Abstract
To elucidate the hormonal change and alteration in cytokine expression in peripheral blood mononuclear cells (PBMC) during the early stage of autoimmune thyroiditis, we have developed a canine model of this disease, in which normal dogs were immunized with bovine thyroglobulin (Tg) and/or canine thyroid extract. Serum samples were collected weekly, anti-canine Tg antibody was measured by enzyme-linked immunosorbent assay (ELISA) and thyroid stimulating hormone (TSH) and total T4 levels by radioimmunoassay. We also assayed T lymphocyte proliferation in response to Tg, as well as measuring cytokine mRNA by semiquantitative reverse transcription–polymerase chain reaction (RT–PCR). All six dogs immunized with bovine Tg had both canine Tg autoantibody and anti-T4 antibody. When the sample from the highest TgAA titre time-point was compared with baseline the expression of mRNA encoding the Th1-type cytokine such as interferon (IFN)-γ, interleukin (IL)-18 and IL-15 was increased during the development of autoimmune thyroiditis. Expression of the Th2-type cytokine, IL-6 showed minimal change and IL-4 expression was not detected in any of the samples. Expression of the T suppressive cytokine, IL-10 and transforming growth factor (TGF)-β was increased in the presence of antigen stimulation. These findings suggest that, although autoimmune thyroiditis is an organ-specific autoimmune disease, systemic cytokine mRNA expression is also changed.
Keywords: autoimmune disease, autoimmune thyroiditis, cytokine, dog, PBMC, RT–PCR, Th1:Th2
Introduction
Hypothyroidism is the most common endocrine disease in humans and dogs, with the majority of cases resulting from autoimmune mediated mechanisms [1,2]. Animal models of autoimmune thyroiditis provide the opportunity to understand the development and/or progression of this disorder and to identify disease-associated pathways [3–5]. Immunization-induced models of autoimmune thyroiditis generally consist of immunization of appropriate strains of mice or rats with thyroglobulin (Tg) mixed with a variety of adjuvants. However, the relatively short life span of mice and rats, together with their small body weight and organ size, limit the ability to determine the serial pathophysiological changes associated with autoimmune thyroiditis, as well as limiting the ability to obtain sufficient samples of materials such as blood. There are a number of potential advantages in using dogs rather than mice or rats. Dogs are not inbred and thus have an advantage in replicating some aspects of the human disease. A canine model of autoimmune thyroiditis can therefore serve as a surrogate for studying the immunopathogenesis of the disease and for evaluating the long-term therapeutic effects of new drugs. To date, however, only a few studies have utilized a canine model of autoimmune thyroiditis [6,7].
Cytokines are thought to mediate the initiation and perpetuation of autoimmune thyroiditis, but most of these results derived from in vitro and intrathyroid studies. Although several studies have measured intrathyroidal cytokine gene expression in autoimmune thyroid disease, it is difficult to apply these findings to clinical practice because surgical specimens are obtained mainly at a late stage in the disease [8]. To date, the Th1 : Th2 cytokine balance and cytokine profiles in peripheral blood, in relation tothyroglobulin autoantibody production, during the development of autoimmune thyroiditis have not been determined.
In this study, we describe a canine model of autoimmune thyroiditis in which dogs were immunized with bovine Tg, an easily obtained reagent. We also measured the expression of cytokine mRNA expression in this model.
Materials and methods
Experimental animals
A total of 11 (three beagles and eight mongrels) dogs confirmed to be healthy after routine physical examination, complete blood counts (CBC), serum biochemistry analysis and urinalysis were used under protocols approved by the Institutional Animal Care and Use Committee of Seoul National University. All efforts were made to minimize animal suffering. The dogs were housed separately in metal cages and maintained on commercial dry dog food and water ad libitum. The cages were kept at a constant temperature (22–25°C) and humidity (50–55%). The animals were acclimatized at least 5 weeks prior to use.
