Abstract
Previously we reported that during protracted morphine abstinence rats show reduced conditioned place preferences (CPP) for food-associated environments, compared to non-dependent subjects. To determine the brain regions involved in this altered reward behavior, we examined neural activation (as indexed by Fos-like proteins) induced by a preference test for a food-associated environment in five-week morphine-abstinent vs non-dependent animals. The results indicate that elevated Fos expression in the anterior cingulate cortex (Cg) and basolateral amygdala (BLA) correlated positively with preference behavior in all groups. In contrast, Fos expression in stress-associated brain areas, including the ventral lateral bed nucleus of the stria terminalis (VL-BNST), central nucleus of the amygdala (CE), and noradrenergic (A2) neurons in the nucleus tractus solitarius (NTS) was significantly elevated only in morphine-abstinent animals. Furthermore, the number of Fos positive neurons in these areas was found to correlate negatively with food preference in abstinent animals. These results indicate that the altered hedonic processing during protracted morphine withdrawal leading to decreased preference for cues associated with natural rewards may involve heightened activity in stress-related brain areas of the extended amygdala and their medullary noradrenergic inputs.
Keywords: Morphine Dependence, Conditioned Place Preference, Extended Amygdala, Opiate Withdrawal, Food Conditioning, Amygdala
1. Introduction
During the process of addiction, the transition from recreational drug use to compulsive use may be caused by neurochemical changes in the brain that produce a dysregulation of normal brain reward function. One current hypothesis [32] for such a process suggests that drugs can over-stimulate brain reward circuitry leading to a decrease in overall reward function. Evidence for a decrease in the rewarding value of various types of natural rewards has been shown following withdrawal from a number of different drugs [1,3,4,14,24,37,40,44]. Furthermore, in conjunction with these changes in reward function, drug use leads to an increase in the stress response and increased aversive mental states (i.e., anxiety and dysphoria) which may also negatively impact the value of natural rewards [26,32,33,39].
These maladaptive changes that mediate the dysregulation in reward function are hypothesized to occur specifically within extended amygdala circuitry [31]. The extended amygdala includes the bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CE) and select intervening areas that share similar cytoarchitecture and circuitry [28]. The extended amygdala receives afferents from limbic structures including the basolateral amygdala (BLA) [28], and from medullary structures like the A2 noradrenergic region of the nucleus tractus solitarius (NTS) [11,12,42,46]. The BLA conveys sensory information important for conditioning processes [49], and the NTS transmits visceral responses that have been linked to emotional processes [41,46]. As a result, both the CE and BNST could be expected to integrate viscerally pertinent information from the NTS with stimulus information from the BLA in order to label particular stimulus events as emotionally relevant. This illustrates one way that the extended amygdala and its medullary noradrenergic inputs could be involved in reward dysregulation. Support for this idea can be found in a number of studies that implicate the extended amygdala in the affective disorders associated with drug withdrawal [12,21,29].
Previously we reported that after five weeks of morphine abstinence rats show enhanced preferences for morphine-associated cues relative to placebo-pretreated (i.e., non-dependent) subjects [22]. This enhanced preference correlated specifically in morphine-withdrawn animals with elevated Fos expression in the ventral lateral BNST (VL-BNST) [23]. We also reported that at this same withdrawal time-point, when morphine-abstinent animals show enhanced preferences for morphine-associated cues, they also exhibit significantly decreased preferences for food-associated cues [24]. The brain areas involved in this withdrawal-induced decreased preference for natural reward-associated cues are unknown. Here, we sought to identify such brain areas by comparing Fos expression (as a marker of neural stimulation) in several brain areas during conditioned place preference (CPP) testing for food in five-week morphine abstinent vs non-dependent rats. This paradigm is thought to reflect a conditioned approach response to cues previously associated with reward. The brain areas examined included the anterior cingulate cortex (Cg) and BLA (regions that we previously identified as being correlated with the expression of conditioned place preference) [23], the dorsomedial hypothalamus (DMH) (an area involved in both homeostatic regulation and feeding behavior) [10,13], and the VL-BNST, CE and NTS (areas known to be associated with morphine withdrawal aversion) [12,55].
2. Materials and Methods
2.1 Subjects
Male Sprague-Dawley rats (200–250 g) from Harlan (Indianapolis, IN) were used in all experiments. Rats were group housed in accordance with NIH guidelines on a 12-h light/dark cycle with food and water available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. A total of 26 animals were used with individual group numbers of six to seven animals.
