Abstract
Previously, we have demonstrated that macrophages exhibited cytotoxicity toward mouse bladder cancer MBT-2 cells upon bacille Calmette–Guérin (BCG) stimulation. In this study, we have investigated the role of Th1-stimulating cytokines in BCG-induced macrophage cytotoxicity. Thioglycollate-elicited peritoneal exudate cells (PECs) were used as a conventional source for macrophages and the induction of PEC effector functions (cytolytic activity and cytokine production) by BCG was evaluated in vitro. The BCG-activated PECs showed potent cytotoxicity and killed MBT-2 cells in a dose-dependent manner. Depletion of T cells, natural killer (NK) cells, or both, in PEC preparations exhibited a marginal or small reduction of MBT-2 cell killing, suggesting that macrophages played a primary role in PEC cytotoxicity. Transwell assays indicated that the maximal PEC cytotoxicity required both direct cell–cell contact and soluble factors such as interferon (IFN)-γ and tumour necrosis factor (TNF)-α. Neutralizing endogenous cytokines interleukin (IL)-12, IL-18, IFN-γ or TNF-α reduced PEC cytotoxicity by 38%, 22%, 15% and 94%, respectively. Supplementation of BCG with recombinant (r)IL-2, rIL-12 or rIL-18 increased PEC cytotoxicity by approximately twofold. Compared with control BCG for PEC stimulation, rBCGs expressing IL-2 or IL-18 showed enhanced MBT-2 cell killing by PECs. Increased cytokine production (IFN-γ, TNF-α and IL-6) was also observed in rBCG-stimulated PEC cultures. Taken together, these results suggest that Th1-stimulating cytokines play an important role in BCG-induced macrophage cytotoxicity and that combination of BCG with selected Th1-stimulating cytokines, either supplemented or expressed by BCG, may enhance the effect of BCG in the treatment of bladder cancer patients.
Keywords: BCG, bladder cancer, cytokine, cytotoxicity, macrophage
Introduction
Intravesical bacille Calmette–Guérin (BCG) therapy has been used successfully in the treatment of superficial bladder cancer for more than two decades [1,2]. Although the exact mechanisms through which BCG mediates anti-tumour immunity remain unclear, a local non-specific immune reaction reflecting various activities of immune cells has been proposed [2–5]. Several lines of evidence suggest that macrophages actively mediate BCG's anti-bladder cancer activity. Following BCG instillation, immune cell infiltration in the bladder wall of patients has been observed, including T cells, natural killer (NK) cells and macrophages [5–8]. A marked increase of macrophages, along with a large number of lymphocytes in patients' voided urine after intravesical BCG therapy, has also been reported [9–11]. Moreover, induction of an array of cytokines by BCG has been detected in patients' urine as well as in immunocompetent cell cultures [11–16]. These BCG-induced cytokines include those expressed predominantly by macrophages such as interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-12, IL-18, interferon (IFN)-γ and tumour necrosis factor (TNF)-α[17–20]. Although the specific role each of these cytokines in orchestrating BCG-induced anti-tumour immunity is not clear, a high expression of Th1 cytokines (IL-2, IL-12 and IFN-γ) has been observed to be associated with BCG responders [14,15,21]. These observations suggest that effective BCG therapy of bladder cancerdepends largely on proper induction of the Th1 immune pathway.
Macrophages exhibit multiple pivotal functions in host immune responses. Macrophages act as a first line of defence against microorganism infection in the innate immune system. In the case of BCG infection, anti-mycobacterial immunity develops in the host, which involves the initial ingestion of BCG by immature macrophages, the early growth of BCG in infected macrophages and the late inhibition and destruction of BCG by activated macrophages [22,23]. Once activated, macrophages gain effector functions and act as inflammatory, microbicidal and tumoricidal cells through cell–cell contact and/or release of soluble factors such as cytotoxic cytokines and nitrate oxide (NO) [4,17–20]. Previously, we have demonstrated that BCG could induce macrophage cytotoxicity toward mouse bladder cancer MBT-2 cells in vitro[24–26]. In this study, we have further explored the effector mechanisms of BCG-induced macrophage cytotoxicity. We have demonstrated that Th1-stimulating cytokines play an important role in BCG-induced macrophage cytotoxicity and that supplementation of Th1-stimulating cytokines can augment BCG for induction of macrophage cytotoxicity.
