Abstract
Ro60 kDa is a member of the Ro/LaRNP ribonucleoprotein complex and its major linear B cell epitope, corresponding to the region 169–190aa, has been found to be the initial target of the autoimmune response in patients with systemic lupus erythematosus. This sequence contains one serine and two arginine amino acid residues, which can potentially be modified post-translationally by phosphorylation or citrullination, respectively. The aim of this study was to develop an immunoassay for anti-Ro60 kDa epitope antibody detection and to investigate the changes in the antigenicity of the Ro60 kDa epitope when it is post-translationally modified, by either citrullination or phosphorylation. Peptide analogues corresponding to the unmodified form of the epitope, its phosphorylated form, and a form with both arginine residues citrullinated were synthesized. The peptide coating conditions were investigated and it was found that the use of highly hydrophilic surfaces increase the efficiency of the coating, as well as the sensitivity of the method for anti-peptide antibody detection. All peptides were tested by the optimized enzyme-linked immunosorbent assay (ELISA) against 119 sera from patients with primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis with anti-Ro/SSA reactivity, 20 sera from patients with systemic diseases without anti-Ro/SSA immune reactivity, as well as against 65 sera from normal individuals. A large proportion of the tested sera reacted against all three peptide analogues, although with a preference for the unmodified form of the epitope. In conclusion, post-translational modifications of the major Ro60 kDa B cell epitope can alter the autoantibody binding.
Keywords: autoantibodies, B cell epitopes, post-translational modifications, Ro60 kDa, Sjögren's syndrome
Introduction
Post-translational modifications (PTMs) occur in a variety of proteins of eukaryotic cells and appear to be significant for a number of cellular functions and the maintenance of homeostasis. The most frequently observed PTMs include glycosylation, phosphorylation, acetylation, citrullination and ubiquitination. During recent years, several studies have shown that PTMs and especially citrullination and phosphorylation can affect autoantibody binding onto different autoantigens in various systemic autoimmune diseases [1–4]
Autoantibodies against the Ro/LaRNP ribonucleoprotein complex are directed against the protein components Ro60 kDa, Ro52 kDa and La/SSB and they are found with increased prevalence in sera of patients with systemic lupus erythematosus (SLE) and primary Sjögren's syndrome (pSS). Previous studies from our laboratory, using epitope mapping techniques, demonstrated that the Ro60 kDa autoantigen contains two linear B cell epitopes, spanning the sequences 169–190aa and 211–232aa [5,6]. The epitope 169–190aa was recognized mainly from sera of patients with SLE, and the epitope 211–232aa from sera of patients with pSS. However, screening of a large panel of autoimmune sera disclosed that the sensitivity of the autoantibody detection, using peptide-based assays, was low, suggesting that Ro60 kDa contains conformational rather than linear epitopes [7–9].
Interest in the Ro60 kDa epitope 169–190aa has been renewed recently, as McClain and colleagues [10]demonstrated that this sequence could be involved, through molecular mimicry mechanisms, in the initiation of the autoimmune response in patients with SLE. They also showed that purified antibodies against this epitope cross-reacted with the 58–72aa amino acid sequence of the latent viral protein Epstein–Barr virus nuclear antigen-1 (EBNA-1). These observations, along with our recent findings that the phosphorylation of the linear B cell epitope 349–368aa of La/SSB autoantigen enhances the autoantibody binding and its relative avidity [11], prompted us to investigate whether specific PTMs of the moiety of the Ro60 kDa major linear B cell epitope 169–190aa can alter the antigenicity of this molecule. Furthermore, with the aim of developing a more efficient solid phase assay for the detection of anti-peptide antibodies, we utilized highly hydrophilic enzyme-linked immunosorbent assay (ELISA) surfaces and compared them to other commonly used ELISA plates.