2.2 Chronic drug treatment
Two 75-mg morphine tablets (provided by the National Institute on Drug Abuse) were subcutaneously implanted under halothane anesthesia to induce morphine dependence. Non-dependent rats were implanted at the same time with two inert placebo pellets. Previous studies have shown that morphine pellets are a reliable way to induce physical dependence [19,56]. Deprivation withdrawal (a model of abstinence) was induced by removing any remaining pellet residue after 14 days. Somatic signs of withdrawal in this abstinence model were very mild (slight weight loss, rhinorrhea, lacrimation), and disappeared within a few days.
2.3 Conditioned place preference procedure
The place preference procedure was carried out in a Plexiglas apparatus consisting of 2 distinct compartments, as previously described [24]. One compartment had a grid floor with black walls, and the second compartment had a mesh floor with black and white stripes on the walls. The apparatus was equipped with photocells to automatically record the time that animals spent in each compartment (MED Associates, East Fairfield, VT). One week prior to conditioning, animals were pre-exposed to the food (oat flavored Lucky Charms cereal) to avoid a neophobic response. Because the food was highly desirable, it was not necessary to use food deprivation. On the first day of the conditioned place preference procedure, animals were allowed to freely explore all of the apparatus for 15 min, and the amount of time spent in each compartment was recorded (n = 6 per group). None of the animals had a substantial initial bias for either compartment, and therefore rats were randomly assigned to one compartment for food conditioning in a balanced design. Animals were conditioned four weeks after pellet removal, long after all somatic signs of opiate withdrawal had dissipated. For conditioning sessions, animals were alternately placed into one compartment that contained 6 pieces of cereal, or the other compartment which had no food but contained an empty plastic dish similar to the one used to hold the cereal in the other compartment. Conditioning sessions occurred in the morning and afternoon and the presentation of the food was alternated between the morning and afternoon sessions on three separate days. Conditioning sessions were 20 min long and all rats consumed all the food presented during the sessions. The preference test occurred five days following conditioning during which the animals were given free access to the apparatus without the plastic dishes or food for 15 min. The amount of time spent in each compartment was recorded.
Non-conditioned control animals were also exposed to morphine or placebo pellets and withdrawn for 4 weeks (n = 4 per group). These animals spent the same amount of time in each of the place conditioning compartments as the conditioned animals but they had no exposure to the cereal reward. On what would have been the conditioning test day, these animals were allowed to freely explore both compartments. The amount of time spent in each compartment was recorded.
2.4 Fos protein immunoreactivity
Two hours after the preference test animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and transcardially perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4. The brains were removed and stored overnight in 4% paraformaldehyde. They were then transferred to a 20% sucrose solution and stored at 4°C for 5 days. Coronal sections (40 um) were cut using a freezing microtome. Sections from placebo and morphine animals were processed together to equalize staining between groups. Sections were placed in a solution of 0.1 M PBS with 0.3% Triton-X added (PBS-Tx, pH 7.4) containing 2% normal donkey serum for 3 hours. Sections were incubated overnight at room temperature in this same solution with the addition of primary antibody (rabbit antiserum against Fos-related antigens at 1:20,000, Oncogene Sciences, Cambridge, MA). Sections were rinsed 3 times in PBS-Tx and then incubated for 2 hours with the secondary antibody (biotinylated donkey anti-rabbit 1:1000, Jackson Immunoresearch Laboratories, West Grove, PA). After 3 rinses in PBS-Tx, sections were transferred to an avidin-biotin complex (1:1000, Jackson Immunoresearch Laboratories) for 1.5 hours. Sections were again rinsed 2 times with PBS-Tx and once with 0.05 M Tris buffer. Fos related antigen-positive (denoted here as simply Fos) cells were visualized by placing tissue in 3,3′-diaminobenzidine (DAB, 0.02%, Sigma, St Louis, MO) with 0.0002% H2O2 and 0.6% nickel ammonium sulfate in 0.01 M Tris buffer for 3.5 min. This reaction was arrested by immediate transfer into 0.05 M Tris buffer. The sections were mounted on gelatin coated slides, stained with neutral red to identify specific structures, dehydrated through graded alcohols, cleared in xylene, and coverslipped with Permount.
Sections from the NTS were counterstained with tyrosine hydroxylase (TH) to identify noradrenergic neurons of the A2 group. For these sections, after completion of the Fos reaction described above, sections were placed in primary antibody against TH overnight at room temperature (mouse antiserum at 1:6000, ImmunoStar, Hudson WI). The reaction was then carried out the same as the Fos reaction (secondary anti-mouse 1:500, Jackson Immunoresearch Laboratories), except without nickel intensification. Double-labeled cells were easy to identify as TH cell bodies were stained brown and Fos-positive nuclei (nickel intensified) were stained black.