Materials and methods
Reagents, cytokines and antibodies
Thioglycollate and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, MO, USA). Mouse rIFN-γ, rTNF-α, rIL-2 and rIL-12 were purchased from BD PharMingen (San Diego, CA, USA). Mouse rIL-18 was obtained from Hayashibara (Hyogo, Japan). Paired capture and detecting antibodies for enzyme-linked immunosorbent assay (ELISA) were purchased from Endogen (Woburn, MA, USA) for IFN-γ and from BD PharMingen for TNF-α and IL-6. Antibodies for NK cell depletion (Pan-NK, clone DX5) were purchased from BD PharMingen and for T cell depletion (Thy-1·2, clone F7D5) from Serotec (Oxford, UK). Low toxic rabbit complement was purchased from Cedarlane Laboratories (Ontario, Canada). Cytokine-neutralizing antibodies were obtained from BD PharMingen for mIL-12 (clone C15·6, rat IgG1), mIFN-γ (clone XMG1·2, rat IgG1) and mTNF-α (clone MP6-XT3, rat IgG1), and from Hayashibara for mIL-18 (rabbit polyclone). Species and isotype matched control antibodies were obtained from Sigma for rabbit IgG and from BD PharMingen for rat IgG1.
Mice
C3H/HeN mice (female, 6–8 weeks) were purchased from the National Cancer Institute (NCI) and allowed free access to food and water. All animal studies were approved by the University of Iowa Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animals prepared by the National Institutes of Health.
Cell culture
MBT-2 cells, a bladder cancer line derived from C3H/He mice treated with N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide (FANFT) [27], were grown in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 units/ml of penicillin and 100 µg/ml of streptomycin at 37°C in a humidified 5% CO2 incubator. Cells were free of mycoplasma as determined by polymerase chain reaction (PCR)-based analysis using the Stratagene Mycoplasma PlusTM PCR Primer Set (La Jolla, CA, USA).
BCG culture
Pasteur strain BCGs that contained an empty vector (BCG) or a vector expressing IL-2 (rBCG-IL-2) or IL-18 (rBCG-IL-18) were grown in Middlebrook 7H9 media (Difco, Detroit, MI, USA) supplemented with 10% Albumin Dektrose Catalase (ADC) (5% bovine serum albumin fraction V, 2% dextrose and 0·85% NaCl), 0·05% Tween 80, and 30 µg/ml kanamycin [24,28]. Both rBCGs typically secreted ∼3000–5000 pg/ml of recombinant cytokines under the standardized conditions described previously [24,28].
Peritoneal exudate cell (PEC) cytotoxicity
PECs were prepared by standard intraperitoneal thioglycollate induction and used as a conventional source for macrophages, as described previously [24–26]. Briefly, mice were injected intraperitoneally with 2 ml of 3% thioglycollate. After 3 days, PECs were collected by washing out the peritoneal cavity, suspended in RPMI-10% FBS media, and seeded (1 × 105 cells/well) in 96-well flat-bottomed plates (Nunc, Rochester, NY, USA). After attachment, BCG (0·005 OD/well) was added for PEC activation. After culturing for 24 h, 51Cr-labelled MBT-2 cells were added at the indicated effector : target cell (E : T) ratios. After further culturing for 20 h, the supernatants were harvested and the released 51Cr were counted. Spontaneous 51Cr release was determined by incubating radiolabelled target cells in the absence of PECs. Maximal 51Cr release was determined by incubating the target cells in 2% Triton X-100. The cytotoxicity was calculated according to the formula : % lysis = {[experimental counts per minute (cpm) − spontaneous cpm)/(maximal cpm − spontaneous cpm]} × 100.
To evaluate the impact of T cells and NK cells on BCG-induced PEC cytotoxicity, depletion assays were performed as described [25,26]. Briefly, after PEC attachment, anti-Thy-1·2 and/or anti-NK antibodies together with complement were added to each well. After incubation for 2 h, non-adherent cells were removed by aspiration and the remaining cells were stimulated with BCG. To dissect the effector components of PEC cytotoxicity, a transwell apparatus with a 3-µm pore size (Fisher Scientific, Pittsburgh, PA, USA) was used to separate PECs from MBT-2 targets but allows free penetration of soluble factors. In some experiments, culture supernatants of PECs stimulated by BCG for 24 h were collected and evaluated for cytotoxicity against MBT-2 cells. To evaluate the roles of endogenous Th1-stimulating cytokines in induction of PEC cytotoxicity, neutralizing antibodies to IL-12, IL-18, IFN-γ and TNF-α were used during BCG stimulation of PECs. To test whether exogenous Th1 cytokines could enhance BCG-induced PEC cytotoxicity, rIL-2, rIL-12 and rIL-18 were added during BCG stimulation of PECs. For comparison, rBCG-IL-2 and rBCG-IL-18 were also included for PEC stimulation.
