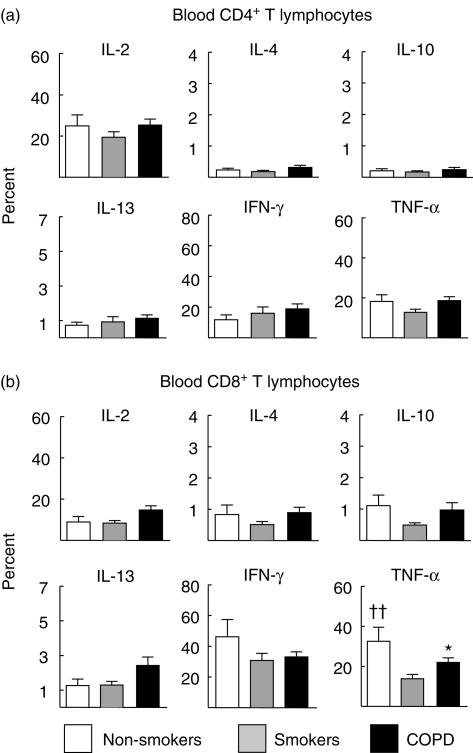
Pattern of intracellular cytokine expression in circulating T lymphocytes
The distribution of CD4+ and CD8+ T cells in peripheral blood samples was not different between groups, although patients with COPD showed a tendency towards higher CD8+ values (Table 3). Figure 4 presents the results obtained in circulating CD4+ (a) and CD8+ T lymphocytes (b). In contrast to the observations made in BAL fluid, in peripheral blood differences between groups were mainly absent, both in CD4+ and CD8+ T lymphocytes. Only in the case of TNF-α did we observe an unexpected lower staining in circulating CD8+ T lymphocytes of smokers with normal lung function compared to never smokers or patients with COPD.
Fig 4.
Percentage (mean ± s.e.m.) of circulating CD4+ (a) and CD8+ T lymphocytes (b) with positive intracellular staining for the different cytokines studied in patients with chronic obstructive pulmonary disease (COPD) (dark columns), smokers with normal lung function (grey columns) and never smokers (white columns). ††P < 0·01 non-smokers versus smokers with normal lung function; *P < 0·05 COPD versus smokers with normal lung function.
Discussion
The main results of our study indicate that: (1) BAL T lymphocytes in patients with COPD produced more cytokines than controls (Fig. 2). This was not the case in circulating T lymphocytes (Fig. 3), suggesting local activation and/or selective homing of T lymphocytes to the lungs; (2) the pattern of cytokine production tended to follow a Tc2 profile, as shown by the higher percentage of CD8+ T cells staining positive for IL-4, IL-10 and, particularly, IL-13 (Fig. 2); and (3) several type 2 cytokines were correlated inversely with the degree of airflow obstruction (Fig. 3). Overall, these results suggest that the profile of cytokines expressed in pulmonary T lymphocytes follows a type 2 pattern, mainly a Tc2, and that this is related to the severity of the disease.
It is now well established that T lymphocytes participate actively in the inflammatory response of the airways and lung parenchyma that characterizes COPD [1,3,17,18]. Several previous studies have attempted to characterize the profile of cytokine production in these patients, but results were conflicting. Mattoli et al. reported that, compared to never smokers or smokers with normal lung function, CD8+ T cell clones harvested from patients with COPD present a Tc2 phenotype [5]. These findings were confirmed later by Miotto et al. [6] and Zhu et al. [7] in bronchial tissue. In contrast, Majori et al. found that peripheral CD4+ T lymphocytes developed a Th1 phenotype in patients with COPD compared to smokers with normal lung function and never smokers [8] and, in keeping with these findings, more recently Di Stefano et al. published that lymphocytes in bronchial tissue of patients with mild to moderate stable COPD showed a Th1/Tc1 pattern [9], although Th-2 cytokines (such as IL-4 or IL-5) were never determined. Furthermore, a Tc2 phenotype has also been associated with interstitial fibrosis and declining lung function in patients with systemic sclerosis [19]. Finally, in a recent paper Grumelli et al. suggested a Th1 pattern of BAL T lymphocytes based on IFN-γ production and chemokine receptor expression [10]. In contrast to these previous investigations, which measured only a reduced number of cytokines, we screened cytokine production by both CD4+ and CD8+ T lymphocytes comprehensively, not only in those harvested from BAL but also in those isolated from the circulating pool. Our results show that the percentage of cytokine-producing BAL T lymphocytes was higher in patients with COPD than in controls for many cytokines (Fig. 2), suggesting a highly effector state of these cells in the lungs of these patients. Interestingly, this was absent in circulating T lymphocytes (Fig. 4), suggesting that lung lymphocytes are activated locally or recruited selectively to the lungs. We also found that BAL CD8+ T lymphocytes in patients with COPD follow a mainly Tc2 pattern because the production of IL-4, IL-10 and IL-13 was higher than in the other two groups (Fig. 2b). This pattern was less well defined in CD4+ T lymphocytes, which showed a higher production of IL-13 and IFN-γ (Fig. 2a). However, CD4+ T cells staining positive for IFN-γ reached statistical significance only when compared to never smokers (Fig. 2a), an observation that may indicate a direct effect of tobacco smoke on the production of this cytokine. Thus, these observations pinpoint towards a mainly Tc2 pattern of response in COPD. In support of this contention is the fact that circulating levels of IL-6 are known to be increased in COPD patients [20], and IL-6 polarizes mainly T cells towards a type 2 immune response [21,22]. A possible explanation of these results is that stimulation of the innate immune system induces the production, among others, of IL-6 in COPD patients, which would commit T cells to produce a type 2 cytokine profile, this pattern being a consequence rather than a cause of the disease.