Induction of autoimmune thyroiditis
We induced experimental autoimmune thyroiditis by injection of thyroid extract (Te), bovine thyroglobulin (Tg 1, 2 and 3) or both (Bo 1, 2 and 3). The thyroid extracts were prepared from freshly isolated thyroid glands of healthy dogs of different breeds. Thyroid glands with an equal amount (w/v) of distilled water were sonicated at a maximum setting and centrifuged at 20 000 g to remove the plasma membrane and insoluble material. The supernatant was supplemented with 100 mg/ml purified bovine thyroglobulin (Tg; Sigma, St Louis, MO, USA). For induction, the thyroid extract (0·5 ml) and/or Tg was emulsified in Freund's complete adjuvant (1 : 1, v/v) and 1 ml was given subcutaneously (s.c.) to each dog. Subsequent injections on days 14, 28, 42 and 56 were given s.c. using thyroid extract and/or Tg in incomplete Freund's adjuvant. For the dogs in the control group (Cp 1, 2 and 3) all procedures were identical, except for using pancreas extracts as control antigen instead of the thyroid extract or Tg. The other dog was used as non-treated normal control (Cn).
Detection of canine anti-thyroglobulin autoantibody
During the experiment, serum samples were collected once per week and kept frozen at −70°C until analysis of anti-canine thyroglobulin autoantibody by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (VT12; Oxford Biomedical Research, Rochester Hills, MI, USA) according to the manufacturer's instructions. The sample was considered to be positive when the value was > 25% for the TgAA assay; < 10% was counted as negative.
Canine thyroid stimulating hormone (TSH) and total T4 measurement
Serum TSH and total T4 levels were measured by radioimmunoassay using Coat-A-Count kit (canine TSH IRMA (IK9T1) and canine T4 (TKC41), respectively; Diagnostic Products Corp., LA, USA) according to the manufacturer's protocols.
Canine free T4 and anti-T4 antibody titre measurement
Serum samples were collected from the experimental group on day 66. Serum free T4 by equilibrium dialysis and anti-T4 antibody titres were determined by referring to ANTECH Laboratory (LA, USA).
Proliferation assay of T lymphocytes
Peripheral blood mononuclear cells (PBMC) were harvested from approximately 6 ml of venous blood, supplemented with sodium heparin as an anti-coagulant. Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation was performed at 400 g for 30 min. PBMC were washed twice with phosphate-buffered saline (PBS) and adjusted to 5 × 105 viable cells per ml of complete endotoxin-free medium RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS; Boehringer Mannheim, Germany), 100 U/ml penicillin and 100 µg/ml streptomycin. A total of 5 × 104 cells/well was cultured in the presence of 5, 10 and 20 µg/ml bovine thyroglobulin in 96-well plates at 37°C in a humidified 5% CO2 air atmosphere. Different concentrations of bovine thyroglobulin or culture medium were added to the wells for a final volume of 200 µl. After 3 days in culture, a bromodeoxyuridine (BrdU) assay was carried out according to the manufacturer's instructions (cell proliferation ELISA BrdU, colorimetric; Roche Diagnostics, Mannheim, Germany). The reaction was quantified by measuring the optical density at a wavelength of 450 nm and a reference range wavelength of 655 nm. The stimulation index value (mean optical density values of thyroglobulin-stimulated cultures/mean optical density values of medium-only cultures) was calculated for each culture treatment.
Semi-quantitative reverse transcription–polymerase chain reaction (RT–PCR) analysis of cytokines
Total RNA was extracted with the TRIzol (Invitrogen, Carlsbad, CA, USA) from PBMC samples collected weekly, according to the manufacturer's instructions. DNase I was treated to remove traces of contaminating DNA and then eluted in a volume of 60 µl RNase-free water. Semi-quantitative RT–PCR was used to measure multiple cytokine gene expression. Primer sequences and optimal PCR annealing temperatures and cycle numbers are listed in Table 1. Interferon (IFN)-γ F and R primers were designed previously by Chamizo et al. [9]. Reactions were carried out in the PCR Thermal Cycler Dice TP600 (TaKaRa, Otu, Japan). A first cycle of 30 min at 45°C, then 5 min at 94°C, was followed by 30 s at 94°C, 30 s at optimal PCR annealing temperatures and 1 min at 72°C for a number of cycles determined [e.g. 30 cycles for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Table 1]. Normalization of the samples was accomplished using RT–PCR for the housekeeping gene GAPDH to control the efficiency of the RNA extraction, its integrity and the amount of RNA present. After gel electrophoresis, the amount of DNA was quantified as band intensities using GelDoc software from Biorad (Hercules, CA, USA). The relative amount of cytokine mRNA was expressed as the ratio of each cytokine mRNA to GAPDH mRNA.
Table 1.