Cytokine ELISA
To evaluate cytokine production by PECs in response to BCG, culture supernatants were collected 24 h after BCG stimulation and analysed by ELISA as described previously [16,24].
Statistical analysis
All determinations were made in triplicate and each result was expressed as a mean ± standard deviation (s.d.). Statistical significance was determined by paired Student's t-test. A P-value of 0·05 was considered significant.
Results
BCG efficiently induces PEC cytotoxicity
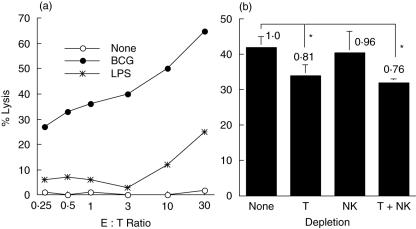
PECs were stimulated with BCG for 24 h followed by co-culturing with 51Cr-labelled MBT-2 targets for 20 h. LPS, a reagent known to activate macrophages, was used for comparison. BCG was more potent than LPS for induction of PEC cytotoxicity (Fig. 1a). The activated PECs showed cytotoxicity toward MBT-2 cells in a dose-dependent manner. The BCG-induced PEC cytotoxicity was so potent that the lysis of MBT-2 cells was observed even at a very low E : T ratio (i.e. 26% lysis at E : T = 0·25 : 1). This killing certainly resulted from PEC cytotoxic activity, as no killing of MBT-2 cells was observed by incubation of MBT-2 cells with BCG alone lacking of PECs (data not shown).
Fig. 1.
Bacille Calmette–Guérin (BCG)-induced peritoneal exudate cells (PECs) cytotoxicity against mouse bladder cancer MBT-2 cells. (a) Thioglycollate-elicited PECs were stimulated with BCG (0·005 OD) or lipopolysaccharide (LPS) (10 µg/ml) for 24 h. Cytotoxicity was evaluated by coculturing the activated PECs with 51Cr-labelled MBT-2 cells at the indicated effector : target (E : T) ratios for 20 h. Supernatants were then harvested, released 51Cr counted, and specific lysis calculated. (b) T cells, natural killer cells or both were depleted from thioglycollate-elicited PECs. The treated PECs were then stimulated with BCG (0·005 OD) for 24 h. After stimulation, induction of PEC cytotoxicity was evaluated as described above (E : T ratio = 10 : 1). *Significantly decreased.
PECs usually consist of ∼90% macrophages and ∼10% T cells and NK cells [25,26]. To investigate the impact of T cells and NK cells on BCG-induced PEC cytotoxicity, depletion assays were performed (Fig. 1b). Depletion of T cells reduced 19% total killing of MBT-2 cells whereas depletion of NK cells reduced only 4% total killing. Depletion of both T cells and NK cells reduced 24% total killing. These observations indicate that macrophages play an important role in PEC cytotoxicity toward MBT-2 cells.
The role of endogenous Th1-stimulating cytokines in BCG-induced PEC cytotoxicity
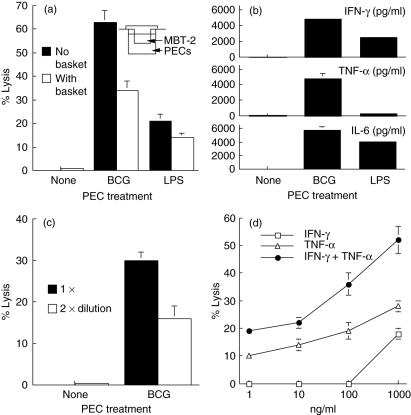
To dissect the effector components in BCG-induced PEC cytotoxicity, a transwell apparatus was used to separate PECs from MBT-2 targets but allow free penetration of endogenously produced soluble factors. Blockage of direct cell–cell contact reduced killing of MBT-2 cells by approximately 50% (Fig. 2a). Supernatants from the same transwell assay were found to contain the high levels of IFN-γ, TNF-α and IL-6 (Fig. 2b). IL-2, IL-10 and IL-12 were not detectable (data not shown). The supernatants were also found to have direct cytotoxicity against MBT-2 cells (Fig. 2c), with a cytotoxic magnitude (30% killing) similar to that of soluble factors observed in the transwell assay (see Fig. 2a). To ascertain that IFN-γ and TNF-α produced endogenously in BCG-stimulated PEC cultures were cytotoxic, MBT-2 cells were exposed to exogenous rIFN-γ, rTNF-α or both (Fig. 2d). MBT-2 cells were susceptible to both cytokines either alone or in a combination. rTNF-α appeared more cytotoxic to MBT-2 cells than rIFN-γ.
