Abstract
Considering the complexity of the immunological events triggered during active visceral Leishmaniasis (VL), the relevance of the segregation of the immune response during human VL into type 1 and type 2 still remains unclear. For this purpose, in individuals living in risk areas for VL, we have evaluated especially asymptomatic individuals and patients with active VL, the plasmatic levels of cytokines and reactive nitrogen species under ex vivo conditions. In addition, we have also performed an analysis of intracellular cytokine patterns of circulating leucocytes after short-term culture, particularly in the absence of antigenic-specific stimulation, in order to reflect dynamic events of immune response in vivo during Leishmania chagasi infection. Although asymptomatic individuals and non-infected subjects presented a similar immunological profile, an outstanding inflammatory/regulatory profile, based on higher plasmatic levels of cytokines such as interleukin (IL)-8, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, IL-6 and IL-10, was associated with clinical status observed in active VL. In this context, we hypothesize that IL-10, through its ability to inhibit anti-leishmanial macrophage activation, associated with the lower frequency of TNF-α+ monocytes and ordinary levels of nitrite and nitrate are the major mechanisms associated with disease onset.
Keywords: cellular immune response, cytometric bead array, flow cytometry, immunoregulation, visceral Leishmaniasis
Introduction
Visceral Leishmaniasis (VL), caused by the intracellular protozoan Leishmania (Leishmania) chagasi, in the New World is a progressive, potentially fatal infection characterized by chronic fever, hepatosplenomegaly, pancytopenia and profound cachexia. Protective immunity to Leishmania infection is cell-mediated and results in the killing of intracellular parasites by activated macrophages and cytotoxic responses. Many reports have previously linked the presence of active VL with an impaired lymphocyte proliferation after in vitro stimulation with Leishmania antigens with suppression of type 1 cytokines, such as interleukin (IL)-2, interferon (IFN)-γ and IL-12, associated with marked up-regulation of type 2 cytokines as IL-4 and IL-10 [1–3]. However, despite such down-regulation of antigen-specific T cell responsiveness, strong IFN-γ and IL-10 expression has been demonstrated in the liver, spleen and bone marrow of infected hamster, used as the preferred model to reproduce active human disease [4]. Moreover, high levels of mRNA for both type 1 and type 2 cytokines has been described further in lymphoid tissue and bone marrow-derived cells during human active VL [5,6]. Consistent with this tissue profile, a mixed type 1/type 2 immune response has been observed when increased levels of circulating cytokines such as IFN-γ, tumour necrosis factor (TNF)-α, IL-6, IL-8 and IL-10 occur simultaneously during active VL [7–10]. Additionally, studies of cellular immune response after in vitro non-specific stimulation with phorbol myristate acetate (PMA) and ionomycin have demonstrated co-expression of IFN-γ and IL-10 by CD4+ T lymphocytes during active VL [11]. However, despite the increased levels of IFN-γ, it seems that the IFN-γ-mediated macrophage activation could be regulated by IL-10 during active VL, blocking leishmanicidal activity and nitrogen (NO)-mediated killing of Leishmania by macrophages, favouring disease onset and progression [12,13]. In the hamster, this observation is supported by the fact that progressive disease is associated with a defect in the generation of NO [4].
It is possible that these controversial data may reflect distinct methodological approaches used to investigate the immune mechanisms associated with human VL. Considering the complexity of the immunological events triggered during active VL, the relevance of such segregation of the immune responses into type 1 and type 2 during active VL still remains unclear in human VL. We have postulated previously that antigen stimulation in vitro is able to switch the immune profile toward a type 2 profile, and it may explain those disagreements observed between previous investigators.
For this purpose, in this study, in individuals living in risk areas for VL, we have evaluated especially asymptomatic individuals and patients with active VL, the plasmatic levels of cytokines and reactive nitrogen species under ex vivo conditions in addition to the analysis of intracellular cytokine patterns of circulating leucocytes after a short-term culture, particularly in the absence of antigenic-specific stimulation in order to reflect dynamic events of immune response in vivo during L. chagasi infection.
Materials and methods
Study groups
The patients included in this study were divided into two groups of L. (L) chagasi-infected individuals. The first group comprised untreated patients with signs and symptoms of active VL (ACT) and the second comprised individuals with asymptomatic VL (AS). The ACT group consisted of 22 patients (16 males and six females), with ages ranging from 3 to 67 years, who acquired their infection in the metropolitan area of Belo Horizonte, the capital of Minas Gerais State or in Montes Claros, the largest city of the north region of Minas Gerais State, Brazil. Their clinical status was defined by independent clinical evaluation by three of us (A. R., R. M. F and T. C. A. F. in Belo Horizonte and S. F. G. C. in Montes Claros). Active infections were determined when the patients presented fever, hepatosplenomegaly, anaemia, leukopenia and a positive serological test for Leishmania (indirect immunofluorescence test). In the majority of cases (63·6%), a positive bone marrow aspirate for Leishmania amastigotes in a Giemsa-stained smear was also documented. In cases without a parasitological confirmatory diagnosis the VL was diagnosed by clinical cure statement after successful pentavalent antimony therapy. The AS group consisted of 22 individuals (eight males and 14 females), with ages ranging from 6 to 66 years, living in Porteirinha, an area endemic for L. (L) chagasi infection, also located in the region refereed above. These individuals had never received VL treatment prior to this study, and remained without clinical signs or symptoms of VL as well as presenting positive serological tests for Leishmania and positive Montenegro skin tests (68% of cases).
