Abstract
We conducted a placebo-controlled, cross-over trial to examine the effect of Lactobacillus casei Shirota (LcS) on natural killer (NK) cell activity in humans. NK cell activity exhibited a declining trend during the period of placebo ingestion, but NK cell activity increased after intake for 3 weeks of fermented milk containing 4 × 1010 live LcS. When human peripheral blood mononuclear cells were cultured in the presence of heat-killed LcS, NK cell activity was enhanced. The ability of LcS to enhance NK cell activity and induce interleukin (IL)-12 production was correlated, and the addition of anti-IL-12 monoclonal antibody reduced the enhancement of NK cell activity triggered by LcS. In addition, separation of NK cells from LcS-stimulated monocytes with membrane filter reduced NK cell activity to the intermediate level and almost deprived monocytes of the ability to produce IL-12. These results demonstrate that LcS can enhance NK cell activity in vivo and in vitro in humans, and IL-12 may be responsible for enhancement of NK cell activity triggered by LcS.
Keywords: cell-to-cell contact, IL-12, Lactobacillus casei, NK cell activity
Introduction
Natural killer (NK) cells are lymphocytes having a natural ability to kill infected cells and cancer cells. There has been accumulating evidence showing the importance of NK cells in defence of the human body. A patient genetically deficient in NK cells and having normal T cells and B cells suffers frequent severe infections with herpes virus [1], and elderly people with low NK cell activity exhibit a higher mortality rate due to infection compared with their counterparts with high NK cell activity [2]. These results support the notion that NK cells are essential for preventing pathogenic infection. An epidemiological study revealed that the population with low NK cell activity had a significantly higher risk of cancer than the population with intermediate or high NK cell activity [3], suggesting a pivotal role of NK cells in the survey and eradication of cancer cells.
It has been reported that genetic and environmental factors are associated with a reduction of NK cell activity. Elderly people have significantly lower NK cell activity than young subjects, while the number of CD16+ cells increases significantly in the elderly [4]. In contrast, CD16-mediated cytotoxicity of purified NK cells do not change with age, but CD94 and NKG2A expression decrease in an age-related fashion [5]. Moreover, lifestyle, such as cigarette smoking and little physical exercise, is correlated significantly with low NK cell activity [6], and drastic changes of life associated with mental stress including bereavement and hospitalization cause a reduction in NK cell activity [7,8]. Therefore, it would be useful to determine a way to keep NK cell activity from declining.
Supplementation of live beneficial bacteria has been found to promote health and reduce the risk of various diseases. In a double-blind, placebo-controlled intervention study [9], oral administration of live Lactobacillus casei Shirota (LcS) prevented the recurrence of superficial bladder cancer and the daily intake of live LcS suppressed atypia of colorectal tumours in subjects who had undergone resection of at least two colorectal tumours [10]. These findings show that ingestion of live LcS could prevent the development of cancer. On the other hand, drinking fermented milk containing LcS was found to restore NK cell activity in healthy subjects and augment NK cell activity in patients with HTLV-1 associated myelopathy [11–13]. These results suggest that LcS may enforce immunosurveillance against cancer through restoring NK cell activity [14].
In this report, the effect on NK cell activity of fermented milk containing LcS was investigated in a placebo-controlled, cross-over trial. Furthermore, possible mechanisms for LcS to activate human NK cells were elucidated in vitro. Our results demonstrate that LcS potentially enhances human NK cell activity by inducing monocytes to generate interleukin (IL)-12.
Materials and methods
Subjects
For the supplementation study, 10 volunteers (aged 69–97 years; three male, seven female) were enrolled. They participated in the study during their stay in hospital for investigation. The inclusion criteria comprised no abnormalities of haematological and biochemical parameters in serum, and a willingness to follow the trial guidelines. The exclusion criteria were any recent history of liver diseases, cancers and immunological disorders. Serum parameters of all the participants changed within the normal range during the supplementation study. We obtained informed consent from all the subjects and carried out the study in accordance with the guidelines of the Helsinki Declaration and under the approval of the ethical committee for clinical studies of the Juntendo University School of Medicine.