Fig. 2.
Maximal bacille Calmette–Guérin (BCG)-induced peritoneal exudate cells (PECs) cytotoxicity requires both cell-to-cell contact and soluble factors secreted from PECs. Thioglycollate-elicited PECs were stimulated with BCG (0·005 OD) for 24 h in 24-well plates. 51Cr-labelled MBT-2 cells (effector : target ratio = 25 : 1) were then added onto the effector cells or into an insert basket (pore size: 3 µm). After culturing for 20 h, supernatants were collected and analysed for PEC cytolytic activity (a) and cytokine production (b). To evaluate the cytotoxicity of soluble factors, 51Cr-labelled MBT-2 cells were incubated with BCG-stimulated PEC culture supernatants collected above at 1× or 2× dilutions for 20 h (c) or with increasing concentrations of interferon-γ, tumour necrosis factor-α or equal mixtures of both cytokines for comparison (d).
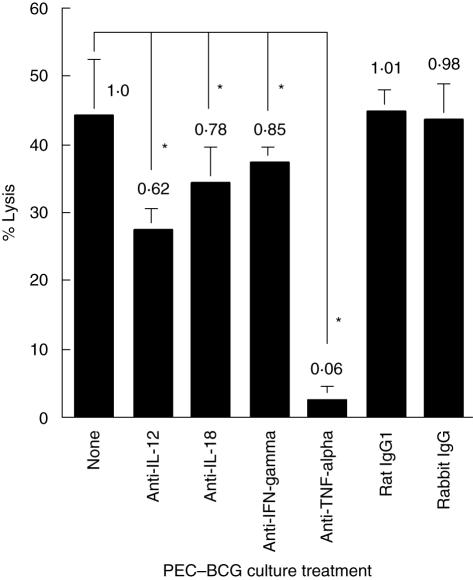
Because Th1-stimulating cytokines are known to be important modulators in BCG-mediated immune cell activation [16], we have investigated the role of endogenous Th1-stimulating cytokines in BCG-induced PEC cytotoxicity (Fig. 3). Neutralizing IL-12 reduced PEC cytotoxicity by 38%, IL-18 by 22%, IFN-γ by 15% and TNF-α by 94%, respectively. Among them, TNF-α appeared to play the most vital role in PEC cytotoxicity against MBT-2 targets. This observation, together with the observations in Fig. 2, indicates that the maximal BCG-induced PEC cytotoxicity requires both direct effector–target cell contact and the biological activities of endogenously produced soluble cytokines.
Fig. 3.
The role of endogenous Th1-stimulating cytokines in bacille Calmette–Guérin (BCG)-induced peritoneal exudate cells (PECs) cytotoxicity. Thioglycollate-elicited PECs were stimulated with BCG (0·005 OD) in the absence or presence of various neutralizing antibodies (3 µg/ml for each) for 24 h. 51Cr-labelled MBT-2 cells (effector : target ratio = 10 : 1) were then added and co-cultured for 20 h. Cytotoxicity was evaluated by measuring 51Cr release in culture supernatants. Species and isotype matched control antibodies (rabbit IgG and rat IgG1) were used in parallel. *Significantly decreased.
