Abstract
Autoantibodies to insulin (IAA) are one of the first markers of the autoimmune process leading to type 1 diabetes (T1D). While other autoantibodies in T1D have been studied extensively, relatively little is known about IAA and their binding specificities, especially after insulin treatment is initiated. We hypothesize that insulin antibodies (IA) that develop upon initiation of insulin treatment differ in their epitope specificities from IAA. We analysed insulin antibody binding specificities in longitudinal samples of T1D patients (n = 49). Samples were taken at clinical diagnosis of disease and after insulin treatment was initiated. The epitope specificities were analysed using recombinant Fab (rFab) derived from insulin-specific monoclonal antibodies AE9D6 and CG7C7. Binding of radiolabelled insulin by samples taken at onset of the disease was significantly reduced in the presence of rFab CG7C7 and AE9D6. rFab AE9D6 competed sera binding to insulin significantly better than rFab CG7C7 (P = 0·02). Binding to the AE9D6-defined epitope in the initial sample was correlated inversely with age at onset (P = 0·005). The binding to the AE9D6-defined epitope increased significantly (P < 0·0001) after 3 months of insulin treatment. Binding to the CG7C7-defined epitope did not change during the analysed period of 12 months. We conclude that epitopes recognized by insulin binding antibodies can be identified using monoclonal insulin-specific rFab as competitors. Using this approach we observed that insulin treatment is accompanied by a change in epitope specificities in the emerging IA.
Keywords: epitopes, insulin autoantibody, radioligand binding assay, recombinant Fab, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by the specific destruction of the insulin-producing beta cells of the pancreas. While the disorder is T cell-mediated (for reviews see [1,2]), loss of immune tolerance is best reflected in the presence of autoantibodies to three major islet autoantigens, namely the smaller isoform of glutamate decarboxylase (GAD65), the tyrosine phosphatase-like protein IA-2 and insulin. These autoantibodies can be found in the circulation months to years prior to the clinical onset of the disease [3,4]. The presence of these autoantibodies is used to predict future development of diabetes, with risk correlating directly to the number of autoantibodies present [3,4]. Insulin autoantibodies (IAA), in contrast to GAD65Ab and IA-2Ab, recognize a beta cell-specific autoantigen. They are among the first autoantibodies to appear, and are found typically in young children [5–8]. IAA are also found in patients with insulin autoimmune syndrome [9], in first-degree relatives of patients with T1D and in other autoimmune diseases [10,11].
The need for improved diagnosis together with the rise in incidence of T1D, especially in young children [12–14], has increased the focus of many investigations on IAA. The studies of IAA isotypes, subclasses and affinities suggest that IAA may be useful in early prediction of diabetes [15,16].
Once insulin treatment is initiated, insulin antibodies (IA) are routinely detected [17]. These antibodies appear regardless of whether or not the patient was initially IAA-positive. Because IAA are the result of an autoimmune reaction, while IA react with an exogenous protein, the question arises as to whether the antibodies differ in their epitope recognition.This issue has been addressed in part by epitope analyses using phage display and insulin isoforms, and the results suggest that IAA differ from IA [18–20]. However, these studies compared IAA-positive samples obtained from T1D patients at clinical diagnosis and IA-positive samples from type 2 diabetes (T2D) patients treated with insulin. A detailed analysis of possible longitudinal changes in the epitope specificity of insulin-binding antibodies upon initiation of insulin treatment is necessary, however, to understand the mechanism that govern the formation of IA.
Analysis of conformational autoantibody epitopes using recombinant Fab (rFab) has furthered our understanding of the development of disease-specific autoantibodies in T1D [21–23]. Here we use this approach to investigate the epitope specificities of insulin-binding antibodies in longitudinal samples obtained from T1D patients.
Materials and methods
Newly diagnosed IAA-positive T1D patients (n = 28) (median age: 10 years, range: 3–14 years) were part of a study conducted at the St Görans Children Hospital, Stockholm, Sweden. These IAA-positive samples represent 18% of the entire study cohort. The serum samples were obtained at the clinical diagnosis of diabetes.
Another set of newly diagnosed IAA-positive T1D patients (n = 21) (median age: 22 years, range: 15–34 years) were part of the Diabetes Incidence Study in Sweden (DISS). These IAA-positive samples represent 5% of the entire study cohort. The newly diagnosed Swedish insulin-dependent patients were registered in 1992–93.