Materials and methods
Human sera
To assess peptide antigenicity, 119 sera from patients with systemic autoimmune diseases were used. All sera were tested by counter immunoelectrophoresis (CIE) and found to be positive for the presence of anti-Ro/SSA antibodies. These sera were obtained from patients with pSS (n = 42) [12], SLE (n = 41) [13] and rheumatoid arthritis (RA) (n = 36) [14]. Twenty sera from patients with pSS, SLE and RA, which did not contain anti-Ro60 kDa antibodies, were used as disease controls, whereas 65 sera from healthy individuals were used as negative controls. All sera were collected while patients were investigated for diagnostic purposes; written informed consent, allowing part of the serum to be used for research purposes, was obtained from all patients. Ethical approval for the study was obtained from the scientific committee of Laiko General Hospital.
Peptide synthesis
Three peptide analogues of the Ro60 kDa epitope 169–190aa were synthesized for the purposes of this study. These analogues included the following peptides: (i) NH2-169TKYKQRNGWSHKDLLRLSHLKP190-CONH (pep169-190aa), (ii) NH2-169TKYKQ-Cit-NGWSHKDLL-Cit-LSHLKP190-CONH (pep169–190aaCit), with the two arginine residues, Arg174 and Arg184, substituted by citrulline amino acid derivatives (Cit) and (iii) NH2-169TKYKQRNGW-pS-HKDLLRLSHLKP190-CONH (pep169–190aaPh), where the serine amino acid residue at position 178 (Ser178) was phosphorylated (pS). All three synthetic peptides were tested by mass spectrometry for confirmation of the correct synthesis and correct orientation of the chemically modified amino acid residues.
ELISA assays
Specific anti-peptide ELISA assays were used in order to examine the reactivity of the tested sera against the Ro60 kDa epitope and its modified peptide analogues. More specifically, 96-well polystyrene plates with hydrophilic surface (MultisorpTM; Nunc, Roskilde, Denmark) were coated with each peptide diluted in carbonate-bicarbonate buffer pH = 10, at a concentration of 5 µg/ml. Non-specific binding on the plates was blocked using a solution 2% w/v of bovine serum albumin (BSA) in phosphate-buffered saline (PBS) as blocking buffer. The sera that were tested in the ELISA experiments were added to the ELISA plates at a dilution of 1 : 140 in blocking buffer. After a 2-h incubation time the plates were washed three times with PBS; subsequently alkaline phosphatase-conjugated, affinity purified, anti-human IgG (Jackson Immunoresearch, West Grove, PA, USA) diluted 1 : 1400 in blocking buffer, was added. p-Nitrophenyl phosphatase disodium substrate solution (pNPP, Sigma, St Louis, MO, USA) was added and the absorbance was measured at 410 nm by an ELISA reader (Molecular Devices, Sunnyvale, USA). The optimum concentration of the reagents used was selected after preliminary experiments. The results from each ELISA experiment were transformed in binding units (BU) to enable comparisons between different ELISA experiments, according to the formula:
The cut-off value was calculated as the mean BU value obtained by the sera from healthy individuals plus three standard deviations.
Inhibition assays
In order to test the specificity of the immune response of the tested sera against the synthetic peptide analogues of the 169–190aa epitope, we performed inhibition ELISA experiments. For these experiments we selected one serum on the basis of its reactivity against the tested Ro60 kDa peptides. More specifically, serum P1 recognized all three peptides, exhibiting slightly higher reactivity against the citrullinated peptide than the other two synthetic analogues. The serum was preincubated for 2 h in room temperature with different concentrations, ranging from 1 to 50 µg/ml of the pep169–190aa, pep169–190Cit and pep169–190aaPh peptides and was tested subsequently in ELISA assays against the unmodified epitope. The remainder of the ELISA assays were similar to that described above.