Supplementation of exogenous Th1-stimulating cytokines augments BCG-induced PEC cytotoxicity
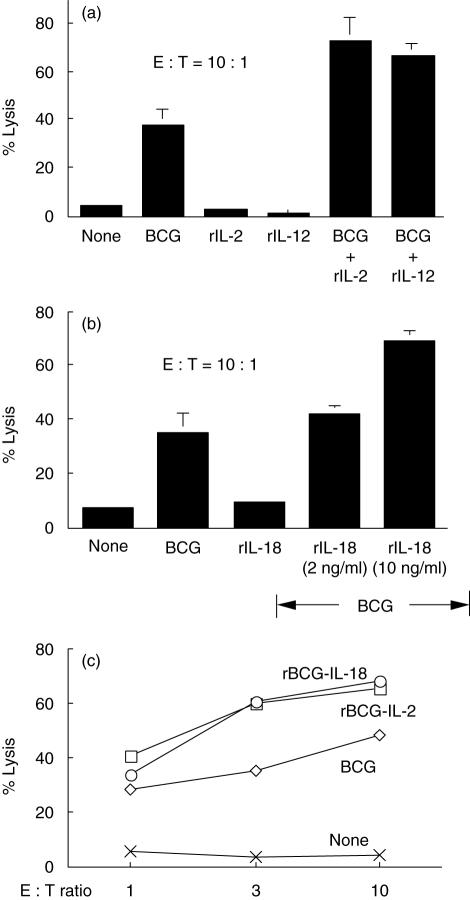
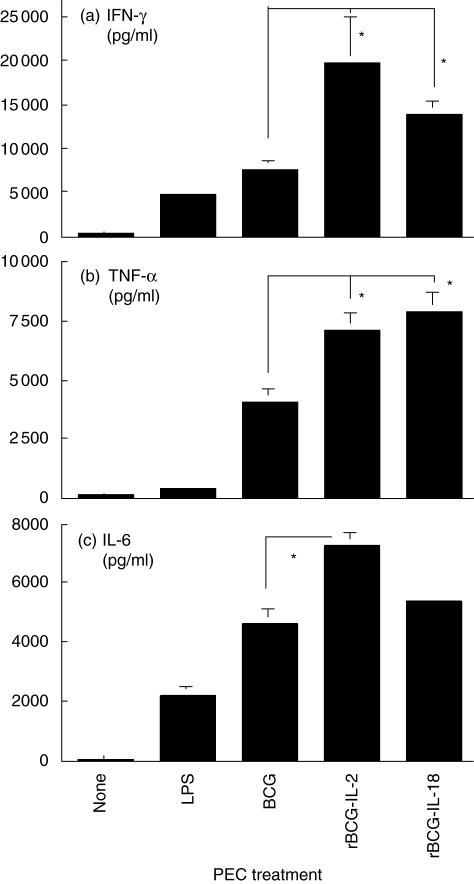
Because endogenous Th1-stimulating cytokines were found to be important effector components in BCG-induced PEC cytotoxicity, we tested whether PEC cytotoxicity could be enhanced by supplementation of exogenous Th1-stimulating cytokines. Although rIL-2, rIL-12 or rIL-18 alone showed no clear effect on induction of PEC cytotoxicity, combination of BCG with any one of these cytokines enhanced PEC cytotoxicity by approximately twofold (Fig. 4a,b). Similarly, the enhanced PEC killing was observed by activating PECs with IL-2- or IL-18-secreting rBCGs known to be capable of augmenting Th1 responses (Fig. 4c) [24,28]. Accordingly, increased expression of PEC-derived cytokines (IFN-γ, TNF-α and IL-6) was also observed in rBCG-stimulated PEC cultures (Fig. 5). Thus, it is clear that supplementation of exogenous Th1-stimulating cytokines can augment PCE effector functions in both cytotoxic activity and cytokine production.
Fig. 4.
Augmentation of peritoneal exudate cells (PECs) cytotoxicity by Th1-stimulating cytokines. (a, b) Thioglycollate-elicited PECs were stimulated with bacille Calmette–Guérin (BCG) (0·005 OD), recombinant interleukin (rIL)-2 (1 ng/ml), rIL-12 (100 ng/ml), rIL-18 (10 ng/ml), or combination of BCG (0·005 OD) with rIL-2 (1 ng/ml), rIL-12 (100 ng/ml) or rIL-18 (2 ng/ml and 10 ng/ml) for 24 h. 51Cr-labelled MBT-2 cells [effector : target (E : T) ratio = 10 : 1] were then added for 20 h and released 51Cr in culture supernatants counted. (c) Thioglycollate-elicited PECs were stimulated with BCG (0·005 OD), rBCG-IL-2 (0·005 OD) or rBCG-IL-18 (0·005 OD) for 24 h. After stimulation, induction of PEC cytotoxicity was evaluated by coculturing PECs with 51Cr-labelled MBT-2 cells (E:T ratio = 1 : 1, 3 : 1 and 10 : 1) for 20 h.
Fig. 5.
Up-regulation of peritoneal exudate cells (PECs) cytokine production by recombinant bacille Calmette–Guérin (BCG) expressing interleukin (IL)-2 and IL-18. Thioglycollate-elicited PECs were stimulated with lipopolysaccharide (10 µg/ml), BCG (0·005 OD) or the cytokine-secreting rBCGs (0·005 OD) for 48 h. Culture supernatants were then collected and measured by enzyme-linked immunosorbent assay for production of interferon-γ (a), tumour necrosis factor-α (b) and IL-6 (c). *Significantly increased.