The non-infected group (NI) comprised 20 volunteers (12 males and eight females), with ages ranging from 6 to 60 years, also living in Porteirinha. These individuals presented negative Montenegro skin tests and serological tests for Leishmania.
All subjects in this study signed an informed consent, approved by the FIOCRUZ Ethics Committee − Protocol 0070/99 (Ministry of Health, Brazil).
Detection of plasmatic cytokine levels by cytometric bead array immunoassay (CBA)
The secreted cytokines analysis by flow cytometry is a methodology in which the simultaneous measurement of multiple cytokines in a single sample is performed [14]. For plasmatic cytokine determination, whole blood samples were collected using ethylenediamine tetraacetic acid (EDTA) as the anti-coagulant. Plasma was maintained for up to 6 months at −20°C in aliquots thawed just before use. The CBA immunoassay kit (Becton Dickinson Biosciences Pharmingen, San Diego, CA, USA) was used for quantitative analysis of plasmatic cytokine levels. The CBA kit uses 7·5 µm polystyrene microbeads, consisting of six distinct populations, unique on their type 3 fluorescence intensity (FL-3) each coupled to monoclonal antibody (MoAb) against one of six cytokines [IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ (human TH1/TH2 kit) or IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α (inflammation kit)] to capture a given cytokine. The captured cytokines were then detected via direct immunoassay using a cocktail of six different MoAbs coupled to phycoerythrin (PE) (FL-2). Briefly, 25 µl of plasma (diluted 1 : 5 in diluent G, supplied by CBA kit) or standard (previously diluted in diluent G, as recommended by the manufacturer) were added to 15 µl of mixture beads and incubated for 90 min at room temperature in the dark. The cytokine standard calibrator mixture was used for each assay. After incubation, the samples and standards were washed with 500 µl of wash buffer (supplied with CBA kit) and centrifuged at 600 g for 7 min at room temperature. Subsequently, 20 µl of detection cocktail consisting of six PE-conjugated MoAbs were added to each tube and the mixture re-incubated for 90 min at room temperature in the dark. Following incubation, the samples and standards were washed again with 500 µl of wash buffer and centrifuged at 600 g for 7 min at room temperature to remove unbound detector reagent. After washing, 250 µl of wash buffer was added to each tube prior to data acquisition using a fluorescence activated cell sorter (FACS)Calibur® flow cytometer (Becton Dickinson).
Although the fluorescently labelled particles in the BD CBA immunoassay are designed to be excited by the 488 nm laser common to all BD flow cytometers, they can also be excited by the red diode laser on dual-laser BD FACSCalibur instruments. The detection of particle emission on the FL-4 channel simplifies the instrument set-up procedure and reduces the need for fluorescence compensation. Thus, a total of 1800 events/gate were acquired after proper set-up of a flow cytometer to measure forward (FSC) and side (SSC) light scatters and dual-colour (FL-4 and FL-2) flow cytometric acquisition, using a dual-laser BD CBA template. Data analysis was performed using BD Bioscience CBA software.
Short-term whole blood culture in vitro
Peripheral blood samples were collected into Vacutainer tubes containing sodium heparin (Becton Dickinson Vacutainer), and 500 µl aliquots were dispensed into individual 17 × 100 mm polypropylene tubes (Falcon® 2059). In this study, analysis of the cytokine profile was evaluated after short-term incubation in vitro in the absence of exogenous stimuli. These conditions were chosen considering that the detection of cytokines, particularly in the absence of exogenous stimuli, may reflect the dynamic events of cytokine production in blood leucocyte subsets in vivo. For this purpose, whole blood samples were incubated for 4 h at 37°C in a 5% CO2 humidified atmosphere in the presence of 500 µl of RPMI-1640 (Gibco, Grand Island, NY, USA) plus 10 µg/ml of Brefeldin A (BFA; Sigma Chemical Company, St Louis, MO, USA) to inhibit cytokine secretion, allowing accumulation at the Golgi complex leading to higher percentages of cytokine-positive cells, which increases sensitivity for further analysis. Following incubation, the cultures were treated with 2 mM EDTA (Sigma) and kept at room temperature for 15 min.