Reverse transcription–polymerase chain reaction (RT–PCR) primer sequences for GAPDH and cytokines.
| Cytokine (GenBank no.) | Primer sequence (5′– 3′) | Product length (bp) | Annealing temp. (°C) | Cycle |
|---|---|---|---|---|
| GAPDH (AB038240) | F: GGT CAC CAG GGC TGC TTT | 209 | 56 | 30 |
| R: ATT TGA TGT TGG CGG GAT | ||||
| IL-18 (Y11133) | F: ATG GCT GCT AAC CTA ATA | 281 | 52 | 35 |
| R: CTA GTG AGG CTA TCT TTA T | ||||
| IFN-γ (AF126247) | F*: CAT TCA AAG GAG CAT GGA TAC C | 203 | 56 | 40 |
| R*: GAC TCC TTT TCC GCT TCC TTA G | ||||
| IL-10 (U33843) | F: CTC TGT TGC TGC CTG GTC | 303 | 56 | 42 |
| R: CTT GAT GTC TGG GTC GTG | ||||
| IL-12p40 (U49100) | F: GTT GGA CTG GCA CCC TGA T | 385 | 56 | 40 |
| R: GAA GCC TCT GCT ACT TTT GA | ||||
| IL-15 (AF479882) | F: ACT TGC ATC CAG TGC TAC TT | 271 | 56 | 38 |
| R: CGA GCG AGA TAA CAC CTA AC | ||||
| IL-1α (XM_849577) | F: GGC ATT TCG TGT CAG TCA TTG TAG | 396 | 62 | 38 |
| R: GCT GTA GGG TGG GCT TTC | ||||
| IL-6 (AF275796) | F: GGC TAC TGC TTT CCC TAC CC | 223 | 62 | 40 |
| R: CTC CAG TTT GGG AAG ATG TAG GTT | ||||
| TGF-α (L34956) | F: CAG AAT GGC TGT CCT TTG ATG | 392 | 62 | 38 |
| R: CAG GCA GAA GTT AGC GTG GT |
Primer designed previously (Chamizo et al.[9]). bp: Base pairs; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IFN: interferon; IL: interleukin; TGF: transforming growth factor.
Histopathology
On day 128, the thyroid glands were removed surgically from dogs Te, Tg1, Bo2 and Cn, fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 µm, and stained with haematoxylin and eosin (H&E) for histopathology. The severity of thyroiditis was graded according to the standard of Ng et al. [10] on a scale of 0–4 as follows: grade 0, normal histology; grade 1, interstitial accumulation of inflammatory cells distributed around one or two follicles; grade 2, one or more foci of inflammatory cells reaching at least the size of one follicle; grade 3, 10–40% of thyroid replaced by inflammatory cells; and grade 4, more than 40% of thyroid replaced by inflammatory cells. Frozen sections were subjected to immunostaining using polyclonal antibodies specific for CD4+ and CD8+ T cells. Serial 5 µm-thick sections were obtained and washed with PBS, pH 7·2, followed by a treatment with 1% PBS/H2O2 solution for blocking endogenous peroxidase. Unspecific binding was blocked by 10% goat non-immune serum. Anti-canine CD4 and anti-canine CD8 mouse IgGs (Serotec Ltd, Oxford, UK) diluted 1 : 10 and 1 : 30, respectively, were used subsequently and incubated overnight at 4°C. The sections were then incubated with biotinylated secondary antibody (Zymed Laboratories Inc., San Francisco, CA, USA) for 20 min, washed three times in PBS and incubated with streptavidin–peroxidase (Zymed Laboratories Incs., San Francisco, CA) for 40 min. Colour was detected using a solution of 0·05% diaminobenzidine and 0·2% H2O2 at room temperature for 10 min. Sections were counterstained with diluted H&E and mounted balsam. Spleen sections were used as positive controls; primary anti-serum was substituted by PBS in some sections constituting the negative control.
Statistical analysis
All results are expressed as means and standard error (s.e.) of the mean. The Mann–Whitney U-test for non-paired data was used to compare T cell proliferation and Wilcoxon's signed-rank test for paired data was used to compare cytokine mRNA levels. Differences with a confidence level of 95% or higher were considered statistically significant (P < 0·05). Statistical analyses were performed using spss version 12·0.