Comparison of the efficiency of different ELISA microplate surfaces for the detection of antipep169–190aa antibodies
Twenty sera positive for anti-Ro60 kDa antibodies were used in ELISA experiments in order to compare the bindingefficacy of two types of ELISA microplates for the pep169–190aa peptide analogue. The plates that we used were the 96-well polystyrene high binding microplates (Costar™; Corning, NY, USA) and the 96-well polystyrene plates with highly hydrophilic surface (Multisorp™; Nunc). All plates were coated with a 5 µg/ml solution of the pep169–190aa peptide, diluted in carbonate–bicarbonate buffer with pH = 10. After the addition of the peptide solution, the plates were incubated for 2 h at room temperature in order to allow the peptide adequate time to interact with the solid phase. Subsequently ELISA assays were performed as described previously.
Statistical analysis
Continuous variables (BU) were compared using the Mann–Whitney test or Student's t-test if normality assumed. Statistical significance was set at P = 0·05.
Results
Comparison of binding efficacy between ELISA microplates
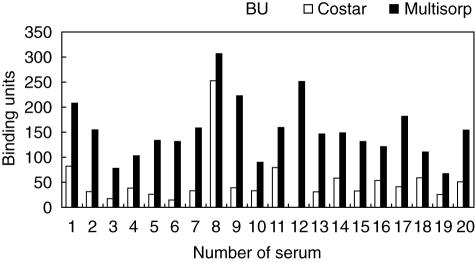
The two different ELISA microplates (Costar™ High Binding and Multisorp™) were tested for their ability to interact with the highly polar peptide analogue of the Ro60 kDa epitope 169–190aa at pH = 10. It was found that the use of an ELISA microplate manufactured especially to interact with hydrophilic highly charged molecules can enhance the binding of the tested peptide (Fig. 1). Thus, Multisorp™ microplates bound the peptide pep169–190aa (at a concentration of 5 µg/ml) with statistically significant greater efficiency (P < 0·001) in comparison with COSTAR™ high binding polystyrene plates.
Fig. 1.
Comparison of the binding efficiency for the coating peptide pep169–190aa between the enzyme-linked immunosorbent assay plate Costar™ and Multisorp™.
Recognition of the peptide analogues of the 169–190aa epitope from sera of patients
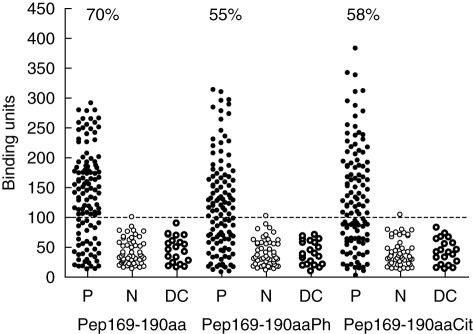
Seventy per cent (83 of 119) of the anti-Ro60 kDa sera gave a positive reaction in the ELISA experiments against the unmodified form of the epitope (pep169–190aa). Fifty-five and 58% of the same sera were found to be positive for antibodies against the phosphorylated (pep169–190aaPh) and citrullinated analogues (pep169–190aaCit), respectively (Fig. 2). Only one from the normal sera and none from the disease control sera reacted against the three synthetic peptides. The binding (as assessed by the mean value of the binding units) of the tested sera onto the unmodified epitope was statistically significantly higher in comparison with the binding of the same sera against the chemically modified peptides (P < 0·05).
Fig. 2.
Recognition of the peptide analogues of the 169–190aa epitope from sera of patients with autoimmune diseases, with anti-Ro/Sjögren's syndrome (SSA) reactivity (P), patients with autoimmune diseases without anti-Ro/SSA reactivity (DC) and from normal subjects (N). The dotted line represents the cut-off value for the experiment. All values above the cut-off value were considered as positive values. Pep169–190aa, pep169–190aaPh and pep169–190aaCit represent the unmodified, the phosphorylated and the citrullinated epitope, respectively.
