Abstract
Macrophages/monocytes and the proinflammatory mediators, such as tumour necrosis factor (TNF)-α, prostaglandin E2 (PGE2), macrophage inflammatory protein (MIP)-1α and MIP-1α, play a critical role in the progression of immunological disorders including rheumatoid arthritis, Behçet’s disease and Crohn’s disease. In addition, the nicotinic acetylcholine receptor-α7 (α7nAChR) subunit is an essential regulator of inflammation. In this study, we evaluated the expression of the α7nAChR subunit on human peripheral monocytes and the effect of nicotine on the production of these proinflammatory mediators by activated monocytes. Fluorescein isothiocyanate (FITC)-labelled α-bungarotoxin demonstrated the cell surface expression of the α7nAchR subunit. Pretreatment with low-dose nicotine caused inhibition of TNF-α, PGE2, MIP-1α and MIP-1α production, and mRNA expression of TNF-α, MIP-1α and MIP-1α and COX-2 in lipopolysaccharide (LPS)-activated monocytes. These suppressive effects of nicotine were caused at the transcriptional level and were mediated through α7nAChR. Nicotine suppressed the phosphorylation of I-κB, and then inhibited the transcriptional activity of nuclear factor-κB. These immunosuppressive effects of nicotine may contribute to the regulation of some immune diseases.
Keywords: monocytes, nicotinic acetylcholine receptor, NF-κB
Introduction
Macrophages/monocytes play a critical role in the innate immune response and nuclear factor (NF)-κB is activated by components of microorganisms such as lipopolysaccharide (LPS), peptidoglycan, dsRNA and others via Toll-like receptors (TLRs). The signalling cascade downstream of the NF-κB activation, including I kappa B kinase (IKK) activation followed by I-κB phosphorylation, NF-κB translocation and subsequent gene transcription, is a common pathway with T cell receptors (TCR) and B cell receptors (BCR). However, upstream signalling cascades leading to NF-κB activation are different between TCR/BCR stimulation and TLR stimulation. These findings suggest that multiple receptor systems are able to converge on NF-κB and the acquired and innate immune responses share the downstream cascade.
Some immunological disorders show key roles of macrophages/monocytes in progressive inflammation, and the production of proinflammatory cytokines including interleukin (IL)-1, IL-6, tumour necrosis factor (TNF)-α and chemokines necessary for Th1 cell accumulation such as macrophage inflammatory protein (MIP)-1 [1–3]. Moreover, the innate immune response via TLRs and subsequent T cell-mediated acquired immune responses may contribute to the pathogenesis of some autoimmune inflammatory diseases [4,5], which suggests that the regulation of proinflammatory mediators produced by macrophages/monocytes is important in overcoming the progression of inflammation. NF-κB regulates the production of proinflammatory mediators at the level of gene transcription and, thus, the modulation of NF-κB activity is critical for the control of both antigen-specific and innate immune responses in these pathogenic conditions.
Nicotine, a major constituent of cigarette smoke and also a selective cholinergic agonist, has been demonstrated to inhibit systemic inflammation and chronic inflammatory bowel diseases [6,7]. Nicotine blocks endothelial cell activation and leucocyte recruitment [8], suppresses dendritic cell function leading to Th1 priming [9,10] and inhibits LPS-induced cardiac apoptosis [11]. Much research finding that nicotine alters gene expression in endothelial cells has been accumulated and one of the most important molecules down-regulated by nicotine is NF-κB [12]. However, the regulatory pathways involved in human macrophages/monocytes remain to be defined. Recent studies have suggested the effect of nicotine on macrophages. Vagus nerve stimulation attenuates the inflammatory response and acetylcholine, which is one of the principle parasympathetic neurotransmitters, significantly inhibits the release of proinflammatory cytokines in the activated macrophage culture [13]. In addition, the cholinergic anti-inflammatory pathway has major implications in the immune response and the nicotinic acetylcholine receptor-α7 (α7nAChR) subunit is essential for inhibiting proinflammatory cytokine synthesis by this pathway [14].