Quantification of Fos positive cells was done using Openlab image processing software (Improvision, Ltd.; Coventry England) on a Macintosh computer that was linked to a microscope and digital camera. Color images of the areas of interest were taken and saved to disc. The numbers of Fos-positive nuclei in regions of interest were counted with a point counter tool on the saved image. This tool simultaneously marked and counted each cell so that no cells could be counted twice and the total number of cells counted was available. Two sections at each level were randomly selected from each animal. The levels chosen corresponded to the following distances from bregma [45]: Cg (+2.20 mm), VL-BNST (−0.26 mm), CE, BLA and DMH (−2.80 mm) and NTS (−14.06) (Fig. 1). Fos-positive neurons in the structures of interest from both the right and left hemispheres from the two sections were counted by a blind observer and averaged into a single score. Because the analysis of Fos staining in the NTS was confined to the region of TH positive cells, we analyzed the Fos levels in this area as the percentage of Fos-positive TH cells.
Fig. 1.
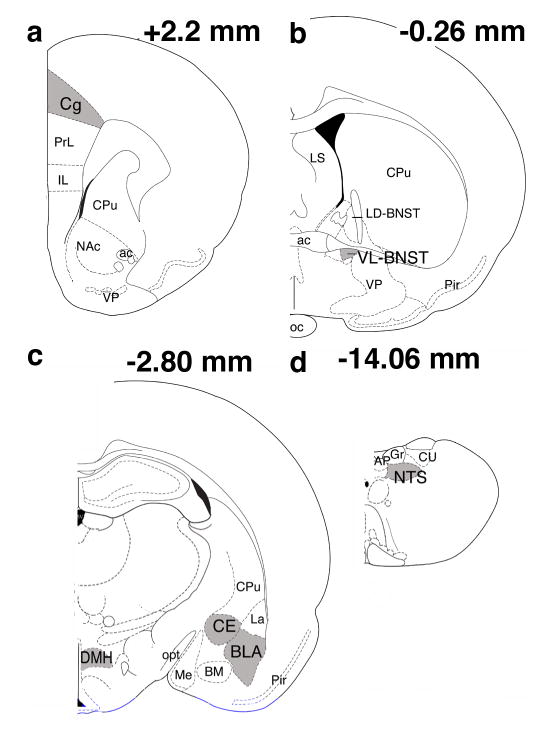
Schematic frontal representations of regions analyzed for Fos-positive neurons. Gray-shaded regions indicate the areas where Fos+ neurons were counted. a. anterior cingulate cortex (Cg). b. ventral lateral portion of the bed nucleus of the stria terminalis (VL-BNST) c. central (CE) and basolateral (BLA) nuclei of the amygdala and dorsomedial hypothalamus (DMH). d. nucleus of the solitary tract (NTS). Numbers in the upper right-hand corner of each brain section represent the distance from bregma. Drawings were adapted from Paxinos and Watson (1998). ac– anterior commissure, AP-area postrema, BM- basomedial amygdala, Cpu- caudate putamen, CU- nucleus cuneatus, Gr- nucleus gracilis, IL- infralimbic cortex, LD-BNST- lateral dorsal portion of the bed nucleus of the stria terminalis, La- lateral amygdala, LS- lateral septum, Me- medial amygdala, NAc- nucleus accumbens, oc- optic chiasm, opt- optic tract, Pir- piriform cortex, PrL– prelimbic cortex, cortex, VP– ventral pallidum.
2.5 Data analysis
Place conditioning data were analyzed by calculating the time spent in the food-paired compartment minus the time spent in the other (non-rewarded) compartment. The resulting difference (preference score) was compared between drug-treated and placebo groups using a two-way analysis of variance (ANOVA; drug treatment (morphine vs placebo) and conditioning (conditioned vs non-conditioned)). In the case of non-conditioned animals alternating sides of the conditioning box were pre-selected prior to any treatment and randomly assigned to be the reference compartment for calculating a preference score. Fos data were also analyzed with a two-way ANOVA. Follow-up comparisons were done using Fisher’s PLSD. Correlations between preferences scores and Fos levels in the various brain areas in each group were performed using the Pearson’s product-moment correlation coefficient (r).
3. Results
3.1 Place conditioning
Fig. 2a shows the results of the food place conditioning. None of the groups were significantly different from each other during the preconditioning test (F(1,22)=0.3, P=.82). After conditioning, significant main effects for conditioning, drug treatment, and the conditioning by drug treatment interaction were found. The F values for these results are presented in Table 1. Follow-up tests revealed that conditioned animals had significantly higher preference scores than non-conditioned animals, and that placebo-pretreated conditioned animals showed significantly greater preferences for the food-paired compartment (P<.01) than morphine-pretreated conditioned animals. Fig. 2b shows the total amount of locomotor activity exhibited by each group on the test day. No significant group differences were seen in locomotor activity between the groups (F(1,22)= 0.2, P=.90).