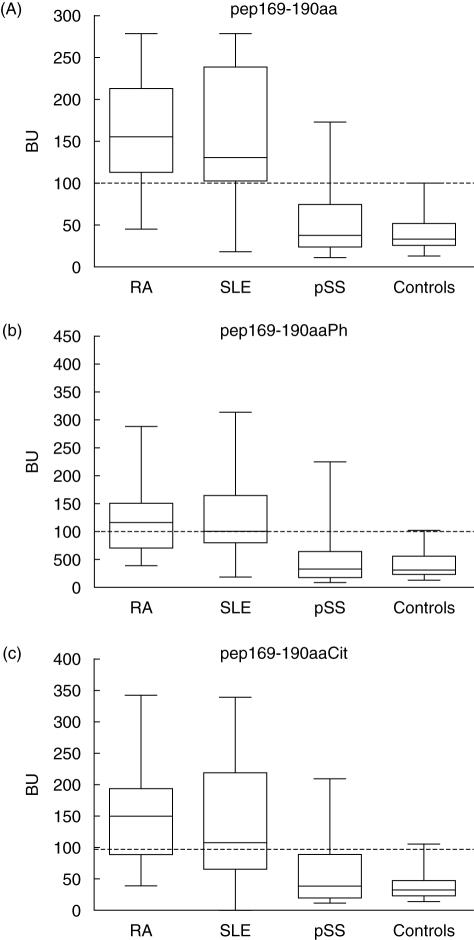
The reactivity of the tested sera against the three synthetic peptides was also assessed with regard to the underlying disease. We found that 86·5% of the RA anti-Ro/SSA positive sera, as well as 78% and 16% of the SLE and pSS anti-Ro/SSA positive sera, respectively, reacted with the unmodified epitope. Additionally 62% of the RA anti-Ro/SSA positive sera, 48% of the SLE anti-Ro/SSA positive sera and 12% of the pSS anti-Ro/SSA positive sera reacted against the phosphorylated peptide pep169–190aaPh. Finally 62%, 59% and 12% of the RA, SLE and pSS anti-Ro/SSA positive sera recognized the citrullinated analogue (Fig. 3). From these results and the statistical analysis performed, it seems that the prevalence of antibodies against pep169–190aa and its modified derivatives is lower in pSS sera, compared with sera from patients with SLE and RA. In fact, pSS sera were marginally statistically significantly different even from the tested normal sera (P = 0·05). This limited recognition of the 169–190aa epitope by anti-Ro60 kDa positive pSS sera is consistent with our previous results [6]. All peptides were recognized preferentially by the majority of SLE and RA sera. The prevalence of autoantibodies against all three peptides of the 169–190aa epitope in RA and SLE sera did not differ statistically significantly (P = 0·05).
Fig. 3.
Immune reactivity of the sera from patients with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), primary Sjögren's syndrome (pSS), as well as from normal subjects (controls) against the synthetic peptide analogues of the 169–190aa epitope: (a) unmodified peptide pep169–190aa, (b) phosphorylated peptide pep169–190aaPh and (c) citrullinated peptide pep169–190aaCit.
Inhibition experiments
The specificity of the peptide recognition was assessed by inhibition experiments. When the P1 serum (with the slightly higher anti-pep169–190aaCit reactivity) was tested against the unmodified peptide pep169–190aa, antibody binding to the solid phase was inhibited at 35% by the same peptide. A slightly higher inhibition percentage (39%) was achieved when pep169–190aaCit was used as a soluble inhibitor. In contrast, when the phosphorylated epitope (169–190aaPh) was used as inhibitor, it did not result in significant inhibition of the binding of the antibodies onto the ELISA plate (12%). Therefore the phosphorylated peptide pep169–190aaPh was not found to be able to inhibit the binding of autoantibodies to the unmodified epitope 169–190aa.
Discussion
The antibody response against the Ro60 kDa autoantigen is diverse, involving different epitopes within the moiety of the protein. Although the majority of the autoantibodies are directed against conformational epitopes, previous studies from our laboratory revealed that Ro60 kDa contains two linear B cell epitopes spanning the sequences 169–190aa and 211–232aa, which possess significant disease specificity [6]. The epitope 211–232aa is associated with pSS and the 169–190aa with SLE. During recent years the sequence 169–190aa has gained significant interest, as McClain and colleagues [10] revealed that it is the initial epitope recognized by sera from SLE patients years before the onset of the disease.