Results
Canine thyroglobulin autoantibody immunoassay
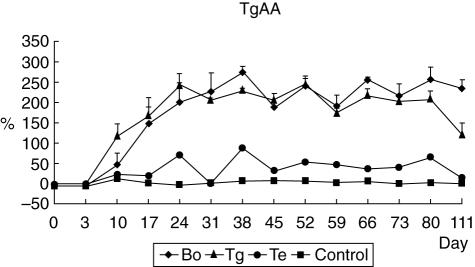
Following the primer and first booster injection with bovine Tg, all seven treated dogs were positive for Tg autoantibody (TgAA) and maintained high antibody titres thereafter, whereas all four control dogs were negative for TgAA and their antibody titres were very low (Fig. 1). The pattern of TgAA in the Tg and Bo groups was very similar, whereas the dog Te had lower TgAA titres than those in the experimental groups (Tg and Bo groups).
Fig. 1.
Detection of canine thyroglobulin autoantibody titres after immunization by the thyroid extract and/or bovine thyroglobulin. Serum samples were collected weekly and anti-canine thyroglobulin autoantibody measured by a commercially available enzyme-linked immunosorbent assay kit. The thyroglobulin autoantibody (TgAA) value was determined by calculation according to the manufacturer's instruction. The sample was considered to be positive when the value was > 25% for the TgAA assay. The value < 10% was counted as negative. Following the primer and first booster injections with bovine thyroglobulin, all seven experimental dogs [thyroid extract (Te), bovine thyroglobulin (Tg) and bovine thyroglobulin and thyroid extract (Bo) group] were positive for TgAA and maintained high antibody titres thereafter. Arrows indicate antigen injections (C: control dogs).
Canine TSH and total T4 measurement
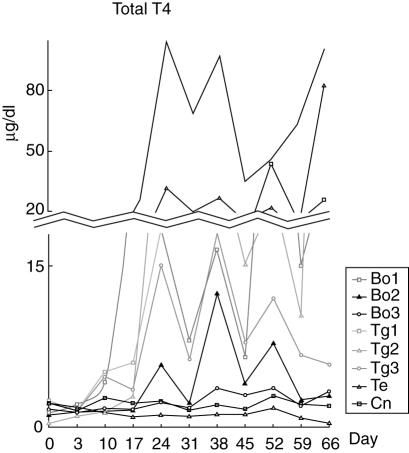
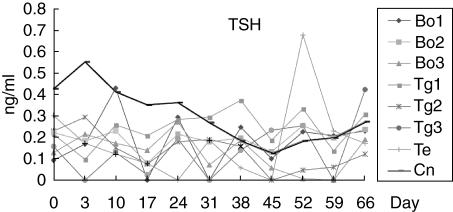
Beginning on day 24, a marked increase in total T4 was noted in five dogs (Bo 1, 2 and Tg 1–3; Fig. 2), with an average maximal total T4 concentration of 52·2 ± 40·2 µg/dl (mean ± s.d.; reference range: 0·73–2·9 µg/dl). The total T4 concentration of dog Te was within the reference range. In contrast, TSH did not change significantly in any of the experimental groups (Fig. 3).
Fig. 2.
The change of total T4 during the development of autoimmune thyroiditis. Beginning on day 24, a marked increase in total T4 was noted in five dogs [bovine thyroglobulin and thyroid extract (Bo) 1, 2 and bovine thyroglobulin (Tg) 1–3; Fig. 2]. The average of maximal total T4a levels of these dogs was 52·2 ± 40·2 µg/dl (mean ± s.d.). Arrows indicate antigen injections. aReference range of T4: 0·73–2·9 µg/dl.
Fig. 3.
The change of thyroid stimulating hormone (TSH) during the development of autoimmune thyroiditis. The TSHa did not change significantly in any of the experimental groups. Arrows indicate antigen injections. (Bo: dogs immunized with bovine thyroglobulin and thyroid extract, Tg: dogs immunized with bovine thyroglobulin, Te: dog immunized with thyroid extract, Cn: normal control dog). aReference range of TSH: < 0·6 ng/ml.
Canine free T4 and anti-T4 antibody titre measurement
Free T4 levels were within reference range in all dogs and anti-T4 antibody titre was very high (4·5–19-fold higher than the upper value of the reference range) in all dogs except for dog Te (Table 2).
Table 2.