Samples in the younger patient group were collected every 3 months after the initial insulin treatment, while samples in the older patient group were collected 1 year after insulin treatment. All patients were treated with recombinant human insulin. A healthy control group (n = 50) (age 21–44 years) was used to determine the positive cut-off level for the IAA-assay.
All subjects in this study, their parents or legal guardians, gave informed consent. Local institutional ethics committee approval was obtained prior to collection of all serum samples.
Monoclonal antibodies
Both insulin-specific monoclonal antibodies used in this study were raised in mice to human insulin. Monoclonal antibodies AE6D9 [24] and CG7C7 [24][American Type Culture Collection (ATCC), Manassas, VA, USA] recognize conformational epitopes located at the A chain loop of insulin [21,24].
Competition studies using natural occurring isoforms of insulin suggest that the antibodies recognize different epitopes [24]. Moreover, both antibodies can bind simultaneously to the insulin molecule [25].
Bacterial expression and purification of recombinant Fab
The heavy and light chain genes were subcloned into the pAK19 expression vector [26] and expressed in Escherichia coli 25F2 cells, as described previously [22]. Briefly, E. coli 25F2 cells containing the recombinant plasmid were grown for 16 h at 30°C in complete morpholinopropanesulphonate (MOPS) medium [27]. Cells were then subcultured and grown in the absence of phosphate at 30°C for 4 h. The recombinant Fab (rFab) was isolated from the bacteria as described previously [22] and purified by two subsequent affinity chromatography steps on Ni-NTA-agarose (Qiagen Inc., Valencia, CA, USA) and protein G Sepharose (PGS) (Zymed Laboratories, Carlton Court, CA, USA). Fractions were examined by immunoblot for the presence of rFab and by radioligand binding for insulin binding. Active fractions were pooled and the protein concentration was determined. The yield of functional purified rFab was ∼ 0·5–1 mg/l bacterial culture.
Radiobinding assay (RBA) for antibodies to insulin
The binding capacity of serum samples, the monoclonal antibodies (MoAbs) and rFab were determined in the insulin antibody RBA as reported previously [28]. Briefly, 15 000 counts per minute (cpm) A14-[125]I-radiolabelled recombinant human insulin (> 2000 Ci/mmol) (Amersham Pharmacia Biotech, Piscataway, NJ, USA) was incubated overnight at room temperature with the serum samples, MoAbs or rFab. Subsequently the immunocomplexes were absorbed by protein-A Sepharose (PAS) (Zymed Laboratories) or PGS (for absorption of rFab). Results were expressed in arbitrary units derived from a standard curve. Samples were considered positive if they had levels above the 97·5th percentile of 50 healthy controls (0·2 units). Our laboratory participated in the Diabetes Antibody Standardization Program (DASP) workshop [29] and the IAA assay showed a sensitivity of 40% and good specificity (92%). We focused our analysis on samples that tested positive repeatedly in our IAA assay to avoid false-positive samples.
Competition experiments using rFab
The capacity of the rFab to inhibit [125]I-insulin binding to human serum was tested in a competitive RBA using PAS as the precipitating agent [21]. Fab lack the CH2 domain of the Fc region and therefore do not bind to PAS. Serum samples were tested at a serum dilution of 1/6. All longitudinal samples from derived from one patient were analysed at the same time to minimize interassay variations. The optimal concentrations of rFab AE6D9 (0·2 µg/ml) and CG7C7 (1 µg/ml) were determined in competition assays using the respective parent mAb as the competitor. Titration experiments confirmed that higher concentrations (0·4 µg/ml and 1 µg/ml, respectively) did not yield significant changes in the competition experiments.
Statistical analyses
Binding of IAA to insulin in the presence of rFab was expressed as follows: cpm of [125]I insulin bound in the presence of rFab/cpm of [125]I insulin bound in the absence of rFab × 100.
The cut-off for specific competition was determined as > 10% by using as a negative control rFab D1·3 (a kind gift from Dr J. Foote, Fred Hutchinson Research Center, Seattle, WA, USA), specific to an irrelevant target, anti-hen egg-white lysozyme, at 5 µg/ml.
All samples were analysed in triplicate and the average intra-assay coefficient of variation was 1·4% (range: 0·04–5·9%). Significance of competition within serum groups was tested using the Wilcoxon matched-pair test. Significant differences in competition between serum groups were tested for with the non-parametric Mann–Whitney U-test. A P-value < 0·05 was considered significant.