For the in vitro study, peripheral blood mononuclear cells (PBMC) were obtained from subjects (aged 22–54 years; 27 male, six female) in general good health. All the participants gave informed consent and these studies were carried out with the approval of the ethical committee of the Yakult Central Institute for Microbiological Research.
Study design
Fermented milk containing LcS was produced by inoculating live LcS into skimmed milk and manufacturing the taste with syrup, and contained 4 × 1010 colony-forming units (CFU) of live LcS per bottle (80 ml). The placebo was the same in components and appearance as the fermented milk, except that LcS was not included. Both fermented milk and placebo contained 0·8 g of protein, 0·08 g of lipid, 11·5 g of carbohydrate and 12 mg of sodium per one bottle.
The effect of intake of fermented milk containing LcS or a placebo was assessed in a cross-over trial. Volunteers were divided randomly into two groups and each subject drank fermented milk containing LcS or a placebo daily for 3 weeks. After a washout period of 7 weeks, subjects drank a placebo or fermented milk containing LcS for 3 weeks. Before and after intake in each study period, peripheral blood was collected using heparin sodium at 5 U/ml as anti-coagulant, and PBMC were prepared to measure NK cell activity. In addition, biochemical parameters in serum were checked to assess the safety of the test samples.
For in vitro analysis, PBMC were prepared from healthy subjects using heparin sodium at 5 U/ml as anti-coagulant and used for the study described below.
Assay of NK cell activity
NK cell activity was assayed as described elsewhere [6]. PBMC were adjusted to 7·8 × 104−2·5 × 106 cells/ml in fetal calf serum (FCS)/RPMI-1640 and 100 µl of the cell suspension was put into each well of a 96-well U-bottomed microtitre plate (NUNC, Roskilde, Denmark). Thereafter, 100 µl of [51Cr]-labelled K562 cell suspension was added (5 × 103 cells/well). The cells were incubated in humidified 5% CO2 in air at 37°C for 4 h. The percentage of specific lysis was calculated according to the following formula: % specific lysis = (experimental release − spontaneous release)/(maximal release − spontaneous release) × 100. One lytic unit (LU) was defined as the cytotoxic activity giving 30% maximal release.
Flow cytometry
Cell constitution was analysed as described elsewhere [12]. Cells were incubated with phycoerythrin (PE)-conjugated anti-CD56 monoclonal antibody (MoAb) (clone B159) and fluorescein isothiocyanate (FITC)-conjugated anti-CD3 MoAb (clone UCHT1), or with PE-conjugated anti-CD14 MoAb (clone M5E2, mouse IgG2aκ; all from BD Pharmingen San Diego, CA, USA), and the percentage of CD3–CD56+, CD3+CD56+ and CD14+ cells was then analysed with a flow cytometer (Epics XL; Beckman Coulter, Inc., Fullerton, CA, USA). Isotype- and concentration-matched irrelevant fluorochrome-conjugated mouse MoAbs (clone MOPC-21 as control for mouse IgG1κ; clone G155-178 as control for mouse IgG2aκ) did not stain PBMC.
In vitro culture
LcS was cultured in Man-Rogosa-Sharpe (MRS) medium (Difco Laboratory, Detroit, MI, USA) and collected at the early stationary phase. After LcS was washed with distilled water, it was freeze-dried, adjusted with PBS to a concentration of 1 mg/ml and autoclaved. PBMC were suspended in AIM-V medium (Gibco brl, Rockville, MD, USA) and cultured at 2 × 106 cells/well in medium alone or in the presence of LcS at 1 µg/ml in humidified 5% CO2 in air at 37°C for 6 days.
NK cells were enriched from fresh and LcS-stimulated PBMC with CD56 microbeads (Miltenyi Biotec GmbH, Gladbach, Germany). Positively selected fractions contained both CD3–CD56+ cells (NK cells) and CD3+CD56+ cells (NK T cells), and the percentage of NK cells and NK T cells in these selected fractions was 91·6 ± 4·7% and 4·6 ± 2·6% in fresh PBMC, and 80·1 ± 10·1% and 10·0 ± 6·5% in LcS-stimulated PBMC, respectively.