Mechanistically speaking, two other aspects of our study deserve comment. First, several type 2 cytokines (IL-4, IL-10 and IL-13) showed a significant negative correlation with the degree of airflow obstruction (Fig. 3). Secondly, among the different cytokines screened, IL-13 was the one showing the highest up-regulation in COPD (both in CD4+ and CD8+ BAL T lymphocytes (Fig. 2a,b). It is known that over-expression of IL-13 in transgenic mice causes emphysema, mucus metaplasia and inflammation [23], all well-known pathological changes of COPD [1,3,17,18]. These observations therefore support a role for type 2 cytokines in general and IL-13 in particular in the pathogenesis of the disease. For instance, the higher percentage of CD8+ T cells staining positive for type 2 cytokines could render them more resistant to apotosis [24], thus increasing their life span and destructive capability in the lungs.
In summary, our study provides a comprehensive description of the activation pattern of CD4+ and CD8+ T lymphocytes in BAL and peripheral blood of patients with COPD. We found that differences were significant in the BAL compartment (much less so in peripheral blood), and that the cytokine profile tends to follow a type 2 pattern, mainly a Tc2 one. Finally, this appears to be related to the severity of the disease, as determined by the degree of airflow obstruction.
Acknowledgments
This study was supported, in part, by Fondo de Investigaciones Sanitarias (FIS PI02/0986, FIS PI05/1059 and Red Respira-RTIC 03/11C), SEPAR, Govern Balear and ABEMAR. The authors thank Carmen Santos and Francisca Bauza for their technical assistance.
References
- 1.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–21. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 2.Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax. 2003;58:832–4. doi: 10.1136/thorax.58.10.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 4.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–73. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- 5.Mattoli S, Kleimberg J, Stacey MA, Bellini A, Sun G, Marini M. The role of CD8+ Th2 lymphocytes in the development of smoking-related lung damage. Biochem Biophys Res Comms. 1997;239:146–9. doi: 10.1006/bbrc.1997.7444. [DOI] [PubMed] [Google Scholar]
- 6.Miotto D, Ruggieri MP, Boschetto P, et al. Interleukin-13 and -4 expression in the central airways of smokers with chronic bronchitis. Eur Respir J. 2003;22:602–8. doi: 10.1183/09031936.03.00046402. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Majumdar S, Qiu Y, et al. Interleukin-4 and interleukin-5 gene expression and inflammation in the mucus-secreting glands and subepithelial tissue of smokers with chronic bronchitis. Lack of relationship with CD8(+) cells. Am J Respir Crit Care Med. 2001;164:2220–8. doi: 10.1164/ajrccm.164.12.2009060. [DOI] [PubMed] [Google Scholar]
- 8.Majori M, Corradi M, Caminati A, Cacciani G, Bertacco S, Pesci A. Predominant TH1 cytokine pattern in peripheral blood from subjects with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 1999;103:458–62. doi: 10.1016/s0091-6749(99)70471-9. [DOI] [PubMed] [Google Scholar]
- 9.Di Stefano A, Caramori G, Capelli A, et al. STAT4 activation in smokers and patients with chronic obstructive pulmonary disease. Eur Respir J. 2004;24:78–85. doi: 10.1183/09031936.04.00080303. [DOI] [PubMed] [Google Scholar]
- 10.Grumelli S, Corry DB, Song LZ, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. Plos Med. 2004;1:75–83. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Thoracic Society Official Statement. Standardization of spirometry. 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 12.Roca J, Sanchis J, Agustí-Vidal A, et al. Spirometric reference values for a Mediterranean population. Bull Eur Physiopathol Respir. 1986;22:217–24. [PubMed] [Google Scholar]
- 13.Rennard SI, Aalbers R, Bleecker E, et al. Bronchoalveolar lavage: performance, sampling procedure, processing and assessment. Eur Respir J Suppl. 1998;26:13S–15S. [PubMed] [Google Scholar]
- 14.Wimberley NW, Bass JB, Jr, Boyd BW, Kirkpatrick MB, Serio RA, Pollock HM. Use of a bronchoscopic protected catheter brush for the diagnosis of pulmonary infections. Chest. 1982;81:556–62. doi: 10.1378/chest.81.5.556. [DOI] [PubMed] [Google Scholar]
- 15.Lofdahl JM, Cederlund K, Nathell L, Eklund A, Skold CM. Bronchoalveolar lavage in COPD: fluid recovery correlates with the degree of emphysema. Eur Respir J. 2005;25:275–81. doi: 10.1183/09031936.05.00033504. [DOI] [PubMed] [Google Scholar]
- 16.Pons J, Sauleda J, Ferrer JM, et al. Blunted {gamma}{delta} T-lymphocyte response in chronic obstructive pulmonary disease. Eur Respir J. 2005;25:441–6. doi: 10.1183/09031936.05.00069304. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ. Small Airways in COPD. N Engl J Med. 2004;350:2635–7. doi: 10.1056/NEJMp048102. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 19.Atamas SP, Yurovsky VV, Wise R, et al. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42:1168–78. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation. a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J Immunol. 2003;170:4457–64. doi: 10.4049/jimmunol.170.9.4457. [DOI] [PubMed] [Google Scholar]
- 23.Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–93. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vukmanovic-Stejic M, Vyas B, Gorak-Stolinska P, Noble A, Kemeny DM. Human Tc1 and Tc2/Tc0 CD8 T-cell clones display distinct cell surface and functional phenotypes. Blood. 2000;95:231–40. [PubMed] [Google Scholar]