Results
Sample analysis at time of clinical diagnosis
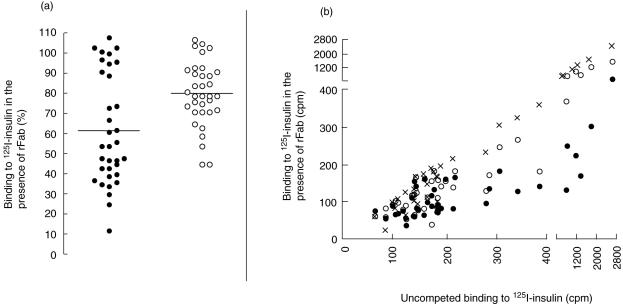
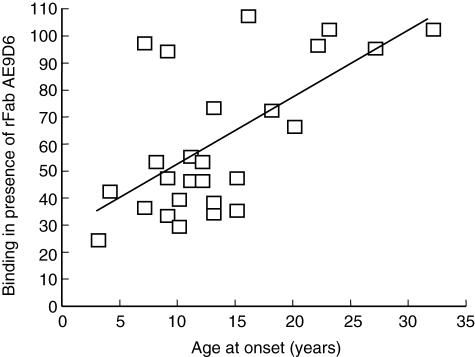
The samples taken at onset of disease were tested for binding to radiolabelled insulin in the presence of rFab CG7C7 and AE9D6 (Fig. 1a,b). We observed a significant reduction in binding to insulin in the presence of either rFab AE9D6 or CG7C7 (median binding in the presence of rFab AE9D6 and CG7C7 of 61% and 78%, respectively). rFab AE9D6 competed binding to insulin significantly better than rFab CG7C7 (P = 0·02). We observed a wide range of competition by both rFab AE9D6 and CG7C7 (Fig. 1a). Competition by the AE9D6-defined epitope was correlated inversely with age at onset (P = 0·005) (Fig. 2). Competition by both rFab was correlated inversely with the titre in the initial sample (P < 0·0001) (Fig. 1b); however, a significant reduction in low IAA-titre samples was observed. We did not find a correlation between IAA-titre and age at onset, although a significantly higher percentage of patients in the younger study group was IAA-positive compared to the older study group (5% versus 18%, P = 0·001). No correlation between the competition by any of the rFab and the patients' HLA or presence of other autoantibodies was observed (data not shown).
Fig. 1.
(a, b) Epitope specificities of autoantibodies to insulin (IAA) at clinical diagnosis of type 1 diabetes (T1D). (a) Samples of patients with newly diagnosed T1D were tested for binding to [125]I insulin in the presence of recombinant Fab (rFab) AE9D6 (black circles) or rFab CG7C7 (white circles). Binding was compared to uncompeted binding (set as 100%). Median binding is indicated. (b) Correlation of IAA-titre and competition. Binding to [125]I-insulin in the presence of rFab AE9D6 (black circles), CG7C7 (white circles), and D1·3 (x) is plotted against the binding to [125]I-insulin of the analysed sample in the absence of competing rFab (presented in counts per minute).
Fig. 2.
Binding to the AE9D6-defined epitope is correlated inversely with age at onset. Binding of type 1 diabetes (T1D) patient sera to [125]I insulin in the presence of recombinant Fab (rFab) AE9D6 was correlated to age at onset (in years). Binding of sera to [125]I insulin in the presence of rFab AE9D6 is presented as percentage in relation to uncompeted binding of sera to [125]I insulin (set as 100%). A linear regression curve is indicated.
Binding to the AE9D6-defined epitope increases longitudinally
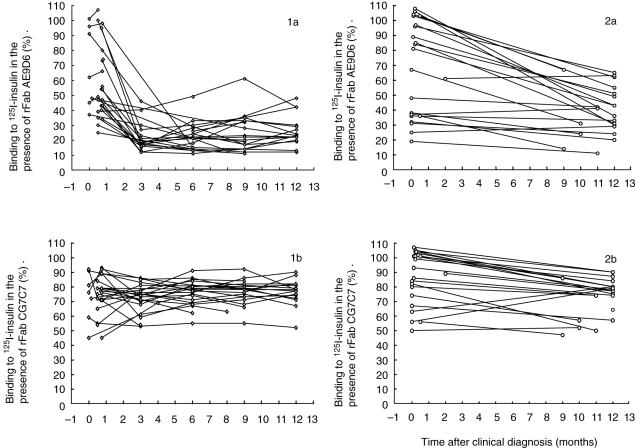
Longitudinal samples obtained every 3 months (0–18-year-old patient group) or after 12 months (15–34-year-old group) after initiation of insulin treatment were analysed for their insulin antibody epitope specificity, as described above (Fig. 3).