In this study, we evaluated functional α7nAChR expression on the cell surface of human monocytes and the effects of nicotine through α7nAChR on the production of proinflammatory mediators by activated monocytes. In addition, we investigated the influence of α7nAChR signalling by nicotine on the NF-κB activation and demonstrate the anti-inflammatory mechanisms that suggest a therapeutic application for immunological disorders of nicotine.
Materials and methods
Reagents
Nicotine was purchased from Sigma (St Louis, MO, USA) and 10−3 M solution was prepared in phosphate-buffered saline (PBS), neutralized to pH 7·2 with HCl, which was prepared freshly prior to each experiment. LPS was also purchased from Sigma and 1 mg/ml stock solution was prepared in PBS. Phorbol myristate acetate (PMA) was purchased from Sigma and 100 µg/ml solution was prepared in dimethyl sulphoxide (DMSO). Purified α-bungarotoxin and fluorescein isothiocyanate (FITC)-conjugated α-bungarotoxin were purchased from Sigma.
Monocyte preparation and cell culture
Peripheral blood was obtained from healthy volunteer non-smokers. Fresh blood, obtained by venipuncture, was layered over Ficoll-Hypaque and sedimented. The peripheral blood mononuclear cells were treated with microbeads conjugated with anti-human CD11b antibody (Miltenyi Biotec, Auburn, CA, USA), and monocytes were separated from the mononuclear cell populations using positive selection column by auto-magnetic affinity cell sorting (MACS) apparatus according to the manufacturer’s protocol (Miltenyi Biotec). The resultant monocytes obtained were more than 99% positive for CD16 and CD14 and were used following experiments. The purified human peripheral monocytes and human monocytic cell line U937 were cultured in RPMI-1640 (Life Technologies, Inc., Gaithersburg, MD, USA) containing 10% heat-inactivated fetal calf serum (FCS) (Life Technologies, Inc.), l-glutamine (2 mM), penicillin (100 µg/ml) and streptomycin (0·1 µg/ml) at 37°C in 5% CO2 atmosphere.
In some experiments, the monocytes were stimulated with 1 µg/ml LPS for appropriate time with or without preincubation with nicotine (10−7−10−8 M) for 1 h. The viability of monocytes, assessed by trypan blue exclusion test, was shown to exceed 95% regardless of treatment condition at 24 h after stimulation with LPS. α-Bungarotoxin alone did not influence the viability and proliferation of monocytes, so we did not assess the further effects of it alone in each experiment.
Flow cytometric and confocal laser microscopic analyses
α-Bungarotoxin was used to detect the cell surface nAChRα7 subunit on monocytes and two-colour staining was performed. Single-cell suspensions of monocytes were incubated with FITC-conjugated α-bungarotoxin and phycoerythrin (PE)-conjugated anti-human CD14 antibody (BD PharMingen, San Diego, CA, USA) or a mixture of FITC–CD3 and FITC–CD20 antibodies (BD Pharmingen) and PE-conjugated control mouse IgG2a (BD PharMingen). Thereafter, the cells were analysed by a flow cytometer [BD fluorescence activated cell sorter (FACS) system, Becton-Dickinson, San Jose, CA, USA]. The cell surface expression of nAChRα7 subunit on monocytes was also examined using a confocal laser microscope (LSM 510, Carl Zeiss, Jena, Germany).