Previous studies for the determination of Ro60 kDa epitopes were carried out using modified polystyrene hydrophobic microplates (Costar™ high binding and Nunc-Maxisorp™) [7,15–17]. Recently, new highly hydrophilic ELISA microplates (Multisorp™; Nunc) have been developed. Thus, we investigated whether the use of the new ELISA surfaces allows a more efficient detection of antibodies against Ro60 kDa epitopes by comparing the Multisorp™ hydrophilic microplates with the hydrophobic high binding microplates (Costar™; Corning). We found that the vast majority of the tested sera exhibited higher binding to the coated peptide pep169–190aa when the hydrophilic polystyrene surfaces were used rather than the hydrophobic microplates. This observation could explain the low reactivity of the sera against the synthetic peptides that were used in the studies for determination of Ro60 kDa B cell epitopes.
In the present study we used two common PTMs (citrullination and phosphorylation), which are associated with increased binding of autoantibodies from different autoimmune diseases. Citrullination is being considered increasingly to be important in RA [18,19], where different citrullinated autoantigens, such as fillagrin [19], vimentin [20], collagen [21] and α-enolase [22], are recognized with high specificity from sera of patients with RA [23]. Phosphorylated proteins, such as the U1–70 kDa autoantigen and the splicing factors SR, are recognized by CD4+ T cells from lupus-prone mice (MRL/lpr mice) and by autoantibodies found in sera from patients with SLE [24,25]. Recently, phosphorylation of the major La/SSB epitope was found to increase significantly its recognition by pSS and SLE sera [11].
We searched web-based post-translational prediction servers to identify possible PTMs that the Ro60 kDa protein is prone to undergo. Using the NetPhos™ 2·0 server [26] we found that 15 serine residues of the molecule are putative phosphorylation sites. Among the 15 residues we selected Ser178, as it lies within the highly antigenic 169–190aa region of the Ro60 kDa autoantigen. This epitope also contains two arginine residues that can be potentially converted in citrulline derivatives.
Previous studies determined the antigenic characteristics and the amino acid residues which contribute to the antigenicity of the epitope 169–190aa [5]. These residues, along with the amino acids which have been modified for this study, are presented in Table 1.
Table 1.
Biochemical characteristics of the major linear B cell epitope 169–190aa of the Ro60 kDa autoantigen. Important amino acid residues connote the amino acids contributing to the antigenicity of the molecule [5]. Minimum length denotes the minimum required length of the epitope, which retains the antigenicity of the molecule [5]. Finally phosphorylated and citrullinated amino acids reveal those residues that were modified for this study.
| Epitope 169–190aa | T | K | Y | K | Q | R | N | G | W | S | H | K | D | L | L | R | L | S | H | L | K | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Important aa | * | * | * | * | * | * | * | |||||||||||||||
| Minimum length | * | * | * | * | * | * | * | * | * | * | ||||||||||||
| Phosphorylated aa | * | |||||||||||||||||||||
| Citrullinated aa | * | * |
This study demonstrates that citrullination and phosphorylation can impair significantly recognition of the modified peptides by autoantibodies. This observation can be explained in two ways. First, the modified residues may contribute directly to antibody binding and a possible modification of one or more of these residues can affect the affinity of the antibodies. This is consistent with our previous work, which has demonstrated that Ser178 is important for antibody recognition. Similarly, Arg184 has been mapped at the end of the restricted epitope 175–184aa and truncation of this residue abolishes the binding of the autoantibodies to this epitope. Therefore, alteration of the chemical structure (by either phosphorylation or citrullination) of these residues can potentially reduce epitope recognition by autoantibodies. The second possibility is that the tested PTMs may have affected the secondary structure of the peptides. This is also highly likely, as both modifications alter the overall electrical charge of the peptides. Thus, both modifications reduce the positive charge of the peptide at neutral pH. Indeed, phosphorylation adds a negatively charged phosphate group to a serine residue, whereas citrullination converts a positively charged arginine residue to the neutral amino acid derivative citrulline.