Fig. 2.
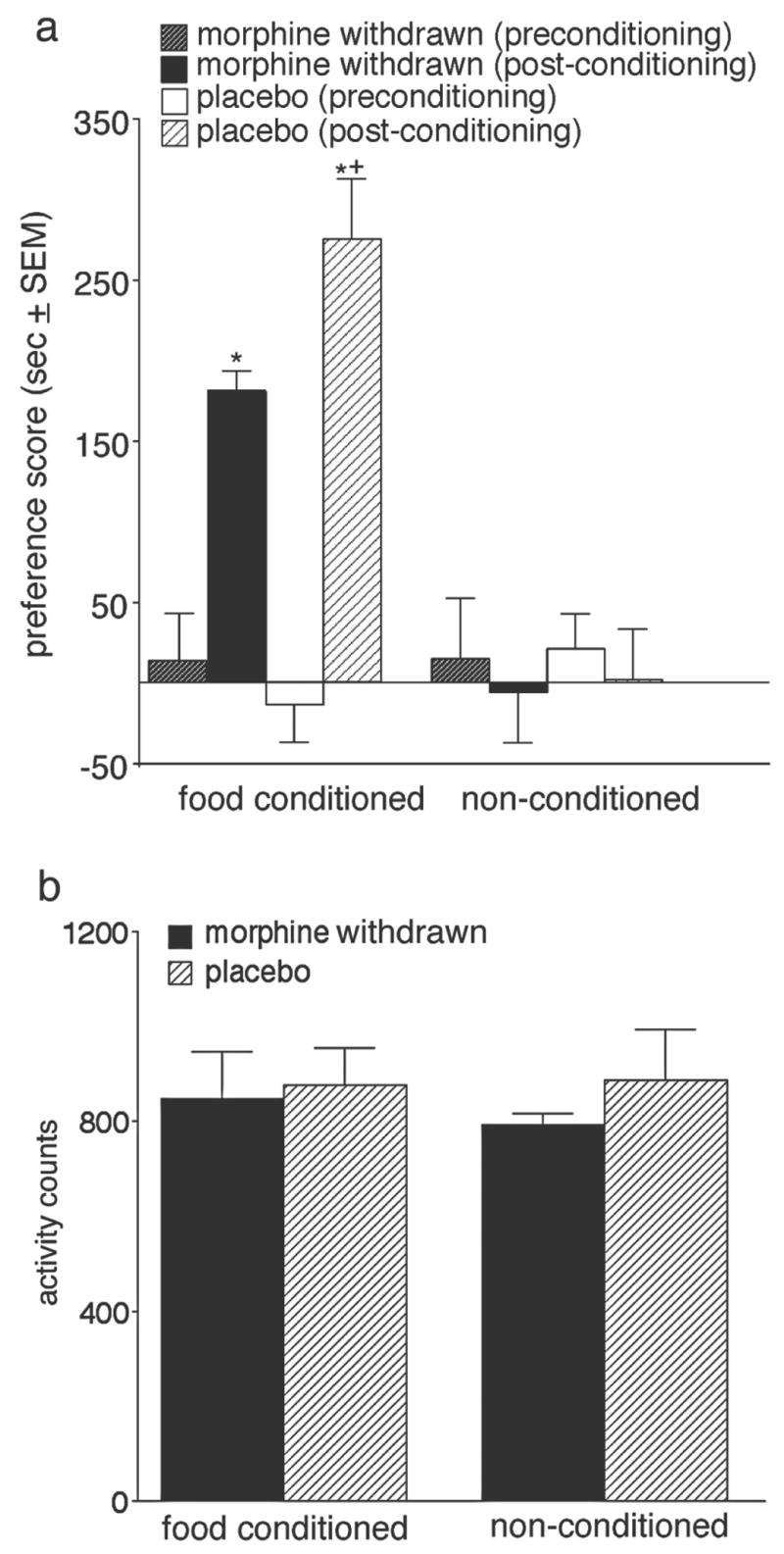
a. Preference scores pre- and post-conditioning for food-conditioned and non-conditioned groups expressed as the mean time in seconds spent in the food-paired or pre-selected reference compartment (in the case of the non-conditioned group) minus the mean time in seconds spent in the other compartment on the test day. *significantly different from preconditioning scores and from non-conditioned animals (P<.01). +denotes significant differences between conditioned groups (P<.01). b. Mean activity scores on the test day ± SEM for conditioned and non-conditioned groups.