Fig. 3.
Longitudinal analysis of insulin binding antibodies in samples taken at clinical diagnosis and the indicated time-points. Samples of the 0–18-year-old patient group (1) and the 15–34-year-old patient group (2) were analysed for their binding to [125]I-insulin in the presence of recombinant Fab (rFab) AE9D6 (a) and CG7C7 (b). Binding is reported as percentage in comparison to uncompeted binding (set as 100%) and plotted against time after clinical onset of type 1 diabetes (T1D) (months).
The IAA/IA titre in the IAA-positive sera increased significantly from onset to 3 months (median increase of 300%) (P = 0·0002), and remained stable for the subsequent longitudinal samples (data not shown). The binding to insulin in the presence of rFab AE9D6 was reduced significantly from a median binding of 56% to 19% (P < 0·0001) after 3 months of insulin treatment. The binding level stayed constant for the next 9 months without significant changes. This longitudinal change in epitope recognition was similar in both age groups with a median decrease of 2·3%/month and 2·8%/month for the younger and the older age group, respectively. Binding to insulin in the presence of rFab CG7C7 did not change during the analysed period of 12 months and remained at a constant median of 80%.
In contrast to the samples taken at onset, no correlation between competition by rFab AE9D6, age at onset or titre of insulin binding antibodies was observed in the samples analysed at 3–12 months.
Discussion
While epitope specificities of both GAD65Ab and IA-2Ab are studied extensively (for review see [30]), much less is known about the binding specificities of IAA [18–20]. Moreover, the development of IA in response to insulin treatment hampers the longitudinal analysis of IAA after the clinical onset of the disease. Previous studies demonstrated differences in the epitope specificities between IAA and IA present in T1D and T2D patients, respectively [18]. To our knowledge the present study addressed for the first time changes in epitope specificities between IAA and IA in longitudinal samples obtained from T1D patients. Our study aimed to determine whether or not treatment with recombinant human insulin elicits IA that differ from the IAA initially present in the sera of newly diagnosed T1D patients. We tracked the progress of epitope specificities of insulin binding antibodies in longitudinal samples obtained from IAA-positive T1D patients. Serum samples taken at onset of disease and after initiation of insulin treatment were studied for their epitope specificities using two rFab derived from insulin-specific monoclonal antibodies with different epitope specificities [21,24]. We observed that while both rFab competed significantly with the antigen binding by the serum samples, binding to the AE9D6-defined epitope was significantly higher than to the CG7C7-defined epitope. Furthermore, we observed that in samples taken at onset the binding to the AE9D6-defined epitope correlated with lower age at onset of the disease. In the longitudinal analysis of the samples the binding to the AE9D6-defined epitope significantly increased over time, while no dynamic changes in the binding to the CG7C7-defined epitope were observed.
In an earlier study the significance of amino acid A13 of the A-chain for the binding of IAA in T1D was demonstrated. Amino acid substitutions of A13 abolished binding of IAA to insulin [31]. The authors concluded that the major binding site for IAA is conformational and includes amino acid residues A8–A13 on the A-chain and B1–B3 on the B-chain. The suggested epitope for AE9D6 involves amino acids A8–A10; moreover, AE9D6 does not bind to the isolated A-chain (Thomas, unpublished observation), suggesting a conformational epitope. Therefore, the binding sites for IAA identified by Castano et al. [31] and in this study overlap.
The observed changes in insulin antibody binding specificities could be due to the loss of antibodies dominating the initial IAA epitope specificities, with other antibodies with epitope specificities identical or similar to that of AE9D6 emerging, leading to a change in the balance between the AE9D6-like antibodies and other insulin-specific antibodies, or to the differentiation of pre-existing B cell precursors. Scatchard analysis of insulin binding sera at onset and after insulin treatment suggest that sera at both time-points are polyclonal and that the overall affinity to insulin of the polyclonal patient sera does not change after insulin treatment (data not shown). This confirms earlier findings [32]. However, we cannot exclude that the affinities of antibody subgroups change. A recent study of insulin-binding isotypes after insulin treatment did not show the appearance of IgM isotypes, suggesting that IA do not originate from the new generation of antibodies [33]. Therefore, we hypothesize that many IA arise from similar precursors as B cells that differentiate to generate IAA.