Reverse transcription–polymerase chain reaction (RT–PCR)
The monocytes were incubated with LPS for 4 h with or without preincubation with nicotine/α-bungarotoxin and total RNA was extracted. Then 500 ng of total RNA was reverse-transcribed and complementary DNA was synthesized. The primers used and the expected size of amplified PCR products were as follows: α-actin [314 base pairs (bp)] sense TCCTGTGGCATCCACGAAACT and anti-sense GAAGCATTTGCGGTGGACGAT; nAChRα7 subunit (574 bp) sense TTGATAGCCCAGTACTTCGC and anti-sense TGCAGATGATGGTGAAGACC; TNF-α (682 bp) sense GAGCACTGAAAGCATGATCCG and anti-sense AAAGTAGACCTGCCCAGACTCGG; Cox-2 (305 bp) sense TTCAAATGAGATTGTGGGAAAATTGCT and anti-sense AGATCATCTCTGCCTGAGTATCTT; MIP-1α (195 bp) sense CGCCTGCTGCTTCAGCTACACCTCCCGGCA and anti-sense TGGACCCCTCAGGCACTCAGCTCCAGGTCG; MIP-1α (186 bp) sense ACCCTCCCACCGCCTGCTGCT TTTCTTACA and anti-sense GTTCCAGGTCATACACG TACTCCTGGACCC. Cycling parameters were as follows: a hot start at 94°C for 2 min, denaturing at 94°C for 1 min, annealing at 60°C for 1 min and elongation at 72°C for 2 min. The reaction was repeated for 30 cycles and followed by elongation at 72°C for 10 min. The amplified DNA fragments were separated on 2% agarose gels and visualized by staining with ethidium bromide.
Enzyme-linked immunosorbent assay (ELISA) for TNF-α, MIP-1α, MIP-1α and prostaglandin E2 (PGE2)
Monocytes were cultured in the presence of LPS with or without nicotine/α-bungarotoxin. The culture supernatant was collected 24 h after stimulation with LPS, and stored at −20°C until analysed. Concentrations of TNF-α, MIP-1α, MIP-1α and PGE2 in the culture supernatants were determined by commercially available ELISA kits according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA). Statistical differences between treatment conditions were analysed by one-way analysis of variance (anova), with the level of significance set at P < 0·05.
Western blot analysis
The monocytes were cultured in the presence of LPS with or without nicotine/α-bungarotoxin for 30 min and the cells were lysed. The cell lysates were electrophoresed on 7·5% sodium dodecyl sulphate–polyacrylamide gel (SDS-PAGE) and proteins were transferred electrically onto nitrocellulose membranes. The membranes were incubated with anti-I-κBα antibody (Cell Signalling Technology Inc., Danvers, MA, USA) or anti-phosphorylated (Ser32) I-κBα antibody (Cell Signalling Technology Inc.), and followed by incubation with a horseradish peroxidase (HRP)-conjugated second antibody. Detection was performed by enhanced chemiluminescence.
NF-κB luciferase reporter assay
U937 cell line was maintained in RPMI-1640 culture medium and preincubated with 10 nM PMA for 48 h for induction of monocytic differentiation. Thereafter, U937 cells were transfected transiently with the vector pNFκB-Luc containing four tandem copies of the κB enhancer element upstream of the firefly luciferase reporter gene (Clontech, Tokyo, Japan) and pRL-TK Renilla luciferase reporter plasmid (Promega, Tokyo, Japan). At 18 h after transfection, the cells were pretreated with nicotine for 1 h. The cells were then stimulated with 1 µg/ml of LPS and 10 ng/ml of PMA for 6 h. Dual luciferase activity was measured using a Dual-GloTM luciferase reagent (Promega). The experiments were conducted in duplicate and the same experiment was repeated at least three times to confirm reproducibility; representative results are presented.
Results
Nicotinic acetylcholine receptor (nAChR) α7 is expressed on human peripheral monocytes
Recently, a variety of nAChRs have been identified and the anti-inflammatory role of α7 nAChR has been suggested in α7 subunit knock-out mice. We investigated whether the nAChRα7 was expressed on the cell surface of human peripheral monocytes. Positive selection using microbeads-conjugated anti-CD11b and a magnetic cell sorter (autoMACS apparatus) enabled us to purify the monocytes, more than 99% of which were positive for CD14 and CD11b. Flow cytometric analysis demonstrated that FITC-conjugated α-bungarotoxin bound to purified human peripheral monocytes (93·1%), suggesting the expression of nAChRα1, α7 and α9, to which α-bungarotoxin can bind selectively (Fig. 1a,b). Similarly, confocal laser microscopic analysis confirmed cell surface nAChR expression on CD14 positive human monocytes using FITC-conjugated α-bungarotoxin (Fig. 1c–e). However, the α-bungarotoxin-positive CD14 cells using whole blood were 51·7 ± 17·6% (mean ± s.d., n = 6), suggesting the possibility that the positive selection using CD11b antibody stimulates human monocytes and may up-regulate the expression of nAChRs.