Flow cytometric immunostaining for intracellular cytokine acquisition and analysis
After EDTA treatment, whole blood culture samples were washed with 6 ml of FACS buffer containing 0·015 M phosphate buffered saline (PBS), 0·5% bovine serum albumin (BSA) and 0·1% sodium azide (Sigma) by centrifugation at 600 g for 7 min at room temperature. After resuspension in 1 ml of FACS buffer, 200 µl aliquots were dispensed into two 12 × 75 mm polystyrene tubes (Falcon® 2052) and stained individually with anti-human fluorescein isothiocyanate (FITC) MoAbs (Becton Dickinson Biosciences Pharmingen) at 0·5 µg/ml, including isotypic control (clone X40), anti-CD14 (clone M0P9), anti-CD16 (clone B73·1), anti-CD19 (clone 4G7), anti-CD4 (clone RPA-T4) and anti-CD8 (clone HIT8a) for 30 min at room temperature in the dark. Stained samples were treated by vortexing gently with 2 ml of FACS lysing solution (Becton Dickinson Biosciences Pharmingen) and re-incubating for an additional 10 min at room temperature. After erythrocyte lysis was completed, the samples were centrifuged at 600 g for 7 min at room temperature, the supernatant discarded and the cell pellet resuspended and kept for 10 min at room temperature in the dark with 2 ml of FACS permeabilizing solution, containing FACS buffer supplemented with 0·5% of saponin (Sigma). Following incubation, the samples were centrifuged at 600 g for 7 min at room temperature, the supernatant decanted gently and the cell pellet washed with 3 ml of FACS buffer. After centrifugation, the cells were resuspended in 200 µl of FACS buffer and distributed in 30 µl aliquots over 96-well U-bottomed microtitre plates (Thomas 9383-A90). Cells were then stained with 20 µl of PE-labelled anti-cytokine MoAbs, including anti-IFN-γ (clone B27), anti-TNF-α (clone MAB11), anti-IL-4 (clone MP4–25D2) and anti-IL-10 (clone JES3–9D7) at a final concentration of 25 µg/ml, diluted previously 1 : 50 in sterile FACS permeabilizing solution, by incubation for 30 min at room temperature in the dark. After incubation, the cells were washed twice, first with 150 µl of FACS permeabilizing solution and then with 200 µl of FACS buffer. The cell preparations were then fixed in 200 µl of FACS fixing solution (10 g/l paraformaldehyde, 10·2 g/l sodium cacodylate and 6·63 g/l sodium chloride, pH 7·2) and stored at 4°C in the dark prior to flow cytometry analysis within 24 h. A total of 30 000 events/tube were acquired using a FACScan® flow cytometer (Becton Dickinson) properly set up to measure FSC and SSC light scatters, FITC (FL-1) and PE (FL-2) fluorescence. CELLQuestTM software provided by the manufacture was used for data acquisition and analysis.
Distinct gating strategies were used to analyse the cytokine profile of specific leucocyte subpopulations. Selective analysis of neutrophils was performed by establishing a specific scatter gate using the combination of anti-cell surface antigens and laser SSC to discriminate, and the neutrophils were gated as SSChighCD16high+. Flow cytometric assessment of eosinophils was based essentially on autofluorescent cells using a non-related FL-3 channel versus FSC. Identification of natural killer (NK) cells, B lymphocytes and T CD4+ or T CD8+ lymphocytes was performed initially by a lymphocyte scatter gate set-up, using FSC versus SSC dot plots, followed by immunophenotyping using anti-CD16-FITC, anti-CD19-FITC, anti-CD4-FITC or anti-CD8-FITC MoAb by determination of the subpopulation of interest. Analysis of monocytes was performed by staining immunophenotyping using SSC versus FL-1/anti-CD14-FITC dot plots to select the monocytes as SSClowCD14high+ cells. Following the selection of leucocyte populations, the prevalence of cytokine-expressing cells was determined using quadrant statistics over FL-2/anti-cytokine-PE versus FL-1/anti-cell surface marker-FITC dot plots, except for eosinophils, where the cytokine-expressing cells were identified using FL-2/anti-cytokine-PE versus FSC dot plots. All results were first expressed as percentages of cytokine-positive cells for different gated leucocyte subpopulations analysed in this study, selected as described above. This procedure was used to obtain the cytokine profile at single-cell level and also as the baseline to calculate the absolute number of cytokine-expressing leucocyte subpopulations. In order to obtain the percentage of cytokine-positive cells within total circulating leucocytes, the results were calculated as:
Final data were expressed as the absolute number of cytokine-expressing leucocyte subpopulations (cells/mm3 of peripheral blood). These values were calculated taking the percentage of cells that express the cytokine of interest in the upper right quadrant multiplied by the absolute number leucocyte subpopulation obtained by haematological analysis.
Nitrite (NO2–) and nitrate (NO3–) detection by the vanadium III/Griess method
The nitrite and nitrate measurement by the vanadium III/Griess method is an assay that combines both nitrate reduction and nitrite detection in a single step. In this study, we evaluated the nitrite and nitrate levels according to a protocol adapted from the original methodology described by Miranda et al. [15].
Human plasma were collected and stored at −20°C until use. Subsequently, 200 µl aliquots were deproteinized by centrifugation at 16000 g for 10 min at room temperature, in the presence of 20 µl of ZnCl2 1 M (Sigma, St Louis, MO, USA). A NaNO2 and NaNO3 (Sigma) standard solution (50 µl final) was serially diluted (generally from 100 to 0·8 µM) in non-pirogenic water plus 10% ZnCl2 1 M, in 96-well, flat-bottomed, polystyrene microtitre plates (Nunc Brand Products, Copenhagen Denmark). The dilution medium was used as blank. After loading the plate with 50 µl of samples, 50 µl of Griess reagent [1 : 1 solution of sulphanilamide 1% in CH3COOH 30% and N-(1-napthyl)ethylenediamine 0·1% in CH3COOH 60% (Sigma)], premixed immediately prior to application to the plate, were added to each well. At this point, nitrite only was measured at 560 nm spectrophotometrically (Emax microplate reader; Molecular Devices, Sunnyvale, CA, USA). For the detection of nitrite plus nitrate levels, 100 µl of vanadium solution (1 : 1 solution of vanadium 0·4 g/50 ml of HCl 0·1 N; Sigma) were added to each well and the plates were incubated for 90 min at 37°C in a 5% CO2 humidified incubator. After incubation, the concentrations of both nitrite plus nitrate were measured at 560 nm spectrophotometrically.