In summary, we have demonstrated that epitopes of insulin binding antibodies can be characterized using competition with monoclonal insulin-specific rFab. Using this approach we identified longitudinal dynamic changes in the specificities of insulin binding antibodies upon insulin treatment.
Acknowledgments
This study was supported by the National Institutes of Health (RO1 DK53456–05A2). C. S. H. was supported through a Career Development Award from the American Diabetes Association. C. J. P. received travel awards from Novo-Nordisk (administered by the Society for Endocrinology, Metabolism and Diabetes of South Africa) and the National Health Laboratory Service.
References
- 1.Notkins AL, Lernmark Å. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–52. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 3.Bingley PJ, Christie MR, Bonifacio E, et al. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994;43:1304–10. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- 4.Kulmala P, Savola K, Reijonen H, et al. Genetic markers, humoral autoimmunity, and prediction of type 1 diabetes in siblings of affected children. Childhood Diabetes Finland Study Group. Diabetes. 2000;49:48–58. doi: 10.2337/diabetes.49.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes. the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–8. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 6.Vardi P, Keller R, Dib S, Eisenbarth GS, Soeldner JS. Log-linear correlation of CIAA with age in new onset type I diabetics. Diabetes. 1988;37:28A. [Google Scholar]
- 7.Roll U, Christie MR, Fuchtenbusch M, Payton MA, Hawkes CJ, Ziegler AG. Perinatal autoimmunity in offspring of diabetic parents. The German Multicenter BABY-DIAB study: detection of humoral immune responses to islet antigens in early childhood. Diabetes. 1996;45:967–73. doi: 10.2337/diab.45.7.967. [DOI] [PubMed] [Google Scholar]
- 8.Naserke HE, Bonifacio E, Ziegler AG. Prevalence, characteristics and diabetes risk associated with transient maternally acquired islet antibodies and persistent islet antibodies in offspring of parents with type 1 diabetes. J Clin Endocrinol Metab. 2001;86:4826–33. doi: 10.1210/jcem.86.10.7931. [DOI] [PubMed] [Google Scholar]
- 9.Hirata Y, Uchigata Y. Insulin autoimmune syndrome in Japan. Diabetes Res Clin Pract. 1994;24(Suppl.):S153–7. doi: 10.1016/0168-8227(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 10.Wilkin T, Armitage M, Casey C, et al. Value of insulin autoantibodies as serum markers for insulin-dependent diabetes mellitus. Lancet. 1985. pp. 480–2. [DOI] [PubMed]
- 11.Srikanta S, Ganda OP, Rabizadeh A, Soeldner JS, Eisenbarth GS. First-degree relatives of patients with Type 1 diabetes mellitus: islet-cell antibodies and abnormal insulin secretion. N Engl J Med. 1985;313:462–4. doi: 10.1056/NEJM198508223130801. [DOI] [PubMed] [Google Scholar]
- 12.EURODIAB. Infections and vaccinations as risk factors for childhood type I (insulin-dependent) diabetes mellitus: a multicentre case–control investigation. EURODIAB Substudy 2 Study Group. Diabetologia. 2000;41:47–53. doi: 10.1007/s001250050006. [DOI] [PubMed] [Google Scholar]
- 13.Pundziute-Lycka A, Dahlquist G, Nystrom L, et al. Type I diabetes in the 0–34 years group in Sweden. Diabetologia. 2002;45:783–91. doi: 10.1007/s00125-002-0845-2. [DOI] [PubMed] [Google Scholar]
- 14.Weets I, Siraux V, Daubresse JC, et al. Relation between disease phenotype and HLA-DQ genotype in diabetic patients diagnosed in early adulthood. J Clin Endocrinol Metab. 2002;87:2597–605. doi: 10.1210/jcem.87.6.8613. [DOI] [PubMed] [Google Scholar]
- 15.Hoppu S, Ronkainen MS, Kimpimaki T, et al. Insulin autoantibody isotypes during the prediabetic process in young children with increased genetic risk of type 1 diabetes. Pediatr Res. 2004;55:236–42. doi: 10.1203/01.PDR.0000100905.41131.3F. [DOI] [PubMed] [Google Scholar]