Free T4 and anti-T4 antibody titre in the dogs immunized with bovine thyroglobulin and/or canine thyroid extract.
| Dog | Free T4 (pmol/l) | Anti-T4 antibody | TgAA (%) | Total T4 (µg/dl) |
|---|---|---|---|---|
| Bo 1 | 23 | 23·1 | 258·8 | 100·8 |
| Bo 2 | 11 | 9·2 | 262·4 | 2·933 |
| Bo 3 | 21 | 7·9 | 245·4 | 3·366 |
| Tg 1 | 38 | 9·1 | 229·3 | 25·7 |
| Tg 2 | 19 | 12·5 | 185·5 | 82·6 |
| Tg 3 | 17 | 10·7 | 237·8 | 5·85 |
| Te | 18 | 1·2 | 36·64 | 1·276 |
| Reference range | 8–40 | 0–2 | < 10 | 0·73–2·9 |
Serum samples were collected from experimental group on day 66. Bo: dogs immunized with bovine thyroglobulin and thyroid extract; Tg: dogs immunized with bovine thyroglobulin; Te: dog immunized with thyroid extract.
Proliferation of T lymphocytes
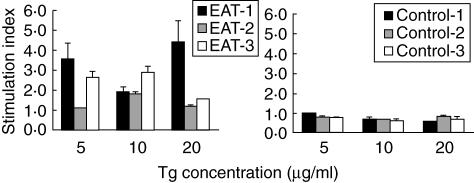
T lymphocytes obtained from animals immunized with thyroid extract and/or Tg (Bo 1, Bo 3 and Tg 3) when cultured in the presence of bovine Tg showed a significant proliferative response when compared with lymphocytes obtained from control dogs (Cp 1–3, Fig. 4).
Fig. 4.
T cell proliferation against thyroglobulin. T lymphocytes obtained from dogs immunized with the thyroglobulin and/or thyroid extract (EAT) when cultured in the presence of bovine thyroglobulin showed significant proliferative response when compared with lymphocytes obtained from control dogs (P < 0·05).
Semi-quantitative analysis of cytokines
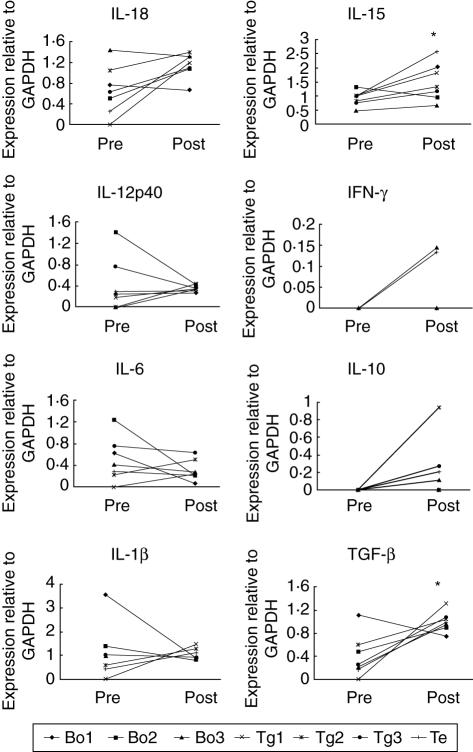
Among the Th1-type cytokines, IFN-γ mRNA expression was increased in two dogs, but was not detected in the other five dogs. Interleukin (IL)-18 was increased in five of seven dogs (P = 0·063) and IL-15 was increased in six of seven dogs (P < 0·05) when the RNA samples at the point of the highest TgAA titre (days 80, 66, 38, 24, 52, 52 and 38 for dogs Bo 1–3, Tg 1–3 and Te, respectively) were compared with baseline (Fig. 5). Among the Th2-type cytokines, IL-6 was decreased in five dogs (Fig. 5) and IL-4 expression was not detected in any of the samples. The T suppressive cytokine IL-10 was increased in five dogs and was not detected in the other two dogs (P = 0·068), and transforming growth factor (TGF)-α was increased in six of seven dogs (P < 0·05). The expression patterns of IL-10 and TGF-α correlated with antigen stimulation (data not shown). IL-12p40 was increased in five dogs and IL-1α was increased in three dogs, but these changes were not statistically significant.
Fig. 5.
Comparison of cytokine mRNA expression in dogs with experimentally induced autoimmune thyroiditis. Interleukin (IL)-15 and transforming growth factor (TGF)-β were increased significantly (P < 0·05) when the RNA samples at the point of the highest thyroglobulin autoantibody (TgAA) titre were compared with baseline.