Statistical analysis
Statistical analysis was performed by a non-parametric analysis using an analysis of variance (anova) Kruskal–Wallis test followed by Bonferroni’s multiple comparison test provided by the software GraphPad Prism version 3·0·3 (San Diego, CA, USA). Spearman’s (rS) rank correlations were computed to assess correlations between plasmatic and intracellular parameters. Statistically significant differences were considered when P < 0·05.
Results
Cytokine plasmatic levels in VL
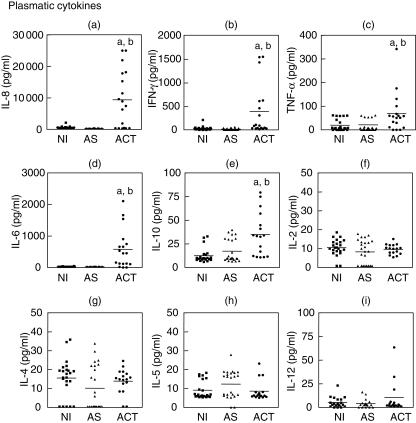
Quantitative detection of soluble cytokines by flow cytometry, particularly in the ex vivo context, have become a recent strategy to establish the immune response balance that closely reflects the in vivo immunoregulation status of patients in different clinical situations. Using the cytometric bead array we have quantified the plasmatic cytokine levels (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, TNF-α and IFN-γ) of L. (L) chagasi-infected individuals, both ACT and AS, and compared to those observed for NI subjects, all living in risk areas for infection. Our data demonstrate that ACT presented higher levels of circulating IL-8, IFN-γ, TNF-α, IL-6 and IL-10 in comparison to the NI and AS groups (Fig. 1a–e). No significant differences were observed for IL-2, IL-4, IL-5 and IL-12p70 plasmatic levels among NI, AS and ACT individuals (Fig. 1f–i). The IL-1β plasmatic levels were below the detection limit for the assay.
Fig. 1.
Circulating cytokine profile from NI (▪), AS (▴) and ACT (•) individuals. The plasmatic cytokine levels were measured using cytometric bead array (CBA) immunoassay. The results are expressed as scattering of individual values and mean of interleukin (IL)-8 (a), interferon (IFN)-γ (b), tumour necrosis factor (TNF)-α (c), IL-6 (d), IL-10 (e), IL-2 (f), IL-4 (g), IL-5 (h) and IL-12p70 (i) plasmatic levels. Letters (a) and (b) represent statistically significant differences (P < 0·05) for the non-infected (NI) and asymptomatic (AS), respectively.
Frequency of cytokine expression by circulating leucocytes in VL
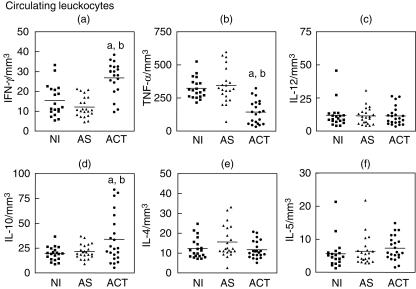
In order to evaluate the dynamic events of cytokines production that reflect the in vivo baseline immune response in human VL, the intracellular cytokine profile of circulating leucocytes was investigated after short-term incubation in vitro in the absence of exogenous stimuli. For this purpose we analysed IFN-γ, TNF-α, IL-12, IL-10, IL-4 and IL-5 cytokines at single-cell level in circulating leucocyte populations (Fig. 2), selected by scatter gate set-up using FSC versus SSC dot plots. In order to verify the main sources of each cytokine, we have further evaluated the contribution of specific subpopulations, including neutrophils, eosinophils, monocytes and lymphocytes. Significant differences are listed in Table 1. Complementary non-significant data are listed in the text.
Fig. 2.
Intracellular cytokine profile of circulating leucocytes in peripheral blood from NI (▪), AS (▴) and ACT (•) individuals, after short-term in vitro incubation, particularly in the absence of exogenous stimuli. The results are expressed as scattering of individual values and mean absolute numbers of interferon (IFN)-γ+ (a), tumour necrosis factor (TNF)-α+ (b), interleukin (IL)-12+ (c), IL-10+ (d), IL-4+ (e) and IL-5+ (f) leucocytes. Letters (a) and (b) represent statistically significant differences (P < 0·05) for the non-infected (NI) and asymptomatic (AS), respectively.
Table 1.