Table 1.
ANOVA results
| drug treatment main effect | conditioning main effect | interaction | |
|---|---|---|---|
| CPP | F=8, P<.01 | F=123,P<.01 | F=7, P<.05 |
| Cg | F=6, P<.05 | F=49, P<.01 | F=7, P<.05 |
| BLA | F=14,P<.01 | F=187,P<.01 | F=19,P<.01 |
| VL-BNST | F=25,P<.01 | F=220,P<.01 | F=6, P<.05 |
| CE | F=8, P<.01 | F=45, P<.01 | F=6, P<.05 |
| NTS | F=23,P<.01 | F=187,P<.01 | F=6, P<.05 |
DF=(1,22) CPP=conditioned place preference, Cg=anterior cingulate cortex, BLA=basolateral amygdala, VL-BNST=ventral lateral bed nucleus of the stria terminalis, CE=central nucleus of amygdala, NTS=nucleus of the solitary tract
3.2 Fos measurements
In each of the brain areas where Fos measurements were analyzed significant main effects for conditioning, drug treatment, and conditioning X drug treatment were found (see Table 1). Fos counts for the non-conditioned animals were very low in each of the brain areas chosen for analysis except for the DMH. No significant group differences in Fos counts in the DMH were found (F(1,22)=0.69, P=.40, group means ranged from 51 to 41 cell counts with a standard error of 4).
Fig. 3 shows the average Fos counts for each brain area in which group differences were found to be significant together with one representative photograph of the Fos staining from one animal in each of the conditioned groups. The Fos results in the Cg and BLA mirrored the place conditioning data (Fig. 3). The post-hoc analysis revealed that Fos expression in each of these areas was significantly greater in conditioned animals pretreated with placebo pellets than in morphine-withdrawn conditioned animals (P<.01 for each).
Figure 3.
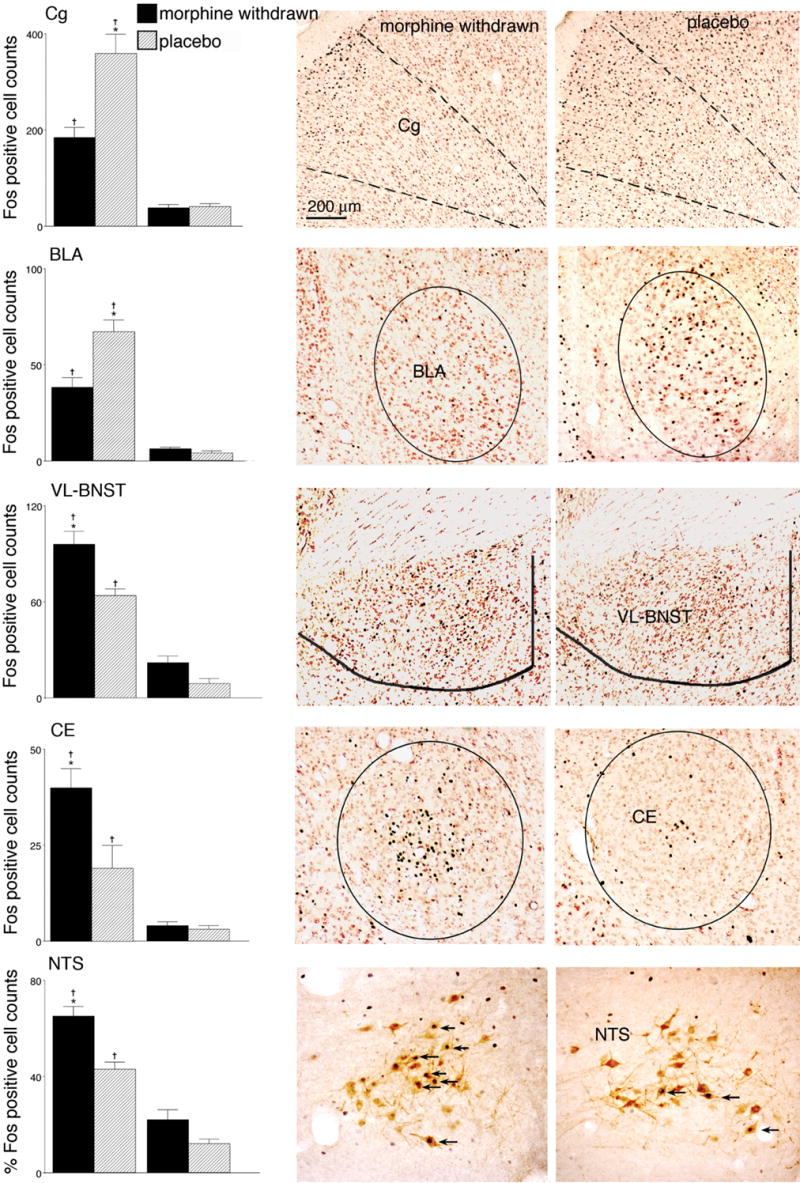
The left panels depict mean counts of Fos-activated neurons for placebo and morphine withdrawn animals in conditioned and non-conditioned groups. Data are mean ± SEM counts of Fos+ neurons in the anterior cingulate cortex (Cg), basolateral amygdala (BLA), ventrolateral portion of the bed nucleus of the stria terminalis (BNST), and central amygdala nucleus (CE), and percentages of TH+ neurons that were also Fos+ in the nucleus of the solitary tract (NTS), as indicated . tsignificantly different from non-conditioned groups (P<.01). *depicts significant differences between conditioned groups (P<.01). The right panels contain photomicrographs of frontal sections from corresponding brain areas showing Fos responses from one animal in each of the two conditioned groups (the placebo group is on the right and morphine withdrawn group is on the left). In all cases medial is on the right-hand side of the photograph. Arrows on the NTS photomicrograph indicate cells that were double labeled for Fos and tyrosine hydroxalase.