Fig. 1.
Expression of nicotinic acetylcholine receptor (nAChR) α7 on cell surface of human peripheral monocytes. (a) Two-colour staining for monocyte marker (CD14) and α7 nAChR using fluorescein isothiocyanate (FITC)-conjugated α-bungarotoxin by flow cytometry. (b) Control staining of (a). (c) Confocal laser microscopic image of cell surface CD14 on human peripheral monocytes using phycoerythrin (PE)-conjugated anti-CD14 antibody. (d) Confocal image of α7 nAChR expression on cell surface of the monocytes. (e) Merged image of cell surface CD14 (a) and α7 nAChR (b) expressions. (f) Messenger RNA expression of human peripheral blood monocytes (PBMC) from non-smokers and human monocytic cell line (U937) by reverse transcription–polymerase chain reaction.
We next examined the mRNA expression of nAChRα7 subunit by RT–PCR. The U937 human monocytic cell line and purified human peripheral monocytes expressed nAChRα7 mRNA (Fig. 1f). On the contrary, human peripheral lymphocytes expressed α3, α5 and α4 subunit mRNA (data not shown). Taking into consideration that monocytes/macrophages generally expressed neither α1 nAChR nor α9 subunit [14], our findings indicate that human peripheral monocytes specifically expressed α7 nAChR.
α7 nAChR stimulation by nicotine inhibited production of proinflammatory mediators by LPS-stimulated human peripheral monocytes
We evaluated the production of proinflammatory mediators by human monocytes and whether nicotine influences monocyte functions. LPS-stimulated human peripheral monocytes produce TNF-α, MIP-1α, MIP-1α and PGE2 (Fig. 2a–e). However, the production of TNF-α, MIP-1α and MIP-1α by monocytes was inhibited significantly by preincubation with nicotine 10−8 M. In addition, nicotinic suppression of production was prevented completely by the addition of α-bungarotoxin, which is an antagonist of nicotine and binds to α7 nAChR with high affinity. These findings suggest the functional cell surface expression of α7 nAChR on human monocytes and that the production of proinflammatory mediators by LPS-stimulated monocytes is inhibited by nicotine through the α7 nAChR signalling pathway. On the contrary, PGE2 production was inhibited by nicotine and prevented by α-bungarotoxin to a lesser degree, suggesting that PGE2 production and its regulation may be different from those of the other proinflammatory mediators (Fig. 2b). To examine further the mechanisms of nicotinic regulation of proinflammatory mediator production by monocytes, we investigated mRNA expression. RT–PCR demonstrated that LPS enhanced mRNA expression of TNF-α, MIP-1α and COX-2 (Fig. 2e). MIP-1α mRNA expression was expressed even in the non-stimulated human monocytes and was not enhanced by LPS stimulation. Nicotinic stimulation (10−8 M) completely inhibited the mRNA expression of TNF-α, MIP-1α and MIP-1α, but not COX-2, confirming the results obtained by ELISA, and suggesting that the nicotinic regulation of TNF-α, MIP-1α and MIP-1α is occurring upstream of the transcriptional level.
Fig. 2.