To examine the involvement of cell-to-cell contact between monocytes and NK cells in LcS-triggered enhancement of NK cell activity, CD14+ cell-enriched fraction (CD14+ Fr.) and CD14+ cell-depleted fraction (CD14– Fr.) were prepared from PBMC with CD14 microbeads (Miltenyi Biotec GmbH). CD14+ Fr. contained CD14+ cells in a proportion of 80·2 ± 8·8%, and the percentage of CD3–CD56+, CD3+CD56+ and CD14+ cells in CD14– Fr. was 7·6 ± 3·6%, 2·1 ± 1·2% and 0·3 ± 0·1%, respectively.
CD14– Fr. was plated at 1·6 × 106 cells/well of 12F-well plates, and 5 × 105 cells of CD14+ Fr were added into a 12F-well plate or the inside of a transwell plate (Corning Inc., Corning, NY, USA) with LcS at 1 µg/ml. In culture, rIL-12 (R&D Systems, Inc., Minneapolis, MN, USA) was added at 4 ng/ml into a transwell plate. After 6 days’ culture, culture supernatants were recovered for measurement of IL-12p40 and surviving cells in the well of the 12F-well plate were measured for NK cell activity.
To neutralize IL-12, anti-IL-12 MoAb (mouse IgG1, clone 8·6) or control mouse IgG1 (clone MOPC21; both from BD Pharmingen) was added at 10 µg/ml together with LcS, and PBMC were cultured for 6 days. To assess the direct effect of IL-12 on NK cell activity, PBMC suspended in AIM-V medium were cultured in medium alone or in the presence of rIL-12 (0·1, 1 or 10 ng/ml) for 6 days.
Measurement of cytokines
The PBMC culture supernatant was recovered and kept at −30°C until analysis. Concentration of IL-12p40, interferon (IFN)-γ and IL-6 was measured by enzyme-linked immunosorbent assay (ELISA) with anti-IL-12 MoAbs (clone A08E6E5 and clone A25C4B6), anti-IFN-γ MoAbs (clone 350B10G6 and 67F12A8) and anti-IL-6 MoAbs (clone 677B6A2 and clone 505E23C7), respectively (all from Biosource International, Camarillo, CA, USA).
Statistical analysis
Significant differences between groups was evaluated by unpaired Mann–Whitney statistics using two-sided tests.
Results
Change of NK cell activity by intake of fermented milk containing LcS
The volunteers drank fermented milk containing LcS or a placebo for 3 weeks, with cross-over repetition of the supplementation course after 7 weeks. Before intake of either fermented milk containing LcS or a placebo, NK cell activity was comparable (Fig. 1a). In contrast, NK cell activity after the intake of fermented milk containing LcS or a placebo was significantly different (Fig. 1b). The average increase of NK cell activity by intake of fermented milk containing LcS was 3·7% at an effector : target (E : T) ratio = 20 and 2·2% at E : T = 10, while the average change of NK cell activity by intake of placebo was −10·5% at E : T = 20 and −5·7% at E : T = 10. As a result, change in NK cell activity by fermented milk intake containing LcS was significantly greater than that by placebo intake.
Fig. 1.
Change of natural killer (NK) cell activity in peripheral blood mononuclear cells (PBMC) by intake of fermented milk containing Lactobacillus casei Shirota (LcS) or a placebo. NK cell activity in PBMC was measured before (a) and after (b) intake of fermented milk containing LcS (•) or a placebo (○). *P < 0·05. (c) Relationship between change of NK cell activity 3 weeks after the beginning of intake of fermented milk containing LcS (•) or a placebo (○) and the level of NK cell activity before intake.
The relationship between NK cell activity before intake and change of NK cell activity by intake was examined. Change of NK cell activity by placebo intake was correlated inversely with NK cell activity before intake. Similarly, change of NK cell activity by fermented milk intake containing LcS was related negatively with the value before intake, and the regression line obtained during intake of fermented milk containing LcS shifted upwards compared with that obtained during placebo intake (Fig. 1c).