Our experiments revealed that SLE, as well as the RA sera, reacted significantly more frequently with all peptide analogues of the 169–190aa epitope compared to pSS sera (P < 0·001). Our previous studies have shown that this particular epitope is recognized preferentially from SLE sera and not from sera from patients with pSS, while the other major Ro60 kDa B cell epitope, 211–232aa, reacts preferentially with pSS sera [5]. Our data support the selected specificity of the epitope 169–190aa for SLE sera, also indicating that this preferential recognition over the pSS sera is not affected by chemical modifications of the amino acid sequence. The anti-Ro60 kDa positive RA sera were also found to react with high frequency with the peptide analogues of the Ro60 kDa epitope. Autoantibodies against the Ro60 kDa autoantigen are detected in 5–10% of the RA sera [27,28], and the data presented here indicate that 86·5% of these sera recognized the unmodified 169–190aa epitope. Thus, the epitope 169–190aa could represent the major target of the B cell response against the Ro60 kDa in RA. Similarly to pSS and SLE sera, modifications of the 169–190aa epitope affected negatively its recognition by RA sera.
When the specificity of the immune response was assessed, a small inhibition was observed (35–39%). It is known that small peptides in solution cannot compete efficiently with the immobilized peptides in the solid surface, due to the competition of divalent versus monovalent binding of antibodies. In our previous studies the attachment of the peptides to a synthetic oligopeptide carrier (SOC2) allowed to achieve inhibition rates of 60% [6]. However, in the present study the Ro60 kDa epitope analogues were used in their monovalent form and therefore the inhibition rates were significantly lower.
In conclusion, we found that immunoassays, based on the hydropathic pattern of the target peptide, are essential for the detection of autoantibodies against linear B cell epitopes. Using these assays it was found that PTMs of the B cell epitope 169–190aa of the autoantigen Ro60 kDa can reduce recognition of this epitope by autoantibodies found in the sera from patients with autoimmune diseases. This observation may help understanding of the mechanisms of the recognition of this epitope by autoantibodies and the initiation of the autoimmune response against Ro60 kDa.
Acknowledgments
The authors wish to thank Dr Panayiotis Ziakas for his valuable assistance with the statistical analysis of the data presented in this paper. This work has been supported by a grant (PENED-01ED164) from the Hellenic Secretariat for Research and Technology.
References
- 1.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001;22:443–9. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosen A, Casciola-Rosen L. Altered autoantigen structure in Sjögren's syndrome: implications for the pathogenesis of autoimmune tissue damage. Crit Rev Oral Biol Med. 2004;15:156–64. doi: 10.1177/154411130401500304. [DOI] [PubMed] [Google Scholar]
- 4.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–54. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Routsias JG, Sakarellos-Daitsiotis M, Tsikaris V, Sakarellos C, Moutsopoulos HM, Tzioufas AG. Structural, molecular and immunological properties of linear B-cell epitopes of Ro60KD autoantigen. Scand J Immunol. 1998;47:280–7. doi: 10.1046/j.1365-3083.1998.00301.x. [DOI] [PubMed] [Google Scholar]
- 6.Routsias JG, Tzioufas AG, Sakarellos-Daitsiotis M, Sakarellos C, Moutsopoulos HM. Epitope mapping of the Ro/SSA60KD autoantigen reveals disease-specific antibody-binding profiles. Eur J Clin Invest. 1996;26:514–21. doi: 10.1046/j.1365-2362.1996.186316.x. [DOI] [PubMed] [Google Scholar]