Discussion
To understand more effectively the role of macrophages in BCG immunotherapy of bladder cancer, we have investigated macrophage cytotoxicity developed in response to BCG stimulation. We have demonstrated that BCG efficiently induces cytotoxic activity of macrophages toward mouse bladder cancer MBT-2 cells. We have also demonstrated that Th1-stimulating cytokines play an important role in BCG-induced macrophage cytotoxicity.
Multiple effector mechanisms are considered to be involved in killing of cancer cells by BCG-stimulated macrophages. Under our experimental settings, both direct effector–target cell contact and release of certain soluble cytotoxic cytokines are involved in MBT-2 cell killing. As macrophages act as a first line of defence in innate immunity against BCG infection [22,23] it is likely that, in the case of BCG intravesical therapy of bladder cancer, macrophages initiate BCG-induced anti-bladder cancer responses and at the same time also kill bladder cancer cells [29]. As observed, such BCG-induced macrophage cytotoxicity is notably potent (26% lysis at E : T = 0·25 : 1) and is superior to lymphokine-activated killer (LAK) cells under the similar experimental settings (data not shown).
Soluble factors secreted by BCG-activated macrophages have been found to account for approximately 50% of the total killing of MBT-2 cells. The soluble components contained the high levels of IFN-γ, TNF-α and IL-6. The former two cytokines are known to be tumoricidal cytokines for both human and mouse bladder cancers [30–32]. They were also found to be cytotoxic toward MBT-2 cells in this study. Both cytokines are also known to be a potent inducer of NO, a macrophage effector molecule [33,34]. IL-6 may contribute to BCG-induced PEC cytotoxicity through its influence on secretion of cytotoxic cytokines [35]. Neutralizing endogenous PEC cytokines (IL-12, IL-18, IFN-γ and TNF-α) all resulted in reduced PEC cytotoxicity. However, among them, TNF-α played a critical role, as the total killing of MBT-2 cells was reduced by 94% when it was neutralized. Because soluble components were observed to account for only approximately 50% killing of MBT-2 cells, TNF-α probably played multiple roles in PEC-mediated killing. For instance, in addition to its tumoricidal activity, TNF-α is known to act as an autocrine cytokine essential for macrophage activation [19,36]. These observations indicate that the maximal macrophage cytotoxicity requires various endogenously produced Th1-stimulating cytokines other than the direct contact of effector–target cells.
Because PEC preparation contained certain numbers of T cells (∼5%) and NK cells (∼5%) [25,26], we evaluated the possible impacts of these cells on BCG-induced macrophage cytotoxicity. Although NK cells appeared to play a minimum role in MBT-2 cell killing, T cells contributed substantially to such killing as a 19% reduction of killing was observed after the removal of T cells. This observation suggests that T cells are potentially more potent cytolytic effector cells than macrophages in this system. The same observation also suggests that BCG-activated macrophages are particularly effective at promoting cytotoxic T cell responses. In addition to their cytotoxic activities, both T cells and NK cells could also have played an important role in amplifying macrophage cytotoxicity. IL-12, IL-18 and TNF-α, the major cytokines produced predominantly by activated macrophages, are known to enhance BCG for induction of IFN-γ from T cells and NK cells [16,37,38], which in turn could feed back positively to augment macrophage phagocytosis and production of soluble cytotoxic factors.
As endogenous Th1-stimulating cytokines play a vital role in BCG-induced macrophage cytotoxicity, we have tested whether such cytotoxicity can be enhanced by supplementation with exogenous Th1-stimulating cytokines. Indeed, enhanced macrophage-mediated killing was observed when PECs were stimulated with combination of BCG plus rIL-2, rIL-12 or rIL-18. Similarly, enhanced killing was also observed when PECs were stimulated with rBCGs expressing IL-2 or IL-18, the two previously reported rBCG strains known to be capable of enhancing host Th1 immune responses and anti-mycobacterial infection [24,28,39]. Accordingly, elevated cytokines (IFN-γ, TNF-α and IL-6) were also observed in PEC cultures stimulated with both rBCGs. These results suggest that rBCGs expressing Th1-stimulating cytokines may serve as a better agent than wild-type BCG for bladder cancer treatment.
The present study demonstrates the killing of mouse bladder cancer MBT-2 cells by BCG-activated PECs. The similar PEC-mediated killing has also been observed for MB49 cells, another commonly used mouse bladder cancer cell line with C57BL/6 genetic background (unpublished observations). Thus, it is likely that macrophages are capable of killing bladder cancer cells in general in BCG immunotherapy of bladder cancer. The observations that BCG-induced macrophage cytotoxicity can be enhanced by supplementation of Th1-stimulating cytokines provide a rationale for the further studies of combined therapy in the treatment of bladder cancer patients.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R21CA104308) and Department of Defense (W81XWH-04-1-0070).