Biochemical parameters in serum were checked before and after the intake of a placebo or fermented milk containing LcS. Most parameters (total protein, albumin, creatinine, cholesterol, glutamic-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), alkaline phosphatase (ALP) and γ-GTP) did not change by the intake of either placebo or fermented milk containing LcS. Neutral lipid and uric acid levels after the placebo intake were slightly higher than those before the placebo intake, but after cessation of placebo intake levels returned to those before intake (data not shown).
Enhancement of NK cell activity of PBMC by LcS
To examine the effect of LcS on human NK cell activity, we added LcS to the culture of PBMC at 1, 10 or 100 µg/ml and found that the addition of LcS at 1 µg/ml stimulated proliferation and IFN-γ production of PBMC to the greatest extent (data not shown). When PBMC were cultured in medium alone for 6 days, NK cell activity almost disappeared. In contrast, PBMC cultured in the presence of LcS at 1 µg/ml exhibited significantly higher NK cell activity than fresh PBMC (Fig. 2a). The relative proportion of NK cells was not different between fresh PBMC and cultured PBMC in the presence of LcS (data not shown), suggesting that LcS could enhance NK cell activity on a cellular basis.
Fig. 2.
In vitro effect of Lactobacillus casei Shirota (LcS) on natural killer (NK) cell activity. (a) Peripheral blood mononuclear cells (PBMC) were cultured in medium alone (none) or in the presence of LcS at 1 µg/ml (LcS) for 6 days, and NK cell activity of the cultured cells was compared with that of fresh PBMC. Data are shown as mean ± s.e. of 17 subjects. ***P < 0·001 compared with fresh PBMC. (b) Relationship between the ability of LcS to induce interleukin (IL)-12 production and enhance NK cell activity. PBMC were cultured with LcS at 1 µg/ml for 6 days, and IL-12 concentration in the culture supernatant and NK cell activity of surviving cells was measured.
In fresh PBMC, NK cell activity was detected in CD56+ cell-enriched fraction and CD56+ cell-depleted fraction had negligible NK cell activity. Similarly, NK cell activity was detected in the CD56+ cell-enriched fraction, and NK cell activity in the CD56+ cell-enriched fraction prepared from LcS-stimulated PBMC was significantly higher than that from fresh PBMC (CD56+ cells in fresh PBMC, 39·2 ± 7·0 IU/106 cells, n = 4; CD56+ cells in LcS-stimulated PBMC, 129·9 ± 10·3 IU/106 cells, n = 4; P < 0·05). These results support LcS-enhanced NK cell activity at a cellular basis.
It is known that LcS induces macrophages to produce IL-12, the cytokine capable of enhancing NK cell activity [15]. To investigate the mechanism of how LcS augments NK cell activity in vitro, we examined the ability of LcS to induce IL-12 production and enhance NK cell activity. As a result, concentrations of IL-12 in the culture supernatant and NK cell activity of surviving cells after culturing PBMC in the presence of LcS were correlated (Fig. 2b).
Involvement of IL-12 in LcS-triggered enhancement of NK cell activity
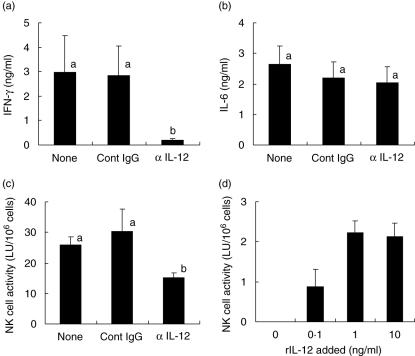
To explore further the involvement of IL-12 in the enhancement of NK cell activity by LcS, anti-IL-12 MoAb was added to the culture of PBMC together with LcS. IFN-γ production was significantly inhibited by anti-IL-12 MoAb but not by control IgG1 (Fig. 3a). On the other hand, neither anti-IL-12 MoAb nor control IgG1 inhibited IL-6 production (Fig. 3b), confirming the specificity of anti-IL-12 MoAb. Control IgG1 had no effect on NK cell activity enhanced by LcS. In contrast, anti-IL-12 MoAb significantly reduced the average NK cell activity enhanced by LcS, although a significant level of NK cell activity was still detected in the presence of anti-IL-12 MoAb (Fig. 3c).