Nicotinic effects on the production of proinflammatory mediators by human monocytes. (a–d) Tumour necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1α, MIP-1α and prostaglandin E2 (PGE2) release from monocytes were measured using enzyme-linked immunosorbent assay (ELISA). Lipopolysaccharide (LPS)-stimulated monocytes induce tumour necrosis factor (TNF)-α (a), PGE2 (b), MIP-1α (c) and MIP-1α (d) production, which were inhibited significantly by nicotine 10−8 M (P < 0·01). The nicotinic suppression of proinflammatory mediators produced by monocytes was prevented by addition of α-bungarotoxin (BTX). (e) Messenger RNA expression of COX-2, TNF-α, MIP-1α and MIP-1α in monocytes using reverse transcription–polymerase chain reaction. MIP-1α mRNA was expressed at high level even in the non-stimulated monocytes. Lipopolysaccharide enhanced the mRNA expression of COX-2, TNF-α and MIP-1α but not MIP-1α. Nicotine (10−8 M) completely inhibited the mRNA expression of TNF-α, MIP-1α and MIP-1α, and partially that of COX-2.
Nicotine inhibits phosphorylation of I-κBα and suppresses the transcriptional activity of NF-κB
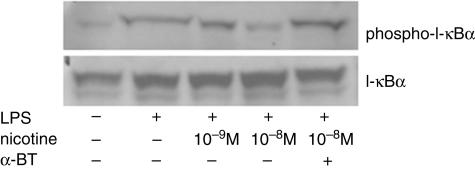
Activation of NF-κB is caused by phosphorylation on specific serine residues of the inhibitory protein I-κB by IKK, which then allows the active form of NF-κB to translocate into the nucleus to initiate gene transcription. To identify the mechanisms by which the transcription of the proinflammatory mediators is suppressed by nicotine, we investigated the effect of nicotine on IKK activity by evaluating I-κBα phosphorylation. In addition, we investigated the effect of nicotine on I-κBα degradation by detecting the I-κBα protein levels. The monocytes were pretreated with nicotine for 1 h or left without nicotine pretreatment and then activated with LPS for 30 min. Thereafter cytosolic proteins of the monocytes were extracted. The protein levels of I-κBα were not changed by LPS stimulation, regardless of pretreatment with nicotine (Fig. 3). However, phosphorylation of I-κBα was induced by LPS stimulation. The LPS-induced I-κB phosphorylation was inhibited by pretreatment with nicotine, which was prevented by the addition of α-bungarotoxin. These findings suggest that the inhibitory effect of nicotine is due to the suppression of IKK activity and the resultant inhibition of I-κBα phosphorylation, followed by decreased NF-κB activation.
Fig. 3.
Nicotinic effects on I-κBα phosphorylation and degradation in lipopolysaccharide (LPS)-stimulated human monocytes. Cytosolic proteins were extracted 30 min after LPS stimulation with or without pretreatment with nicotine/α-bungarotoxin (BTX) and analysed by Western blotting using specific antibodies against I-κBα and phosphorylated (Ser32) I-κBα. Nicotine (10−8 M) strongly inhibited the phosphorylation (Ser32) of I-κBα, and the inhibition was reversed by the treatment with α-bungarotoxin (BTX).
Nicotine inhibits the transcriptional activity of NF-κB
To confirm the suppressive effect of nicotine on NF-κB activation, we performed a luciferase assay and evaluated the transcriptional activity of NF-κB in the presence of nicotine. We tested a construct that contained four tandem repeats of the NF-κB element linked to the reporter gene luciferase in the transient expression system (Fig. 4). The NF-κB luciferase vector was transfected into the U937 cells, which had been cultured with PMA for 2 days to induce monocytic differentiation. The transfected cells were then treated with LPS for 6 h. pRL-TK vector was co-transfected with the NF-κB-luciferase vector. Treatment of the transfected U937 cells with LPS increased luciferase activity reproducibly (Fig. 4). Pretreatment with nicotine of the transfected U937 cells yielded a reproducibly reduced luciferase activity in response to LPS stimulation.
Fig. 4.