Intracellular cytokine profile of leucocytes subpopulations in peripheral blood from NI, AS and ACT individuals*.
| Groups | |||
|---|---|---|---|
| Leucocytes/cytokines | NI | AS | ACT |
| Innate immunity | |||
| Neutrophils | |||
| IFN-γ | 5·6 ± 3·7 | 5·7 ± 3·9 | 11·5 ± 6·5a,b |
| IL-4 | 5·4 ± 3·9 | 7·4 ± 6·0 | 9·5 ± 5·4a |
| Eosinophils | |||
| IFN-γ | 2·1 ± 1·5 | 0·8 ± 0·8a | 3·2 ± 2·3a,b |
| Monocytes | |||
| TNF-α | 243·6 ± 71·8 | 234·1 ± 122·0 | 66·4 ± 50·5a,b |
| NK cells | |||
| IFN-γ | 1·7 ± 1·0 | 1·1 ± 0·9 | 3·1 ± 2·4a,b |
| Adaptive immunity | |||
| Lymphocytes | |||
| CD4+ T cells | |||
| IFN-γ | 2·9 ± 1·6 | 2·4 ± 1·4 | 7·7 ± 4·5a,b |
| CD8+ T cells | |||
| IFN-γ | 2·8 ± 2·2 | 2·4 ± 1·2 | 6·0 ± 4·9a,b |
| IL-10 | 2·1 ± 1·6 | 2·2 ± 1·3 | 4·7 ± 3·2a,b |
| IL-4 | 1·6 ± 0·9 | 2·1 ± 1·2 | 3·3 ± 2·7a |
| CD19+ B cells | |||
| IL-10 | 3·2 ± 1·5 | 3·4 ± 1·9 | 5·9 ± 3·8a,b |
Intracellular cytokine profile was determined by mean absolute numbers of cytokine-expressing cells ± s.d. Letters a and b represent statistically significant differences (P < 0·05) between the active VL (ACT) and the non-infected (NI) and asymptomatic (AS), respectively. IFN: interferon; IL: interleukin; TNF: tumour necrosis factor.
As shown in Fig. 2a, an increased frequency of IFN-γ-expressing leucocytes was observed in ACT compared to NI and AS groups, upholding our results of evaluation plasmatic levels of these cytokines. We have further focused our attention on the identification of the main cell sources of IFN-γ in ACT patients. Our data demonstrate a similar contribution of the innate and adaptive immune response to this enhanced frequency of IFN-γ-producing cells, with increased levels of neutrophils, eosinophils, NK cells, CD4+ T lymphocytes and CD8+ T lymphocytes expressing IFN-γ (Table 1).
On the other hand, our data failed to show an association between the elevated plasmatic levels of TNF-α and the frequency of TNF-α+ circulating leucocytes. Distinct from the high plasmatic level of TNF-α, we observed a decreased frequency of TNF-α+ leucocytes (Fig. 2b) in ACT compared to NI and AS. We then investigated the contribution of leucocyte subpopulations to this phenomenon and identified the circulating monocytes, which are known to be the major source of this cytokine, as the main target of lower TNF-α synthesis in ACT (Table 1). No significant contribution of neutrophils, eosinophils and lymphocytes was observed (neutrophils: NI = 20·5 ± 10·8/mm3; AS = 19·3 ± 8·1/mm3; ACT = 14·3 ± 10·5/mm3; eosinophils: NI = 6·0 ± 4·1/mm3; AS = 4·0 ± 2·1/mm3; ACT = 4·0 ± 2·4/mm3; NK cells: NI = 2·1 ± 1·9/mm3; AS = 3·7 ± 2·5/mm3; ACT = 2·2 ± 1·9/mm3; CD4+ T cells: NI = 4·3 ± 1·6/mm3; AS = 9·5 ± 5·6/mm3; ACT = 3·5 ± 1·9/mm3; CD8+ T cells: NI = 3·4 ± 1·8/mm3; AS = 5·6 ± 2·9/mm3; ACT = 3·7 ± 3·2/mm3; and B cells: NI = 4·3 ± 2·5/mm3; AS = 4·5 ± 2·5/mm3; ACT = 5·5 ± 3·9/mm3).
Although the role of IL-12 determines a type 1 immune response, such as we observed in plasmatic analysis, no difference in the frequency of IL-12+ leucocytes was detected among NI, AS and ACT individuals (Fig. 2c).
Considering the importance of IL-10 on immunoregulatory mechanisms and the high plasmatic levels of IL-10 observed in our study, we have additionally investigated the synthesis of IL-10 by circulating leucocytes at intracytoplasmatic level. Similarly, we found an increased frequency of IL-10-producing leucocytes in ACT compared to NI and AS groups (Fig. 2d). The restricted sources of IL-10 were cells related to the adaptive immune response, with increased levels of IL-10+ CD8+ T lymphocytes and IL-10+ B lymphocytes (Table 1). No significant contribution of neutrophils, eosinophils, monocytes and CD4+ T lymphocytes was observed (neutrophils: NI = 6·7 ± 3·9/mm3; AS = 6·3 ± 3·3/mm3; ACT = 7·1 ± 4·9/mm3; eosinophils: NI = 1·1 ± 0·7/mm3; AS = 1·0 ± 0·8/mm3; ACT = 1·7 ± 1·5/mm3; monocytes: NI = 4·0 ± 2·3/mm3; AS = 3·5 ± 2·5/mm3; ACT = 3·2 ± 3·0/mm3; and CD4+ T cells: NI = 2·6 ± 1·1/mm3; AS = 4·4 ± 3·0/mm3; ACT = 4·0 ± 2·3/mm3).