Fig. 3.
Involvement of interleukin (IL)-12 in Lactobacillus casei Shirota (LcS)-triggered enhancement of natural killer (NK) cell activity. Peripheral blood mononuclear cells (PBMC) were cultured with LcS at 1 µg/ml for 6 days in the absence (none) or presence of control mouse IgG1 (Cont IgG) or anti-IL-12 monoclonal antibody (MoAb) (αIL-12). (a) Interferon (IFN)-γ production; (b) IL-6 production; (c) NK cell activity were analysed. Data are mean ± s.e. of eight subjects. Means without a common letter differ (P < 0·05). (d) Effect of rIL-12 on NK cell activity. PBMC were cultured in medium alone or in the presence of rIL-12 at 0·1, 1 or 10 ng/ml for 6 days, and NK cell activity of the cultured cells was measured. Data are mean ± s.e. of three subjects.
We checked the direct effect of rIL-12 on human NK cell activity. When PBMC were cultured in medium alone for 6 days, NK cell activity had almost disappeared. In contrast, addition of rIL-12 to the PBMC culture enhanced NK cell activity and the maximal effect was achieved at 1 ng/ml (Fig. 3d). However, rIL-12-induced enhancement of NK cell activity was not as great as that induced by LcS (compare with data shown in Figs 2 and 3).
Participation of cell-to-cell contact in LcS-triggered enhancement of NK cell activity
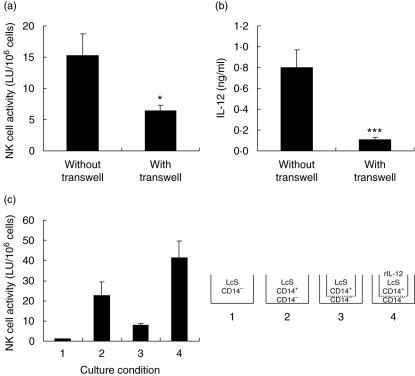
As NK cell activity was enhanced by LcS even in the presence of anti-IL-12 MoAb (Fig. 3c), we tried to examine the effect of other mechanisms. CD14+ cell-depleted cells (CD14– Fr.) and CD14+ cell-enriched cells (CD14+ Fr.) were prepared from fresh PBMC and were cultured together or by separating them using transwell. When CD14– Fr. and CD14+ Fr. were cultured in the presence of LcS, NK cell activity was enhanced. In contrast, after culturing CD14– Fr. without making contact with LcS-triggered CD14+ Fr., NK cell activity was reduced to intermediate level (Fig. 4a). Additionally, IL-12 was produced when CD14– Fr. and CD14+ Fr. were cultured together, but the level of IL-12 produced by LcS-triggered CD14+ Fr. separated from CD14– Fr. was markedly lower (Fig. 4b).
Fig. 4.
Participation of cell-to-cell contact in Lactobacillus casei Shirota (LcS)-triggered enhancement of natural killer (NK) cell activity. CD14+ cell-enriched fraction (CD14+ Fr.) were cultured in 12F-well plate together with CD14+ Fr. in the presence of LcS at 1 µg/ml (without transwell) or left in 12F-well plates without making contact with CD14+ Fr. and LcS at 1 µg/ml given inside transwell (with transwell). After 6 days’ culture, NK cell activity of the surviving cells (a) and interleukin (IL)-12 concentration in the culture supernatants (b) were measured. Data are shown as mean ± s.e. of eight subjects. *P < 0·05; ***P < 0·001. (c) CD14– Fr. and/or CD14+ Fr. were cultured in the conditions shown for 6 days, and NK cell activity of the surviving cells was measured. Data are mean ± s.e. of three subjects.
Finally, we examined the effect of IL-12 on NK cell activity in CD14– Fr. separated from LcS-triggered CD14+ Fr. by transwell. CD14– Fr. alone, even when stimulated by LcS, scarcely maintained NK cell activity (Fig. 4c, condition 1). The addition of CD14+ Fr. to CD14– Fr. together with LcS induced enhancement of NK cell activity (Fig. 4c, condition 2) and separation of CD14– Fr. from LcS-triggered CD14+ Fr., induced an intermediate level of NK cell activity (Fig. 4c, condition 3). When rIL-12 was added at 4 ng/ml into the transwell, NK cell activity of CD14– Fr. was recovered (Fig. 4c, condition 4).