Nicotinic effect on the transcriptional activity of nuclear factor (NF)-κB in human monocytic U937 cells. U937 cells were cultured with phorbol myristate acetate to induce monocytic differentiation. The cells were co-transfected with the pNFκB-Luc containing four tandem copies of the κB enhancer element and phRL-TK; 18 h after transfection, half the cells were stimulated with lipopolysaccharide (LPS) for 12 h, and the remainder was kept unstimulated. phRL-TK was used to compare the transfection efficiency, and accordingly the luciferase activity of the promoter assays was corrected. We found that pNFκB-Luc responded reproducibly to LPS stimulation, whereas pretreatment of the cells with nicotine (10−8 M and 10−7 M) reduced luciferase activity. The results shown are representative of five independent experiments with essentially the same result.
Discussion
nAChRs have been studied in neuronal synapses and neuromuscular junctions and 10 α (α1–10), four β (β1–4), γ, δ and ɛ subunits have been identified. In this study, flow cytometric and confocal laser microscopic analyses demonstrated that FITC-labelled α-bungarotoxin bonds to the cell surface of human monocytes. In addition, mRNA of α7nAChR was detected in human peripheral monocytes and the U937 human monocytic leukaemia cell line. In a sharp contrast with monocytes, human lymphocytes expressed α3, α5 and α4 subunits (data not shown). In general, α-bungarotoxin binds to nicotinic α1, α7 and α9 subunits but macrophages/monocytes do not express the α9 subunit, and therefore monocytes are deficient in α9nAChR [14]. Similarly, macrophages/monocytes failed to express the δ subunit, which is necessary for α1 heteropentameric nAChR, indicating that monocytes lack α1nAChR [14,15]. Thus, these findings confirmed the specific cell surface expression of α7nACR on human monocytes. Subsequent experiments in this study using α-bungarotoxin demonstrated the competition for binding to α7nAChR with nicotine, also suggesting the functional α7nAChR expression on human monocytes, and that the nicotinic modulation of human monocyte activation is mediated by α7nAChR.
Stimulation of human macrophages with LPS induces the transcription of a number of proinflammatory cytokines. In this study, we demonstrated LPS-induced COX-2 expression and PGE2 production in addition to the release of proinflammatory cytokines (TNF-α) and chemokines (MIP-1α and MIP-1α) by human monocytes. In addition, nicotinic stimulation through α7nAChR significantly inhibited LPS-induced production of proinflammatory mediators. According to a previous report [13], acetylcholine and muscarine as well as nicotine inhibited the TNF-α production by LPS-stimulated human macrophages, suggesting the physiological anti-inflammatory effects of these neurotransmitters through α7nAChR on human monocytes. However, the influence of nicotine on COX-2 expression and PGE2 production is controversial. In the rat RAW264·7 macrophage cell line, nicotine does not influence the LPS/IFN-γ-induced COX-2 expression and PGE2 production [16]. Nicotine, on the other hand, enhances COX-2 and PGE2 in spite of the down-regulation of TNF-α in LPS-stimulated rat microglial culture [17]. Moreover, nicotine and smokeless tobacco extract increase PGE2 production but decrease IL-1α release from LPS-treated human gingival and peripheral blood monocytes [18,19]. In contrast, nicotine inhibits both COX-2 and IL-1 expression in the LPS-stimulated human monocytic cell line U937 [20]. One possible explanation accounting for these different results is the concentration of nicotine used in these experiments. We and others who have observed the inhibiting effects of nicotine on COX-2 expression and PGE2 production used low, pharmacological concentrations of nicotine (less than 10 µM) [20]. Taken together, pharmacological concentrations of nicotine have an inhibiting effect on COX-2 and PGE2 in human monocytes and the effects are strictly cell type-specific.