Histopathology
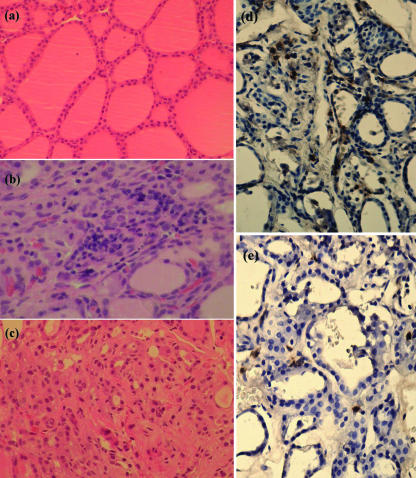
Mild infiltration of plasma cells and lymphocytes and mild follicular atrophy were observed in treated dogs (Fig. 6a–c). On day 128, two dogs (Bo2, Te) were within grade 1, and one dog (Tg 1) was in grade 0. Immunoperoxidase staining of frozen sections showed in that CD4+ and CD8+ T cells had infiltrated between follicles in dogs with experimentally induced autoimmune thyroiditis (Fig. 6d,e).
Fig. 6.
Histopathology of thyroids. On day 128, the thyroid glands were resected surgically from dogs immunized with bovine thyroglobulin with/without thyroid extract and stained with haematoxylin and eosin (H&E) using routine histological technique. Immunoperoxidase staining of frozen sections was also performed. (a) Control normal gland (× 200, H&E); (b) mild infiltration of plasma cells and lymphocytes ( × 400, H&E); and (c) mild follicular atrophy (× 200, H&E) were observed in dogs with experimentally induced autoimmune thyroiditis. (d) CD4+ T cells infiltrating between follicles (× 400, immunoperoxidase staining); and (e) CD8+ T cells infiltrating between follicles (× 400, immunoperoxidase staining) were observed in dogs with experimentally induced autoimmune thyroiditis.
Discussion
We have described here a canine autoimmune thyroiditis model in which the disease was induced by immunization with bovine Tg with or without thyroid extract. The dog Te, which was immunized with thyroid extract alone, showed lower TgAA titres than the dogs immunized with bovine Tg (Tg group) or both bovine Tg and thyroid extract (Bo group). While it was likely that the lower antibody titre observed in dog Te was due to not purifying thoroughly the canine thyroid antigen, the titre in this dog was over the TgAA positive standard (> 25%), suggesting that this dog may also serve as a model of autoimmune thyroiditis if purified or concentrated thyroid extract is used. In the Tg and Bo groups, TgAA titres were high throughout the entire experimental period. Because the concentrations of fT4 and TSH in all the groups were in the range of reference values, it is thought unlikely that they had advanced to the stage of hypothyroidism. In agreement with previous findings [11], dog Te had normal total T4 concentrations and normal anti-T4 antibody titres. In contrast, both the Tg and Bo groups of dogs had very high total T4 and high anti-T4 antibody titres. The elevation in T4 concentrations was due to the production of anti-T4 antibody. Autoantibodies to thyroid hormones are indicative of autoimmune thyroid disease and, as such, affected dogs are likely to develop hypothyroidism at some time in the future [12]. Serum total thyroid hormone concentrations are falsely elevated in dogs with anti-thyroid hormone autoantibodies when measured by solid phase [12].
We found that immunization with heterologous Tg could cause experimental autoimmune thyroiditis (EAT) in dogs, and that these animals had both TgAA and anti-T4 antibodies. Circulating autoantibodies against thyroid antigens such as Tg and the thyroid hormones thyroxine (T4) and triiodothyronine (T3) are evident in autoimmune thyroiditis [13]. Although the origins of T3 and T4 autoantibodies are controversial, several studies have reported that dogs with more than one autoantibody may have an increased risk of developing hypothyroidism compared with dogs with TgAA alone [14,15].
T lymphocytes obtained from EAT dogs when cultured in the presence of bovine Tg showed significantly greater proliferation when compared with lymphocytes obtained from the control dogs, but dose-dependence of Tg-induced proliferation was not observed. Using RT–PCR and normalizing against GAPDH mRNA expression, we measured the expression of cytokine mRNA in PBMCs.