Discussion
In this report, we examined the effect of intake of fermented milk containing LcS on human NK cells. The results showed that intake of fermented milk containing LcS for 3 weeks enhanced NK cell activity but intake of a placebo did not. It is of importance to point out that NK cell activity tended to decline during intake of a placebo in this supplementation study. As the volunteers in this study were elderly (aged 69–97 years), it is likely that their NK cell activity is unstable. We therefore measured the NK cell activity of the volunteers three times at intervals within 6 months and found that their basal NK cell activity was relatively stable during this period (K. Takeda, unpublished data). Additionally, it is known that NK cell activity can be influenced easily by lifestyle or various mental stresses [6,7]; therefore, we selected hospitalized subjects with constant lifestyle and food. However, hospitalization may also be a factor in influencing NK cell activity [8], and therefore the participants in this supplementation study might experience mental stress, resulting in vulnerable NK cell activity. We cannot exclude the possibility that a placebo might have some detrimental effects on NK cell activity in the elderly. However, whether drinking either a placebo or fermented milk containing LcS, biochemical parameters scarcely changed during the study.
Intake of fermented milk containing LcS did not increase the proportion of NK cells in PBMC. Therefore, we consider that the major effect of LcS might be enhancement of NK cell activity at a cellular basis. NK cell activity in CD56+ cell-enriched fraction was significantly higher in LcS-stimulated PBMC than fresh PBMC, supporting that LcS can enhance NK cell activity per cell. Several groups have reported the augmentation of NK cell activity by probiotics [16–19]. These results demonstrate that some probiotics may enhance NK cell activity via enhancement of NK cell activity on a cellular basis, but others may augment NK cell activity as a whole through stimulating the expansion of NK cells.
Using human PBMC in vitro culture, we found a correlation between the ability of LcS to induce IL-12 production and enhancement of NK cell activity. LcS, as well as other Lactobacillus strains, could stimulate monocytes or macrophages to secrete IL-12 [20–22], a cytokine capable of augmenting NK cell activity efficiently [15]. The addition of anti-IL-12 MoAb into LcS-stimulated PBMC inhibited IFN-γ production almost completely and significantly reduced NK cell activity, confirming that IL-12 is responsible for the activation of NK cells. However, NK cell activity was still detected even when anti-IL-12 MoAb was added. These results indicate that mechanisms other than IL-12 may be involved in the activation of NK cells.
NK cells expanded dramatically and NK cell activity was enhanced when murine splenocytes were cultured in the presence of IL-12 and IL-18 [23,24]. Moreover, a combination of IL-12 and IL-18 induced production of IFN-γ, IL-6 and tumour necrosis factor (TNF) [23]. As LcS stimulated human PBMC to produce IFN-γ, IL-6 and TNF-α as well as IL-12 (Fig. 3 and data not shown), IL-18 may be produced by LcS-triggered PBMC. In addition, IL-10 augments NK cell proliferation and cytotoxic activity when combined with IL-18 [25], but NK cell activity was increased by neutralization of endogenous IL-10 in patients with tuberculosis [26]. As IL-10 can be detected in the culture supernatant of human PBMC stimulated with LcS (K. Shida, unpublished data), it is of interest to clarify whether IL-10 induced by LcS may assist the enhancement of NK cell activity or suppress NK cell activity.