For the VL-BNST, CE and A2 (NTS), a completely different pattern of results was seen (Fig. 3). Significantly greater Fos expression in all three brain areas was observed in the morphine-withdrawn conditioned group compared to the placebo conditioned group (P<.01). Fos counts in these areas for non-conditioned morphine-withdrawn animals tended to be higher than non-conditioned placebo animals, but these results were not significant. For the NTS Fos expression was primarily seen in TH positive cells (A2 group, Fig 3); these data are presented as percentages of TH+ neurons that were also Fos+.
3.3 Correlations
Table 2 shows the brain areas and the significant R values for correlations between Fos levels and preference scores. Significant correlations were only found in the conditioned groups and not in the non-conditioned groups. Both conditioned groups showed strong positive correlations between the numbers of Fos+ neurons in Cg and BLA and the corresponding preference scores. The morphine withdrawn conditioned group was unique in showing robust negative correlations between Fos activation in the CE, VL-BNST and A2 and preference scores. Therefore, the higher the Fos levels in these areas, the less likely morphine abstinent subjects were to prefer the food-paired compartment. The only brain area in which Fos levels did not show a significant correlation with behavior was the DMH (R values <.23, P>.1). Furthermore, no significant correlations were found between Fos and locomotor activity in any brain area (R values <.40, P values >.1).
Table 2.
CPP and Fos correlation results.
| Placebo | Morphine | |||
|---|---|---|---|---|
| Conditioned | Non-conditioned | Conditioned | Withdrawn Non-conditioned | |
| Cg | .90* | −.22 | .86* | .21 |
| BLA | .90* | .36 | .83* | −.22 |
| VL-BNST | .18 | −.31 | −.99* | −.18 |
| CE | .11 | .55 | −.87* | −.02 |
| NTS | .23 | −.53 | −.91* | .48 |
| DMH | .44 | .41 | .44 | .53 |
Correlation coefficients (R values)
P<.01
4. Discussion
The main findings of this study were that the morphine withdrawn conditioned group showed significantly less preference for food-associated cues, and correspondingly less Fos expression in the Cg and BLA, than conditioned animals that were pretreated with placebo pellets. Fos expression in these areas showed significant positive correlations with the level of preference seen for food-associated cues. It is unlikely that these findings reflect an altered interest in the food reward in withdrawn animals, as both abstinent and placebo-pretreated subjects consumed the food during conditioning. Furthermore, the abstinent animals had similar weights as placebo-treated animals, indicating that they had recovered from the withdrawal experience. In addition, we found that morphine-withdrawn conditioned animals showed higher Fos activation in stress-associated brain areas including CE, VL-BNST and A2 than placebo conditioned animals or withdrawn non-conditioned subjects. Interestingly, preference scores and Fos activation in CE, VL-BNST and A2 were strongly negatively correlated in morphine-withdrawn conditioned animals, indicating that enhanced Fos activation in these areas predicted lower food preferences.