For analysis of IL-4 and IL-5 in circulating leucocytes, in the same way, no difference was observed among the groups evaluated (Fig. 2e,f, respectively). Although no significant difference was observed, our data demonstrated higher frequency of IL-4+ neutrophils and CD8+ T lymphocytes in ACT patients compared to the NI group (Table 1). No significant contribution of eosinophils, NK cells, CD4+ T lymphocytes and B cells to a higher frequency of IL-4 were observed (eosinophils: NI = 1·3 ± 0·9/mm3; AS = 1·2 ± 1·4/mm3; ACT = 1·6 ± 0·8/mm3; NK cells: NI = 1·8 ± 1·4/mm3; AS = 1·1 ± 0·8/mm3; ACT = 1·9 ± 1·5/mm3; CD4+ T cells: NI = 1·6 ± 1·1/mm3; AS = 2·7 ± 1·3/mm3; ACT = 2·6 ± 1·6/mm3; and B cells: NI = 2·9 ± 1·9/mm3; AS = 2·4 ± 1·8/mm3; ACT = 2·7 ± 1·6/mm3).
Correlation between IFN-γ and IL-10 in the active VL
Whereas IFN-γ is the major type 1 cytokine involved on macrophage activation, IL-10 is characterized initially as a potent immunoregulatory cytokine, modulating the IFN-γ-induced activation of macrophages during Leishmania infection. Therefore, it is usually expected that these two cytokines present a reverse balance. As we observed the presence of IFN-γ and IL-10 on both plasmatic and intracytoplasmatic analysis, it was of interest to determine whether there was a positive or negative relationship between the levels of these cytokines during active VL. For this purpose, we examined the correlation between the plasmatic levels of IFN-γ and IL-10 as well as the frequency of leucocyte subpopulations expressing these cytokines. As shown in Table 2, a positive correlation between IFN-γ and IL-10 was observed in plasma and circulating leucocytes.
Table 2.
Correlation coefficients interleukin (IL)-10/interferon (IFN)-γ plasmatic and intracellular in active visceral Leishmaniasis (VL) (ACT) individuals*.
| Cytokines correlation | Cell type | Coefficient (rs)** | P** |
|---|---|---|---|
| IL-10/IFN-γ | Plasma | 0·7135 | 0·0006 |
| Total leucocytes | 0·6455 | 0·0016 | |
| Eosinophils | 0·6470 | 0·0006 | |
| Neutrophils | 0·3000 | 0·0423 | |
| CD4+ T lymphocytes | 0·5692 | 0·0030 |
Correlation between plasmatic levels of IL-10 and IFN- γ, including only those with detectable titres and correlation between absolute numbers of cells expressing IL-10 or IFN- γ.
Spearman’s (rS) rank correlations were computed and statistical significance was considered when P < 0·05.
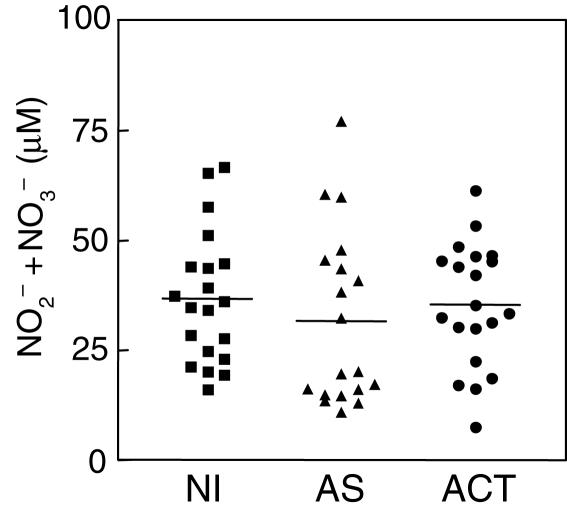
NO2– and NO3– plasmatic levels detection in VL
Considering the importance of reactive nitrogen intermediates in macrophage leishmanicidal activity, and the simultaneous increase of IFN-γ and IL-10 observed in active VL patients, we evaluated the NO2– plus NO3– plasmatic levels by the vanadium III/Griess method of NI, AS and ACT individuals. As showed in Fig. 3, no significant difference was observed for NO2– plus NO3– plasmatic levels among the NI, AS and ACT groups.
Fig. 3.
Measurement of NO2– plus NO3– plasmatic levels from NI (▪), AS (▴) and ACT (•) individuals. The results are expressed as scattering of individual values and mean of NO2– plus NO3– plasmatic levels.
Discussion
Considering the complexity of the immunological events triggered during active VL, the relevance of the segregation of the immune responses into types 1 and 2 has remained unclear in human VL. In this study, we have evaluated the immunological profile of individuals living in risk areas for VL, especially asymptomatic individuals and patients with active VL.
Our data showed an increase of IL-8 plasmatic levels, a strong neutrophil chemotatic and activating cytokine, in active VL patients (Fig. 1a). It is known that the migration of different leucocyte subpopulations to the environment of inflammatory response in active disease is linked to chemokine production [8,16]. This chemokine has also been associated with the anti-apoptotic function of neutrophils induced by Leishmania[17].