- 7.Boire G, Lopez-Longo FJ, Lapointe S, Menard HA. Sera from patients with autoimmune disease recognize conformational determinants on the 60-kd Ro/SS-A protein. Arthritis Rheum. 1991;34:722–30. doi: 10.1002/art.1780340613. [DOI] [PubMed] [Google Scholar]
- 8.Itoh Y, Reichlin M. Autoantibodies to the Ro/SSA antigen are conformation dependent. I. Anti-60 kD antibodies are mainly directed to the native protein; anti-52 kD antibodies are mainly directed to the denatured protein. Autoimmunity. 1992;14:57–65. doi: 10.3109/08916939309077357. [DOI] [PubMed] [Google Scholar]
- 9.Saitta MR, Arnett FC, Keene JD. 60-kDa Ro protein autoepitopes identified using recombinant polypeptides. J Immunol. 1994;152:4192–202. [PubMed] [Google Scholar]
- 10.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85–9. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 11.Terzoglou AG, Routsias JG, Avrameas S, Moutsopoulos HM, Tzioufas AG. Preferential recognition of the phosphorylated major linear B-cell epitope of La/SSB 349–368aa by anti-La/SSB autoantibodies from patients with systemic autoimmune diseases. Clin Exp Immunol. 2006;144:432–9. doi: 10.1111/j.1365-2249.2006.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Barakat S, Meyer O, Torterotot F, et al. IgG antibodies from patients with primary Sjogren's syndrome and systemic lupus erythematosus recognize different epitopes in 60-kD SSA/Ro protein. Clin Exp Immunol. 1992;89:38–45. doi: 10.1111/j.1365-2249.1992.tb06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahren M, Ruden U, Andersson B, Ringertz NR, Pettersson I. Identification of antigenic regions of the human Ro 60 kDa protein using recombinant antigen and synthetic peptides. J Autoimmun. 1992;5:319–32. doi: 10.1016/0896-8411(92)90146-h. [DOI] [PubMed] [Google Scholar]
- 17.Wahren M, Solomin L, Pettersson I, Isenberg D. Autoantibody repertoire to Ro/SSA and La/SSB antigens in patients with primary and secondary Sjogren's syndrome. J Autoimmun. 1996;9:537–44. doi: 10.1006/jaut.1996.0072. [DOI] [PubMed] [Google Scholar]
- 18.Goldbach-Mansky R, Lee J, McCoy A, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–43. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Gabalawy HS, Wilkins JA. Anti-Sa antibodies: prognostic and pathogenetic significance to rheumatoid arthritis. Arthritis Res Ther. 2004;6:86–9. doi: 10.1186/ar1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkhardt H, Sehnert B, Bockermann R, Engstrom A, Kalden JR, Holmdahl R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur J Immunol. 2005;35:1643–52. doi: 10.1002/eji.200526000. [DOI] [PubMed] [Google Scholar]
- 22.Kinloch A, Tatzer V, Wait R, et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1421–9. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundberg K, Nijenhuis S, Vossenaar ER, et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther. 2005;7:R458–67. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monneaux F, Lozano JM, Patarroyo ME, Briand JP, Muller S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur J Immunol. 2003;33:287–96. doi: 10.1002/immu.200310002. [DOI] [PubMed] [Google Scholar]
- 25.Neugebauer KM, Merrill JT, Wener MH, Lahita RG, Roth MB. SR proteins are autoantigens in patients with systemic lupus erythematosus. Importance of phosphoepitopes. Arthritis Rheum. 2000;43:1768–78. doi: 10.1002/1529-0131(200008)43:8<1768::AID-ANR13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–62. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 27.Cavazzana I, Franceschini F, Quinzanini M, et al. Anti-Ro/SSA antibodies in rheumatoid arthritis: clinical and immunologic associations. Clin Exp Rheumatol. 2006;24:59–64. [PubMed] [Google Scholar]
- 28.Skopouli FN, Andonopoulos AP, Moutsopoulos HM. Clinical implications of the presence of anti-Ro (SSA) antibodies in patients with rheumatoid arthritis. J Autoimmun. 1988;1:381–8. doi: 10.1016/0896-8411(88)90008-x. [DOI] [PubMed] [Google Scholar]