We found that expression of Th1-type cytokines, such as IFN-γ, IL-18 and IL-15, was increased during the development of autoimmune thyroiditis, whereas expression of the Th2-type cytokine IL-6 showed little change and IL-4 expression was not detected in any of the samples. We also found that expression of the T suppressive cytokines, IL-10 and TGF-β, was increased and that their pattern of expression correlated with the antigen stimulation. Due to the small sample size we adopted a nonparametric method, in that we measured expression in randomly selected subsamples from the EAT and control groups. To manage these results statistically, we chose the day of highest value of TgAA titre and baseline (day 0) as the points for comparison.
Expression levels of IFN-γ, IL-6, IL-1β, IL-18 and IL-15 in thyroid from the obese strain (OS) of chickens, which suffer from spontaneous autoimmune thyroiditis, were increased compared with their level of expression in thyroids from control birds [16].
A study using the Bio Breeding/Worcester (BB/W) rat as a model [8] found that the expression of TNF-α, IFN-γ, IL-2 and IL-12p40 mRNA was significantly higher in rats with lymphocytic thyroiditis, whereas IL-4 or IL-10 were not expressed. In contrast, we found that the change in IL-12p40 mRNA from PBMC was not significant, whereas IL-15 expression was significantly increased. IL-15 mRNA is present in many normal tissues and its increased production in response to a variety of stimuli suggests that this cytokine plays a role in immune response, allograft rejection and the pathogenesis of autoimmune disorders [17]. Antigen stimulation was associated with a significant increase in TGF-β expression; TGF-β has been shown to be a strong inhibitor of the generation and cytolytic activity of cytotoxic T cells, natural killer (NK) cells and lymphokine activated killer (LAK) cells, and to suppress the natural and lymphokine-activated killing by large granular lymphocytes [18,19]. TGF-β has also been shown to down-regulate the expression of IFN-γ-induced major histocompatibility complex (MHC) class II antigen by both lymphoid and non-lymphoid cells, providing further evidence for the strong immunosuppressive effect of TGF-β on lymphocytes [20].
Histopathological changes on day 128 were milder than expected. As this was the only time-point examined, it is uncertain whether these lesions are representative of the full course of the disease. According to a report on EAT [21], granulomatous experimental autoimmune thyroiditis (G-EAT) was induced by adoptive transfer of spleen cells from mTG-sensitized donors acitivated with mouse thyroglobulin (mTg) and IL-12 in vitro. After G-EAT lesions reached maximal severity 19–21 days after cell transfer, thyroid lesions either resolved or the thyroids became atrophic and fibrotic by days 35–50. Our thyroid resections were conducted on day 128, or 72 days after the final immunization, suggesting that the thyroid lesions had resolved. This is supported further by our finding that, on day 111, almost all cytokine levels had returned to baseline. We did not confirm whether or not severe histopathological changes had occurred in the EAT dogs. Slight alterations have been observed in the thyroids of dogs immunized with pooled homologous extract over a period of 1–11 months [7]; specifically, five of 10 dogs showed no alterations, three showed a few small interstitial infiltrates of lymphoid cells and two showed moderate to severe changes in the thyroid. Further study is needed to confirm the histopathological changes in the EAT dogs induced with heterologous Tg.
Acknowledgments
The authors thank Ul-soo Choi for freely providing anti-canine CD4 and anti-canine CD8 mouse IgGs. This work was supported by Korean Research Foundation grants (KRF-005-E00076 and KRF-005-E00078). Further support was also provided by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University and the Brain Korea 21 Program for Veterinary Science.