Mycobacterium bovis bacilli Calmette–Guérin stimulated human NK cells to enhance cytotoxic activity in an IL-12-independent fashion [27], and long-term supplementation of β-carotene enhanced NK cell activity in the elderly while IL-12 production by PBMC was not affected by β-carotene [28]. These results suggest that other mechanisms than IL-12 could be involved in NK cell activation. Haller et al. have shown that L. johnsonii La1 activated human NK cells to induce expression of CD69 antigen and produce IFN-γ when autologous monocytes were co-cultured [29,30], and Sartorius et al. have reported that anti-CD40 MoAb-engaged antigen-presenting cells could enhance NK cell activity [31]. These findings support that NK cells can be activated by cell-to-cell interaction with activated antigen-presenting cells such as macrophages and dendritic cells. We found that separation of CD14– Fr. from LcS-triggered CD14+ Fr. reduced NK cell activity, demonstrating that cell-to-cell contact may be important for LcS to augment NK cell activity fully. Moreover, when cell-to-cell contact of CD14– Fr. with CD14+ Fr. was inhibited, IL-12 production was almost deprived. These findings suggest that IL-12 production by monocytes is dependent on cell-to-cell contact.
It is likely that IL-12 production by monocytes may be stimulated through cell-to-cell interaction between T cells and monocytes. However, T cell-depleted PBMC as well as unfractionated PBMC produced IL-12 and showed enhancement of NK cell activity in response to LcS [32]. Therefore, T cells may not be indispensable in LcS-triggered NK cell activation.
It has been proposed recently that NK cell activity is enhanced by interaction with murine cytomegalovirus-infected CD11b+, dendritic cells and this interaction is mediated by IFN-α and the NKG2D/NKG2D ligand [33]. On the other hand, LcS did not stimulate PBMC to produce IFN-α, and major histocompatibility complex class I-related chain gene A (MICA) expression was not induced on monocytes stimulated with LcS (T. Suzuki, unpublished data). Consequently, other mechanisms than IFN-α and interaction through the NKG2D/NKG2D ligand may be responsible for augmentation of NK cell activity induced by LcS.
This report, together with previous results [11–13], concludes that daily intake of fermented milk containing LcS restores NK cell activity that may deteriorate through mental stress, an undesirable lifestyle or chronic viral infection in humans. Immune surveillance by NK cells is one of the principal defences against cancer and infection. From this viewpoint, supplementation of fermented milk containing LcS may be helpful to health maintenance.
Acknowledgments
We are grateful to Dr Fumiyasu Ishikawa (Yakult Central Institute) for critical reading of the manuscript. We also thank Ms Junko Kiyoshima-Shibata and Ms Noriko Kato-Nagaoka for their excellent technical assistance.
References
- 1.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 2.Ogata K, An E, Shioi Y, et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol. 2001;124:392–7. doi: 10.1046/j.1365-2249.2001.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo G, Balistreri CR, Candore G, et al. Granulocyte and natural killer activity in the elderly. Mech Ageing Dev. 1999;108:25–38. doi: 10.1016/s0047-6374(98)00156-0. [DOI] [PubMed] [Google Scholar]
- 5.Lutz CT, Moore MB, Bradley S, Shelton BJ, Lutgendorf SK. Reciprocal change in natural killer cell receptors for MHC class I. Mech Ageing Dev. 2005;126:722–31. doi: 10.1016/j.mad.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusaka Y, Kondou H, Morimoto K. Healthy lifestyles are associated with higher natural killer cell activity. Prev Med. 1992;21:602–15. doi: 10.1016/0091-7435(92)90068-s. [DOI] [PubMed] [Google Scholar]
- 7.Irwin M, Daniels M, Smith TL, Bloom E, Weiner H. Impaired natural killer cell activity during bereavement. Brain Behav Immun. 1987;1:98–104. doi: 10.1016/0889-1591(87)90011-0. [DOI] [PubMed] [Google Scholar]
- 8.Irwin M, Lacher U, Caldwell C. Depression and reduced natural killer cytotoxicity: a longitudinal study of depressed patients and control subjects. Psychol Med. 1992;22:1045–50. doi: 10.1017/s0033291700038617. [DOI] [PubMed] [Google Scholar]