References
- 1.Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette–Guérin immunotherapy of superficial bladder cancer. J Urol. 1980;124:38–40. doi: 10.1016/s0022-5347(17)55282-9. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell MA, DeWolf DC. Bacillus Calmette–Guérin immunotherapy for superficial bladder cancer. New prospects for an old warhorse. Surg Oncol Clin N Am. 1995;4:189–202. [PubMed] [Google Scholar]
- 3.Emoto M, Emoto Y, Buchwalow IB, Kaufmann SH. Induction of IFN-gamma-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette–Guérin. Eur J Immunol. 1999;29:650–9. doi: 10.1002/(SICI)1521-4141(199902)29:02<650::AID-IMMU650>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Pryor K, Goddard J, Goldstein D, et al. Bacillus Calmette-Guérin (BCG) enhances monocyte- and lymphocyte-mediated bladder tumour cell killing. Br J Cancer. 1995;71:801–7. doi: 10.1038/bjc.1995.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohle A, Gerdes J, Ulmer AJ, Hofstetter AG, Flad HD. Effects of local bacillus Calmette–Guérin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol. 1990;144:53–8. doi: 10.1016/s0022-5347(17)39365-5. [DOI] [PubMed] [Google Scholar]
- 6.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol. 1992;147:1636–42. doi: 10.1016/s0022-5347(17)37668-1. [DOI] [PubMed] [Google Scholar]
- 7.Peuchmaur M, Benoit G, Vieillefond A, et al. Analysis of mucosal bladder leucocyte subpopulations in patients treated with intravesical Bacillus Calmette–Guérin. Urol Res. 1989;17:299–303. doi: 10.1007/BF00262987. [DOI] [PubMed] [Google Scholar]
- 8.Saint F, Patard JJ, Groux Muscatelli B, Lefrere Belda MA, et al. Evaluation of cellular tumour rejection mechanisms in the peritumoral bladder wall after bacillus Calmette–Guérin treatment. Br J Urol. 2001;88:602–10. doi: 10.1046/j.1464-410x.2001.02394.x. [DOI] [PubMed] [Google Scholar]
- 9.De Boer EC, De Jong WH, Van Der Meijden AP, et al. Presence of activated lymphocytes in the urine of patients with superficial bladder cancer after intravesical immunotherapy with bacillus Calmette–Guérin. Cancer Immunol Immunother. 1991;33:411–6. doi: 10.1007/BF01741603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boer EC, de Jong WH, van der Meijden AP, et al. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urol Res. 1991;19:45–50. doi: 10.1007/BF00294021. [DOI] [PubMed] [Google Scholar]
- 11.De Boer EC, De Jong WH, Steerenberg PA, et al. Leukocytes and cytokines in the urine of superficial bladder cancer patients after intravesical immunotherapy with bacillus Calmette–Guérin. In Vivo. 1991;5:671–7. [PubMed] [Google Scholar]
- 12.Bohle A, Nowc C, Ulmer AJ, et al. Elevations of cytokines interleukin-1, interleukin-2 and tumor necrosis factor in the urine of patients after intravesical bacillus Calmette–Guérin immunotherapy. J Urol. 1990;144:59–64. doi: 10.1016/s0022-5347(17)39366-7. [DOI] [PubMed] [Google Scholar]
- 13.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette–Guérin. J Urol. 2000;164:2129–33. [PubMed] [Google Scholar]
- 14.de Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette–Guérin therapy for superficial bladder cancer: processing, stability and prognostic value. J Urol. 1996;155:477–82. [PubMed] [Google Scholar]
- 15.Saint F, Patard JJ, Maille P, et al. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guérin treatment for superficial bladder cancer. J Urol. 2002;167:364–7. [PubMed] [Google Scholar]
- 16.Luo Y, Chen X, O'Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine. 2003;21:17–26. doi: 10.1016/s1043-4666(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Invest. 1999;103:1023–9. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto H, Suzuki K, Tsuyuguchi K, et al. Interleukin-12 gene expression in human monocyte-derived macrophages stimulated with Mycobacterium bovis BCG. cytokine regulation and effect of NK cells. Infect Immun. 1997;65:4405–10. doi: 10.1128/iai.65.11.4405-4410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calorini L, Bianchini F, Mannini A, et al. IFN-gamma and TNF-alpha account for a pro-clonogenic activity secreted by activated murine peritoneal macrophages. Clin Exp Metastasis. 2002;19:259–642. doi: 10.1023/a:1015583322354. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson S, Valadas E, Smith SM, Lukey PT, Dockrell HM. Monocyte-derived macrophage cytokine responses induced by M. bovis BCG. Tuber Lung Dis. 2000;80:197–207. doi: 10.1054/tuld.2000.0247. [DOI] [PubMed] [Google Scholar]