References
- 1.Feldman EC, Nelson RW. Hypothyroidism. In: Feldman EC, Nelson RW, editors. Canine and feline endocrinology and reproduction. 2. Philadelphia: W.B. Saunders Co.; 1996. pp. 187–265. [Google Scholar]
- 2.Catharine RJ, Lynn Guptill-Yoran S. Hypothyroidism. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. 5. Philadelphia: W.B. Saunders Co.; 2000. pp. 1419–29. [Google Scholar]
- 3.Chistiakov DA, Turakulov RI. CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol. 2003;31:21–36. doi: 10.1677/jme.0.0310021. [DOI] [PubMed] [Google Scholar]
- 4.Kotani T, Umeki K, Hirai K, Ohtaki S. Experimental murine thyroiditis induced by porcine thyroid peroxidase and its transfer by the antigen-specific T cell line. Clin Exp Immunol. 1990;80:11–18. doi: 10.1111/j.1365-2249.1990.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassman R, Solic N, Jasani B, Hall R, McGregor AM. Immunological events leading to destructive thyroiditis in the AUG rat. Clin Exp Immunol. 1988;73:410–16. [PMC free article] [PubMed] [Google Scholar]
- 6.Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. J Am Med Assoc. 1957;164:1439–47. doi: 10.1001/jama.1957.02980130015004. [DOI] [PubMed] [Google Scholar]
- 7.Terplan KL, Witebsky E, Rose NR, Paine JR, Egan RW. Experimental thyroiditis in rabbits, guinea pigs and dogs, following immunization with thyroid extracts of their own and of heterologous species. Am J Pathol. 1960;36:213–39. [PMC free article] [PubMed] [Google Scholar]
- 8.Bluher M, Krohn K, Wallaschofski H, Braverman LE, Paschke R. Cytokine gene expression in autoimmune thyroiditis in BioBreeding/Worcester rats. Thyroid. 1999;9:1049–55. doi: 10.1089/thy.1999.9.1049. [DOI] [PubMed] [Google Scholar]
- 9.Chamizo C, Rubio JM, Moreno J, Alvar J. Semi-quantitative analysis of multiple cytokines in canine peripheral blood mononuclear cells by a single tube RT–PCR. Vet Immunol Immunopathol. 2001;83:191–202. doi: 10.1016/s0165-2427(01)00385-3. [DOI] [PubMed] [Google Scholar]
- 10.Ng HP, Banga JP, Kung AW. Development of a murine model of autoimmune thyroiditis induced with homologous mouse thyroid peroxidase. Endocrinology. 2004;145:809–16. doi: 10.1210/en.2003-0656. [DOI] [PubMed] [Google Scholar]
- 11.Haines DM, Penhale WJ. Experimental thyroid autoimmunity in the dog. Vet Immunol Immunopathol. 1985;9:221–38. doi: 10.1016/0165-2427(85)90073-x. [DOI] [PubMed] [Google Scholar]
- 12.Panciera DL. Canine Hypothyroidism. In: Torrande AG, Mooney CT, editors. BSAVA manual of small animal endocrinology. 2. Cheltenham: British Small Animal Veterinary Association; 1998. pp. 101–13. [Google Scholar]
- 13.Haines DM, Lording PM, Penhale WJ. The detection of canine autoantibodies to thyroid antigens by enzyme-linked immunosorbent assay, hemagglutination and indirect immunofluorescence. Can J Comp Med. 1984;48:262–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Graham PA, Nachreiner RF, Refsal KR, Provencher-Bolliger AL. Lymphocytic thyroiditis. Vet Clin North Am Small Anim Pract. 2001;31:915–33. doi: 10.1016/s0195-5616(01)50005-4. [DOI] [PubMed] [Google Scholar]
- 15.Patzl M, Mostl E. Determination of autoantibodies to thyroglobulin, thyroxine and triiodothyronine in canine serum. J Vet Med A Physiol Pathol Clin Med. 2003;50:72–8. doi: 10.1046/j.1439-0442.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser P, Rothwell L, Vasicek D, Hala K. A role for IL-15 in driving the onset of spontaneous autoimmune thyroiditis? J Immunol. 2002;168:4216–20. doi: 10.4049/jimmunol.168.8.4216. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy MK, Park LS, Paxton RJ. Interleukin-15. In: Thomson AW, editor. The cytokine handbook. 3. London: Academic Press; 1998. pp. 443–64. [Google Scholar]
- 18.Roberts AB, Sporn MB. The TGF-αs. In: Sporn MB, Roberts AB, editors. Peptide growth factors and their receptors. Heidelberg: Springer; 1990. pp. 421–72. [Google Scholar]
- 19.Kehrl JH. Transforming growth factor-beta: an important mediator of immunoregulation. Int J Cell Cloning. 1991;9:438–50. doi: 10.1002/stem.1991.5530090502. [DOI] [PubMed] [Google Scholar]
- 20.Czarniecki CW, Chiu HH, Wong GH, McCabe SM, Palladino MA. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–23. [PubMed] [Google Scholar]
- 21.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Mechanisms of spontaneous resolution versus fibrosis in granulomatous experimental autoimmune thyroiditis. J Immunol. 2003;171:6236–43. doi: 10.4049/jimmunol.171.11.6236. [DOI] [PubMed] [Google Scholar]