The changes in Fos in all brain areas except the DMH only occurred in conditioned animals exposed to conditioned cues; non-conditioned animals did not show elevations in Fos despite similar drug exposure, withdrawal, handling and activity. All groups, on the other hand, showed similar levels of Fos expression in DMH. The DMH is known to be critical for the regulation of feeding, body weight, and energy consumption as well as the expression of a variety of behaviors and physiological events controlled by circadian rhythms [2,10,13]. Our results indicate that the DMH is most likely not involved in the expression of a food CPP although recent results have reported its involvement in the entraining of feeding behavior [20]. In that study [20], Fos levels in the DMH were highest at a scheduled meal time and correlated with the entraining of feeding behavior.
The Fos levels were not found to correlate with the activity levels on the test day in any of the brain areas examined. This indicates that Fos expression in these areas was not simply related to locomotor activation. In further support of this notion, non-conditioned groups had similar overall activity levels to the conditioned groups on the test day but, unlike the conditioned groups, did not show significant elevations in Fos expression in the Cg, BLA, VL-BNST, CE or A2.
The finding that Fos expression in Cg and BLA showed significant positive correlations with preference scores in both abstinent and placebo-pretreated conditioned groups is consistent with what we previously reported for morphine place preference conditioning [23]. Thus, our current and previous results indicate that the Cg and BLA are associated with the expression of the positive reinforcing aspects of both food and drugs. In view of the positive correlation between Fos activation in these two areas and preference behavior, it also seems possible that the amount of Fos we observed was influenced by the amount of time spent in the presence of the reward-associated conditioned cues.
The Cg and BLA are both thought to be important for assigning emotional significance to stimuli associated with reinforcement [30,47]. The BLA is known to be important for stimulus-controlled behaviors, and lesions or chemical inactivation of this structure can result in the loss of both CPP acquisition [16,18,59] and cue-induced reinstatement of drug self-administration [17,50,57]. Lesions of the Cg do not impair reward seeking or consumption of rewards per se, but instead disrupt the ability to discriminate between a rewarded stimulus (CS+) and an unrewarded stimulus (CS-), causing an animal to approach both stimuli in an equal manner [5–7,43].
One of the major findings of this study was the significant increase in Fos expression in the NTS, CE and VL-BNST of the withdrawn conditioned group relative to placebo-pretreated conditioned, or withdrawn non-conditioned, animals. Previously we found that morphine-withdrawn animals exhibited more anxiety-like behaviors [25], and greater retention of learning in fear-related paradigms, than placebo-pretreated subjects [24]. As NTS A2 (noradrenergic), CE, and BNST neurons are associated with fear and anxiety [8,48,52,53], and with the aversion that accompanies morphine withdrawal [12,42,55], it is perhaps not surprising that Fos is elevated in these brain areas after morphine withdrawal. What is more surprising is that the Fos responses in these areas were significantly elevated in morphine-withdrawn animals only during CPP testing. This finding may indicate that withdrawn animals experience more frustration and stress than normal in response to the lack of an expected reward (e.g., as on the test day). This is consistent with our prior observation that enhanced anxiety responses are seen in withdrawn animals (compared to placebo-pretreated subjects) [24,25]. Previous studies have found that anxiety associated with protracted morphine withdrawal can decrease responding for food (e.g., in a conditioned suppression paradigm; ref. 24). These findings lead us to hypothesize that increased anxiety during protracted withdrawal may diminish the reward associated with the food-paired compartment and thus produce lower preference scores. This would explain why higher Fos expression in these areas strongly predict lower preference scores.
The CE and BNST are very similar in that both receive noradrenergic input from A2 neurons in the NTS [12,51,58], and both are involved in naloxone-induced opiate withdrawal associated aversions [21,29,42,55] in addition to stress-induced reinstatement of drug seeking [15]. These findings indicate that the BNST and CE may be more susceptible to alterations by morphine exposure than other brain areas. Our data indicate that these areas may continue to be altered even after 5 weeks of morphine abstinence. The noradrenergic input to the VL-BNST has been shown to be involved in the aversive affective components of opiate withdrawal [12], and to be an important area for stress-induced reinstatement of both morphine CPP [54] and cocaine self-administration [38]. The common noradrenergic input to the BNST and CE from the NTS A2 neurons could be an important link between CE and VL-BNST and their roles in drug dependence.
Previously we found that elevated Fos levels in the VL-BNST of morphine withdrawn animals were positively correlated with enhanced morphine preference [27], whereas in the present study elevated Fos levels in the VL-BNST were negatively correlated with food preference. Thus, the BNST may be a site where aversive responses to environmental stressors help to drive drug seeking behavior that can alleviate stress while decreasing the desire for natural rewards that are not effective in alleviating stress.