In accordance with previous observations [5–8], our data clearly demonstrate simultaneous elevation of both plasmatic and intracytoplasmatic levels of IFN-γ and IL-10 in patients with active VL, as determined by ex vivo and after short-term in vitro cultures, respectively (Fig. 1b, 1e, 2a,d and Table 2). IFN-γ is the most potent cytokine for the induction of macrophage activation for the leishmanicidal state [18]. We evaluated the main cellular sources involved in IFN-γ production, and found a similar contribution of cells involved in both innate and adaptive immune response (Table 1). On the other hand, the synthesis of IL-10 was particularly restricted to CD8+ T cells and B lymphocytes (Table 1). The role that these cells play in human leishmaniasis is still unclear. It has been described that during active VL, immunodominance of the CD8+ T cells could play an important role in disease progression through the significant secretion of IL-10, with a less apparent role of CD4+ T cells in determining the ultimate outcome of the infection in humans [19,20]. In this context, B lymphocytes have also been implicated as IL-10-producing cells, suggesting their involvement in the modulation of the immune response through antigen presentation and expression of co-stimulatory molecules driving the T cell-mediated immune response [21–24].
We postulate that the production of IL-10 could influence the outcome of the infection by its direct action on the macrophages. This includes the down-regulation of cytokine production, mainly TNF-α, and other metabolic events associated with macrophage activation, such as reactive nitrogen species [5,13,19]. Perhaps more important could be the powerful effects of IL-10 on partially inhibiting IFN-γ production and blocking the IFN-γ-mediated activation of macrophages to kill intracellular parasites [4,5,13]. Supporting this hypothesis were the ordinary plasmatic levels of nitrite and nitrate observed in ACT (Fig. 3). Furthermore, the plasmatic and intracytoplasmatic levels of IL-12, a key cytokine involved on the development of specific type 1 T cell-mediated immunity [3,25], remained unaltered in these individuals (Figs 1i and 2c, respectively).
Despite the high plasmatic levels of TNF-α (Fig. 1c), decreased frequencies of TNF-α+ leucocytes were observed in ACT (Fig. 2b) due mainly to the low numbers of circulating TNF-α+ monocytes, the major cell population associated with this phenomenon. We believe that other immunological compartments could account for the high plasmatic levels of TNF-α. However, as monocytes are known to be an important source of TNF-α and also the main targets of Leishmania, we suggest that impairment in the production of TNF-α by monocytes can be implicated as one of the fundamental mechanisms for Leishmania survival during disease onset.
A similar role of IL-4 by blocking the IFN-γ function during active VL may be suggested, as our data demonstrated a higher frequency of IL-4+ neutrophils and CD8+ T lymphocytes in ACT (Table 1), despite basal levels of IL-4 detected at the plasmatic level and by absolute counts of IL4+ leucocytes (Figs 1g and 2e, respectively). These findings indicated the importance of cytokine detection at single-cell level and are consistent with the involvement of neutrophils [17] and type 2 CD8+ T-cells [19,20] on immunological mechanisms that hold back leishmanicidal events during VL.
Consistent with our findings of increased plasmatic levels of IL-6 (Fig. 1d) are the hallmarks of active VL, such as polyclonal activation of B cells and hypergamaglobulinaemia with increased levels of circulating immune complexes [8,9,26,27].
Interestingly, our data demonstrated that AS individuals presented a cytokine profile similar to that observed for NI subjects (Figs 1 and 2). However, it is important to note that in vitro, after antigen-specific short-term stimuli, AS individuals presented a distinct cytokine profile when their innate immune response was analysed at single-cell level [28]. We have shown previously that cells of innate immune response from NI individuals are capable of producing the type 2 pattern of response, characterized by the increased frequency of IL-4+ and IL-10+ cells, as well as low levels of TNF-α+ cells [28]. On the other hand, AS were distinguished by a mixed type 1/type 2 immune profile characterized by increased levels of IFN-γ+ cells as well as a high frequency of IL-12+ cells with simultaneous increase of IL-4+ cells and high levels of IL-10+ cells [28]. Taken together, a basal cytokine pattern, similar to that observed for NI, can be identified by ex vivo and control culture protocols. However, as described above, a mixed type 1/type 2 immune profile demonstrated the ability of AS to respond to Leishmania products differently to uninfected individuals.
In conclusion, these findings are relevant to understanding of the dynamic of immunological events associated with the different clinical status of human VL. We have demonstrated here that outstanding inflammatory profiles, especially driven by IL-8, IFN-γ, TNF-α and IL-6 cytokines, are important for the clinical status changes observed in active VL. On the other hand, to guarantee the survival and persistence of Leishmania during active disease the establishment of a regulatory profile is critical, triggered by lowering frequency of TNF-α+ monocytes and unaltered plasmatic levels of nitrite and nitrate. In addition, our data support the hypothesis that the ability of IL-10 in inhibiting anti-leishmanial macrophage activation seems to be the major mechanism associated with active disease.
Acknowledgments
This work was supported by CPqRR/FIOCRUZ and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). We thank Anna Carolina Lustosa Lima from Centro de Pesquisas René Rachou, Fundação Oswaldo Cruz for statistical support.