- 9.Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S for the the BLP Study Group. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. Eur Urol. 1995;27:104–9. doi: 10.1159/000475138. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa H, Akedo I, Otani T, et al. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer. 2005;116:762–7. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 11.Nagao F, Nakayama M, Muto T, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy subjects. Biosci Biotechnol Biochem. 2000;64:2706–8. doi: 10.1271/bbb.64.2706. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto K, Takeshita T, Nanno M, Tokudome S, Nakayama K. Modulation of natural killer cell activity by supplementation of fermented milk containing Lactobacillus casei in habitual smokers. Prev Med. 2005;40:589–94. doi: 10.1016/j.ypmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki T, Saito M, Usuku K, et al. A prospective uncontrolled trial of fermented milk drink containing viable Lactobacillus casei strain Shirota in the treatment of HTLV-1 associated myelopathy/tropical spastic paraparesis. J Neurol Sci. 2005;237:75–81. doi: 10.1016/j.jns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Okumura K. CAM and NK cells. Evid Based Compl Altern Med. 2004;1:17–27. doi: 10.1093/ecam/neh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodama T, Takeda K, Shimozato O, et al. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur J Immunol. 1999;29:1390–6. doi: 10.1002/(SICI)1521-4141(199904)29:04<1390::AID-IMMU1390>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001;21:264–71. doi: 10.1023/a:1010979225018. [DOI] [PubMed] [Google Scholar]
- 17.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–9. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 18.Bunout D, Barrera G, Hirsh S, et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. J Parent Ent Nutr. 2004;28:348–54. doi: 10.1177/0148607104028005348. [DOI] [PubMed] [Google Scholar]
- 19.Parra MD, Martinez de Morentin BE, Cobo JM, Mateos A, Martinez JA. Daily ingestion of fermented milk containing Lactobacillus casei DN114001 improves innate-defense capacity in healthy middle-aged people. J Physiol Biochem. 2004;60:85–91. doi: 10.1007/BF03168444. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen M, Matikainen S, Vuopio-Varkila J, et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–62. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shida K, Kiyoshima-Shibata J, Nagaoka M, Watanabe K, Nanno M. Effective induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci. in press. [DOI] [PubMed]
- 22.Kato I, Tanaka K, Yokokura T. Lactic acid bacterium potently induces the production of interleukin-12 and interferon-γ by mouse splenocytes. Int J Immunopharmacol. 1999;21:121–31. doi: 10.1016/s0192-0561(98)00072-1. [DOI] [PubMed] [Google Scholar]
- 23.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–30. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 24.Hyodo Y, Matsui K, Hayashi N, et al. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–8. [PubMed] [Google Scholar]
- 25.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur J Immunol. 1999;29:2658–65. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Schierloh P, Aleman M, Yokobori N, et al. NK cell activity in tuberculosis is associated with impaired CD11a and ICAM-1 expression: a regulatory role of monocytes in NK activation. Immunology. 2005;116:541–52. doi: 10.1111/j.1365-2567.2005.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esin S, Batoni G, Pardini M, et al. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacilli Calmette–Guérin. Immunology. 2004;112:143–52. doi: 10.1111/j.1365-2567.2004.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos MS, Gaziano JM, Leka LS, Beharka AA, Hennekens CH, Meydani SN. β-carotene-induced enhancement of natural killer cell activity in elderly men: an investigation of the role of cytokines. Am J Clin Nutr. 1998;68:164–70. doi: 10.1093/ajcn/68.1.164. [DOI] [PubMed] [Google Scholar]
- 29.Haller D, Blum S, Bode C, Hammes WP, Schiffrin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect Immun. 2000;68:752–9. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haller D, Serrant P, Granato D, Schiffrin EJ, Blum S. Activation of human NK cells by staphylococci and lactobacilli requires cell contact-dependent costimulation by autologous monocytes. Clin Diag Lab Immunol. 2002;9:649–57. doi: 10.1128/CDLI.9.3.649-657.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartorius R, D’Apice L, Barba P, et al. Induction of human NK cell-mediated cytotoxicity by CD40 triggering on antigen presenting cells. Cell Immunol. 2003;221:81–8. doi: 10.1016/s0008-8749(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 32.Shida K, Suzuki T, Kiyoshima-Shibata J, Shimada S, Nanno M. Essential roles of monocytes in stimulating human peripheral blood mononuclear cells with Lactobacillus casei to produce cytokines and augment natural killer cell activity. Clin Vaccine Immunol. 2006;13 doi: 10.1128/CVI.00076-06. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andoniou CE, van Dommelen SL, Voigt V, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–9. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]