In this study, nicotine inhibited I-κB phosphorylation and subsequent transcriptional activity of NF-κB of the TNF-α promoter. It is unknown whether nicotine modulates the transcription/activation of I-κB kinases (IKK) or inhibits an upstream mediator that prevents I-κB ubiquitination and subsequent degradation. Microarray analysis of nicotine-induced change in gene expression in endothelial cells revealed that nicotine up-regulated expression of the cAMP response element binding protein (CREB), but down-regulated NF-κB expression [12]. The activation of CREB by phosphorylation as a downstream event in the mitogen-activated protein (MAP) kinase cascade activated by nicotine has been well documented in neurones [21–23]. However, the interaction of CREB and NF-κB is unknown and needs to be elucidated. A recent report demonstrated that cAMP-dependent protein kinase (PKA) plays a critical role as a downstream effector to inhibit the NF-κB pathway of BCR and TLR-4 signalling in B lymphocytes [24]. Moreover, nicotine activates the Jak2-STAT3 signalling pathway through α7nAChR and a possible interaction with phosphorylated STAT3 and the NF-κB pathway has been shown in macrophages [25]. Indeed, STAT3 is a negative regulator of the inflammatory response and activation of STAT3 is required for the anti-inflammatory properties of IL-10 [26]. However, IL-10 production is not induced by nicotine from macrophages/monocytes (data not shown) [25], and the deactivation of macrophage induced by vagus nerve stimulation is caused similarly in IL-10-deficient mice, suggesting that molecular mechanisms exerting the anti-inflammatory effect of nicotine through α7nAChR mimics the signalling pathway of IL-10/IL-10R without the requirement of IL-10 itself.
IL-10 signalling suppresses nuclear localization and activation of NF-κB, leading to the inhibition of proinflammatory cytokine (IL-1α, IL-6, IL-8 and TNF-α) gene transcription in human monocytes [27]. STAT3 serves as a dominant-negative inhibitor of NF-κB DNA-binding activity to suppress the proinflammatory cytokine induction via direct interaction with NF-κB p65 [28]. IL-10 signalling also modulates TNF-α mRNA translation by inhibition of the p38/MAPK-activated protein kinase-2 pathway [29]. Thus, IL-10 signalling inhibits I-κB phosphorylation through the suppression of IKK complex activities and NF-κB DNA binding activity [30], and also blocks AU-rich element (ARE)-dependent mRNA translation [29]. Therefore, it is possible that nicotine/α7nAChR signalling leading to STAT3 activation shares a common signalling pathway with IL-10. Nicotine, on the contrary, does not decrease mRNA levels of TNF-α, suggesting that it controls the cytokine at the post-transcriptional level without affecting intracellular mRNA levels. Furthermore, nicotine inhibits high mobility group box 1 (HMGB1) secretion at a post-translational level by modulating HMGB1 acetylation or phosphorylation [7]. Thus, evidence has been accumulating that nicotine controls the proinflammatory mediator production at the translational and post-translational levels. In this study, however, we observed the decrease of proinflammatory mediator mRNAs by physiological concentrations of nicotine in LPS-stimulated human monocytes, as has been observed by others, in the U937 human monocyte cell line [20]. One possible explanation accounting for the differences is the concentration of nicotine used in these experiments, as mentioned above. In addition, a very recent study has demonstrated that LPS/TLR4 signalling induces IKK activation, which leads eventually to the NF-κB translocation and activation, while LPS/TLR4 also induces TNF-α production through the TRIF–IRF3 pathway independently of IKK and the TNF-α positively regulates IKK activity through the TNF receptor-mediated pathway [31]. These two signalling pathways, from LPS/TLR4 to the IKK and downstream NF-κB activation, may be convenient to explain the complex regulatory mechanisms of both NF-κB activation and TNF-α production without transcriptional regulation by the different concentrations of nicotine. Taken together, the pharmacological concentration of nicotine through α7nAChR may inhibit IKK activity and may also suppress NF-κB DNA-binding activity by direct interaction with activated STAT3.
In this study, we have demonstrated that pharmacological concentrations of nicotine cause the inhibition of proinflammatory mediator production, which might be a result of the inhibition of IKK activity, and subsequent suppression of I-κB phosphorylation in addition to suppression of the NF-κB DNA binding activity in human monocytes. This supports the therapeutic use of nicotine in some inflammatory diseases; the NF-κB activation pathway is one of the most critical molecular targets of nicotine therapy.