- 16.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro) insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114:589–97. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis SN, Thompson CJ, Peak M, Brown MD, Alberti KG. Effects of human insulin on insulin binding antibody production in nondiabetic subjects. Diabetes Care. 1992;15:124–6. doi: 10.2337/diacare.15.1.124. [DOI] [PubMed] [Google Scholar]
- 18.Devendra D, Galloway TS, Horton SJ, Evenden A, Keller U, Wilkin TJ. The use of phage display to distinguish insulin autoantibody (IAA) from insulin antibody (IA) idiotypes. Diabetologia. 2003;46:802–9. doi: 10.1007/s00125-003-1107-7. [DOI] [PubMed] [Google Scholar]
- 19.Diaz JL, Wilkin T. Differences in epitope restriction of autoantibodies to native human insulin (IAA) and antibodies to heterologous insulin (IA) Diabetes. 1987;36:66–72. doi: 10.2337/diab.36.1.66. [DOI] [PubMed] [Google Scholar]
- 20.Mirza IH, Wilkin TJ. Antigenicity of the carboxyl terminus of insulin: isolation of human insulin-specific monoclonal antibodies. Immunology. 1988;65:43–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Padoa CJ, Crowther NJ, Thomas JW, et al. Epitope analysis of insulin autoantibodies using recombinant Fab. Clin Exp Immunol. 2005;140:564–71. doi: 10.1111/j.1365-2249.2005.02802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padoa CJ, Banga JP, Madec AM, et al. Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes. 2003;52:2689–95. doi: 10.2337/diabetes.52.11.2689. [DOI] [PubMed] [Google Scholar]
- 23.Gilliam LK, Binder KA, Banga JP, et al. Multiplicity of the antibody response to GAD65 in Type I diabetes. Clin Exp Immunol. 2004;138:337–41. doi: 10.1111/j.1365-2249.2004.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroer JA, Bender T, Feldmann RJ, Kim KJ. Mapping epitopes on the insulin molecule using monoclonal antibodies. Eur J Immunol. 1983;13:693–700. doi: 10.1002/eji.1830130902. [DOI] [PubMed] [Google Scholar]
- 25.Rojas M, Hulbert C, Thomas JW. Anergy and not clonal ignorance determines the fate of B cells that recognize a physiological autoantigen. J Immunol. 2001;166:3194–200. doi: 10.4049/jimmunol.166.5.3194. [DOI] [PubMed] [Google Scholar]
- 26.Carter P, Kelley RF, Rodrigues ML, et al. High level Escherichia coli expression and production of a bivalent humanized antibody fragment. Biotechnology. 1992;10:163–7. doi: 10.1038/nbt0292-163. [DOI] [PubMed] [Google Scholar]
- 27.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–47. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams AJK, Bingley PJ, Bonifacio E, Palmer JP, Gale EAM. A novel micro-assay for insulin autoantibodies. J Autoimmunity. 1997;10:473–8. doi: 10.1006/jaut.1997.0154. [DOI] [PubMed] [Google Scholar]
- 29.Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes. 2003;52:1128–36. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- 30.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl. 2):S52–61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 31.Castano L, Ziegler AG, Ziegler R, Shoelson S, Eisenbarth GS. Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes. 1993;42:1202–9. doi: 10.2337/diab.42.8.1202. [DOI] [PubMed] [Google Scholar]
- 32.Brooks-Worrell BM, Nielson D, Palmer JP. Insulin autoantibodies and insulin antibodies have similar binding characteristics. Proc Assoc Am Physicians. 1999;111:92–6. doi: 10.1046/j.1525-1381.1999.09114.x. [DOI] [PubMed] [Google Scholar]
- 33.Fineberg SE, Kawabata T, Finco-Kent D, Liu C, Krasner A. Antibody response to inhaled insulin in patients with type 1 or type 2 diabetes. An analysis of initial phase II and III inhaled insulin (Exubera) trials and a two-year extension trial. J Clin Endocrinol Metab. 2005;90:3287–94. doi: 10.1210/jc.2004-2229. [DOI] [PubMed] [Google Scholar]