References
- 1.Kharazmi A, Kemp K, Ismail A, et al. T-cell response in human leishmaniasis. Immunol Lett. 1999;65:105–8. doi: 10.1016/s0165-2478(98)00132-1. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho EM, Badaró R, Reed SG, Jones TC, Johnson WD. Absence of gamma interferon and interleukin-2 production during active visceral leishmaniasis. J Clin Invest. 1985;76:2066–9. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacellar O, D’Oliveira A, Jr, Jerônimo S, Carvalho EM. IL-10 and IL-12 are main regulatory cytokines in visceral leishmaniasis. Cytokine. 2000;12:1228–31. doi: 10.1006/cyto.2000.0694. [DOI] [PubMed] [Google Scholar]
- 4.Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. 2001;166:1912–20. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- 5.Ghalib HW, Piuvezam MR, Skeiky YAW, et al. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karp CL, El-Safi SH, Wynn TA, et al. In vivo cytokine profiles in patients with kala-azar. Marker elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–8. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldas A, Favali C, Aquino D, et al. Balance of IL-10 and interferon-γ plasma levels in human visceral leishmaniasis: implications in the pathogenesis. BCM Infect Dis. 2005;5:113–21. doi: 10.1186/1471-2334-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raziuddin S, Abdalla RE, El-Hag El-Awad, Al-Janadi M. Immunoregulatory and proinflammatory cytokine production in visceral and cutaneous leishmaniasis. J Infect Dis. 1994;170:1037–40. doi: 10.1093/infdis/170.4.1037. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Poll T, Zijlstra EE, Mevissen M. Interleukin-6 during active visceral leishmaniasis and after treatment. Clin Immunol Immunopathol. 1995;77:111–4. doi: 10.1016/0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros IM, Castelo A, Salomão R. Presence of circulating levels of interferon-γ, interleukin-10 and tumor necrosis factor-α in patients with visceral leishmaniasis. Rev Inst Med Trop São Paulo. 1998;40:31–4. doi: 10.1590/s0036-46651998000100007. [DOI] [PubMed] [Google Scholar]
- 11.Lagler H, Willheim M, Traunmuller F, et al. Cellular profile of cytokine production in a patient with visceral leishmaniasis: γδ+ T cells express both type 1 cytokines and interleukin-10. Scand J Immunol. 2003;57:291–5. doi: 10.1046/j.1365-3083.2003.01223.x. [DOI] [PubMed] [Google Scholar]
- 12.Gantt KR, Goldman TL, McCormick ML, et al. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol. 2001;167:893–901. doi: 10.4049/jimmunol.167.2.893. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Ghosh S, Jhonson PL, Bhattacharya SK, Majumdar S. Immunomodulatory role of interleukin-10 in visceral leishmaniasis: defective action of protein kinase C-mediated signal transduction events. Infect Immun. 2001;69:1499–507. doi: 10.1128/IAI.69.3.1499-1507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen R, Lowe L, Wilson JD, et al. Simultaneous quantification of six cytokines in a single sample using microparticle-based flow cytometric technology. Clin Chem. 1999;9:1693–4. [PubMed] [Google Scholar]
- 15.Miranda KM, Espey MG, Wink DA. A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide Biol Chem. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 16.Brenier-Pinchart MP, Pelloux H, Derouich-Guergour D, Ambroise-Thomas P. Chemokines in host–protozoan–parasite interactions. Trends Parasitol. 2001;17:292–6. doi: 10.1016/s1471-4922(01)01902-x. [DOI] [PubMed] [Google Scholar]
- 17.Aga E, Katschinski DM, Zandbergen GV, et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho EM, Bacellar O, Brownell C, Regis T, Coffman RL, Reed SG. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–56. [PubMed] [Google Scholar]
- 19.Holaday BJ, Pompeu MML, Jerônimo S, et al. Potential role for interleukin-10 in the immunosupression associated with kala-azar. J Clin Invest. 1993;92:2626–32. doi: 10.1172/JCI116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holaday BJ. Role of CD8+ T cells in endogenous interleukin-10 secretion associated with visceral leishmaniasis. Men Inst Oswaldo Cruz. 2000;95:217–20. doi: 10.1590/s0074-02762000000200013. [DOI] [PubMed] [Google Scholar]
- 21.Amigorena S, Bonnerot C. Role of B-cell and Fc receptors in the selection of T-cell epitopes. Curr Opin Immunol. 1998;10:88–92. doi: 10.1016/s0952-7915(98)80037-x. [DOI] [PubMed] [Google Scholar]
- 22.Brown DR, Reiner SL. Polarized helper-T-cell responses against Leishmania major in the absence of B cells. Infect Immun. 1999;67:266–70. doi: 10.1128/iai.67.1.266-270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smelt SC, Cotterell SEJ, Engwerda CR, Kaye PM. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164:3681–8. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 24.Van Essen D, Kikutani H, Gray D. CD40 ligant-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–3. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 25.Ghalib HW, Whittle JA, Kubin M, Hashim FA, El-Hassan AM. Interleukin 12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 26.Galvão-Castro B, As Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clin Exp Immunol. 1984;56:58–66. [PMC free article] [PubMed] [Google Scholar]
- 27.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–6. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 28.Peruhype-Magalhães V, Martins-Filho OA, Prata A, et al. Immune response in human visceral leishmaniasis: analysis of the correlation between innate immunity cytokine profile and disease outcome. Scand J Immunol. 2005;62:487–95. doi: 10.1111/j.1365-3083.2005.01686.x. [DOI] [PubMed] [Google Scholar]