References
- 1.Stuhlmuller B, Ungethum U, Scholze S, et al. Identification of known and novel genes in activated monocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:775–90. doi: 10.1002/1529-0131(200004)43:4<775::AID-ANR8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Nagafuchi H, Takeno M, Yoshikawa H, et al. Excessive expression of Txk, a member of Tec family tyrosine kinases, contributes to excessive Th1 cytokine production by T lymphocytes in patients with Behçet’s disease. Clin Exp Immunol. 2005;139:363–70. doi: 10.1111/j.1365-2249.2004.02688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura Y, Kurokawa MS, Yoshikawa H, et al. Involvement of Th1 cell and heat shock protein 60 in the pathogenesis of intestinal Behçet’s disease. Clin Exp Immunol. 2005;139:371–8. doi: 10.1111/j.1365-2249.2005.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwahashi M, Yamamura M, Aita T, et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–67. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 5.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sykes AP, Brampton C, Klee S, Chander CL, Whelan C, Parsons ME. An investigation into the effect and mechanisms of action of nicotine in inflammatory bowel disease. Inflamm Res. 2003;49:311–9. doi: 10.1007/s000110050597. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 8.Saeed RW, Varma S, Peng-Nemeroff T, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2004;201:1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinet E, Yoshida K, Nouri-Shirazi M. Nicotine environment affects the differentiation and functional maturation of monocytes derived dendritic cells (DCs) Immunol Lett. 2004;95:45–55. doi: 10.1016/j.imlet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol. 2005;175:2684–91. doi: 10.4049/jimmunol.175.4.2684. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki J, Bayna E, Molle ED, Lew WYW. Nicotine inhibits cardiac apoptosis induced by lipopolysaccharide in rats. J Am Coll Cardiol. 2003;41:482–7. doi: 10.1016/s0735-1097(02)02820-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Day INM, Te S. Microarray analysis of nicotine-induced changes in gene expression in endothelial cells. Physiol Genom. 2001;5:187–92. doi: 10.1152/physiolgenomics.2001.5.4.187. [DOI] [PubMed] [Google Scholar]
- 13.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 15.Ulloa L. The vagus nerve and the nicotine anti-inflammatory pathway. Nat Rev. 2005;4:673–84. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 16.Chen YC, Shen SC, Lin HY, Tsai SH, Lee TJF. Nicotine enhancement of lipopolysaccharide/interferon-gamma-induced cytotoxicity with elevating nitric oxide production. Toxicol Lett. 2004;153:191–200. doi: 10.1016/j.toxlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Simone RD, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflamm. 2005;4:1–10. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne JB, Johnson GK, Reinhardt RA, Dyer JK, Maze CA, Dunning DG. Nicotine effects on PGE2 and IL-1beta release by LPS-treated human monocytes. J Periodont Res. 1996;31:99–104. doi: 10.1111/j.1600-0765.1996.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 19.Bernzweig E, Payne JB, Reinhardt RA, Dyer JK, Patil KD. Nicotine and smokeless tobacco effects on gingival and peripheral blood mononuclear cells. J Clin Periodontol. 1998;25:246–52. doi: 10.1111/j.1600-051x.1998.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 20.Sugano N, Shimada K, Murai S. Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun. 1998;252:25–8. doi: 10.1006/bbrc.1998.9599. [DOI] [PubMed] [Google Scholar]
- 21.Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–65. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–98. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- 23.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–41. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 24.Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T. Adenosine and cAMP are potent inhibitors of the NF-kappaB pathway downstream of immunoreceptors. Eur J Immunol. 2005;35:31–41. doi: 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- 25.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 26.Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation − a continuing puzzle. Immunology. 2004;113:281–92. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappaB (NFkappaB) activation in human monocytes. J Biol Chem. 1995;270:9558–63. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 28.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kontoyiannis D, Kotlyarov A, Carballo E, et al. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760–70. doi: 10.1093/emboj/20.14.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schottelius AJG, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–74. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 31.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–61. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]