- 21.de Reijke TM, De Boer EC, Kurth KH, Schamhart DH. Urinary interleukin-2 monitoring during prolonged bacillus Calmette–Guérin treatment. can it predict the optimal number of instillations? J Urol. 1999;161:67–71. doi: 10.1097/00005392-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Hamerman JA, Aderem A. Functional transitions in macrophages during in vivo infection with Mycobacterium bovis bacillus Calmette–Guérin. J Immunol. 2001;167:2227–33. doi: 10.4049/jimmunol.167.4.2227. [DOI] [PubMed] [Google Scholar]
- 23.Aldwell FE, Wedlock DN, Slobbe LJ, Griffin JF, Buddle BM, Buchan GS. In vitro control of Mycobacterium bovis by macrophages. Tuberculosis. 2001;81:115–23. doi: 10.1054/tube.2000.0280. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Yamada H, Chen X, et al. Recombinant Mycobacterium bovis bacillus Calmette–Guérin (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin Exp Immunol. 2004;137:24–34. doi: 10.1111/j.1365-2249.2004.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada H, Kuroda E, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Prostaglandin E2 down-regulates viable Bacille Calmette–Guérin-induced macrophage cytotoxicity against murine bladder cancer cell MBT-2 in vitro. Clin Exp Immunol. 2002;128:52–8. doi: 10.1046/j.1365-2249.2002.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Enhancing effect of an inhibitor of nitric oxide synthesis on bacillus Calmette–Guérin-induced macrophage cytotoxicity against murine bladder cancer cell line MBT-2 in vitro. Jpn J Cancer Res. 2000;91:534–42. doi: 10.1111/j.1349-7006.2000.tb00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mickey DD, Mickey GH, Murphy WM, Niell HB, Soloway MS. In vitro characterization of four N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide (FANFT) induced mouse bladder tumors. J Urol. 1982;127:1233–7. doi: 10.1016/s0022-5347(17)54305-0. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell MA, Aldovini A, Duda RB, et al. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–14. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 30.O'Donnell MA, Luo Y, Hunter SE, Chen X, Hayes LL, Clinton SK. The essential role of interferon-gamma during interleukin-12 therapy for murine transitional cell carcinoma of the bladder. J Urol. 2004;171:1336–42. doi: 10.1097/01.ju.0000109751.60921.da. [DOI] [PubMed] [Google Scholar]
- 31.Hawkyard SJ, Jackson AM, Prescott S, James K, Chisholm GD. The effect of recombinant cytokines on bladder cancer cells in vitro. J Urol. 1993;150:514–8. doi: 10.1016/s0022-5347(17)35538-6. [DOI] [PubMed] [Google Scholar]
- 32.Hawkyard S, James K, Prescott S, et al. The effects of recombinant human interferon-gamma on a panel of human bladder cancer cell lines. J Urol. 1991;145:1078–81. doi: 10.1016/s0022-5347(17)38538-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhou A, Chen Z, Rummage JA, et al. Exogenous interferon-gamma induces endogenous synthesis of interferon-alpha and -beta by murine macrophages for induction of nitric oxide synthase. J Interferon Cytokine Res. 1995;15:897–904. doi: 10.1089/jir.1995.15.897. [DOI] [PubMed] [Google Scholar]
- 34.Petricevich VL, Alves RC. Role of cytokines and nitric oxide in the induction of tuberculostatic macrophage functions. Med Inflamm. 2000;9:261–9. doi: 10.1080/09629350020027564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997;65:4843–9. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 37.Ahn HJ, Maruo S, Tomura M, et al. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol. 1997;159:2125–31. [PubMed] [Google Scholar]
- 38.Kohno K, Kataoka J, Ohtsuki T, et al. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–50. [PubMed] [Google Scholar]
- 39.Luo Y, Chen X, Szilvasi A, O'Donnell MA. Co-expression of interleukin-2 and green fluorescent protein reporter in mycobacteria: in vivo application for monitoring antimycobacterial immunity. Mol Immunol. 2000;37:527–36. doi: 10.1016/s0161-5890(00)00077-8. [DOI] [PubMed] [Google Scholar]