In conclusion, these results indicate that even at 5 weeks post-withdrawal there is evidence of hypersensitive brain stress systems. This may direct behavior away from natural rewards and relatively increase the attractiveness of drug reward, thereby helping to perpetuate the cycle of addiction. In support of this view, clinical observations of former opiate addicts found a prolonged hyper-responsiveness to stress and an altered hypothalamic-pituitary-adrenal axis, causing augmented release of stress hormones like corticotropin-releasing factor (CRF) and adrenocorticotropin [36]. This altered responsiveness to stress is hypothesized to facilitate relapse in human addicts [9,34–36]. Better understanding of the brain mechanisms that underlie these changes in affective processing could lead to the development of more successful treatments for opiate addiction.
Acknowledgments
This work was supported by PHS grants DA-06214 and DA-017289. The authors declare no competing financial interests. We thank Rachel Smith for helpful comments on the manuscript and Martha Hilton for help with the data analysis.
References
- 1.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 2.Aston-Jones G, Chen S, Zhu Y, Oshinsky M. A neural circuit for circadian regulation of arousal. Nature Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 3.Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacology. 2002;26:286–294. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- 4.Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- 5.Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- 6.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 7.Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–587. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- 8.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 9.Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O'Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treat. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 10.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- 12.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 13.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 14.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 15.Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 16.Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- 19.Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 20.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 21.Gracy K, Dankiewicz L, Koob G. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- 22.Harris G, Aston-Jones G. Altered motivation and learning following opiate withdrawal: Evidence for prolonged dysregulation of reward processing. Neurpsychopharmacology. 2003;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- 23.Harris G, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: Association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- 24.Harris GC, Aston-Jones G. Altered motivation and learning following opiate withdrawal: evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology. 2003;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- 25.Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology. 2001;24:75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- 26.Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- 27.Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: Association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- 28.Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, editor. The Basal Forebrain. Plenum Press; New York: 1991. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- 29.Heinrichs S, Menzaghi F, Schulteis G, Koob G, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- 30.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 34.Kreek MJ. Plasma and urine levels of methadone. Comparison following four medication forms used in chronic maintenance treatment. N Y State J Med. 1973;73:2773–2777. [PubMed] [Google Scholar]
- 35.Kreek MJ. Tolerance and dependence: implications for the pharmacological treatment of addiction. NIDA Res Monogr. 1987;76:53–62. [PubMed] [Google Scholar]
- 36.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 37.Leith NJ, Barrett RJ. Amphetamine and the reward system: evidence for tolerance and post-drug depression. Psychopharmacologia. 1976;46:19–25. doi: 10.1007/BF00421544. [DOI] [PubMed] [Google Scholar]
- 38.Leri F, Flores J, Rodaros D, Stewart J. Blockade of Stress-Induced But Not Cocaine-Induced Reinstatement by Infusion of Noradrenergic Antagonists into the Bed Nucleus of the Stria Terminalis or the Central Nucleus of the Amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maldonado R. The neurobiology of addiction. J Neural Transm. 2003:1–14. doi: 10.1007/978-3-7091-0541-2_1. Suppl. [DOI] [PubMed] [Google Scholar]
- 40.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 41.Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiol Behav. 2002;77:723–729. doi: 10.1016/s0031-9384(02)00925-3. [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134:9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res. 2000;126:263–285. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- 44.Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press, Inc; San Diego, CA: 1998. [Google Scholar]
- 46.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 47.Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- 48.Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur J Pharmacol. 2005;507:117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 49.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 50.See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- 51.Terenzi MG, Ingram CD. A combined immunocytochemical and retrograde tracing study of noradrenergic connections between the caudal medulla and bed nuclei of the stria terminalis. Brain Research. 1995;672:289–297. doi: 10.1016/0006-8993(94)01453-o. [DOI] [PubMed] [Google Scholar]
- 52.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology (Berl) 2003;170:80–88. doi: 10.1007/s00213-003-1504-0. [DOI] [PubMed] [Google Scholar]
- 56.Yoburn BC, Chen J, Huang T, Inturrisi CE. Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J Pharmacol Exp Ther. 1985;235:282–286. [PubMed] [Google Scholar]
- 57.Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- 58.Zardetto-Smith AM, Gray TS. Organization of peptidergic and catecholaminergic efferents from the nucleus of the solitary tract to the rat amygdala. Brain Res Bull. 1990;25:875–887. doi: 10.1016/0361-9230(90)90183-z. [DOI] [PubMed] [Google Scholar]
- 59.Zarrindast MR, Ahmadi S, Haeri-Rohani A, Rezayof A, Jafari MR, Jafari-Sabet M. GABA(A) receptors in the basolateral amygdala are involved in mediating morphine reward. Brain Res. 2004;1006:49–58. doi: 10.1016/j.brainres.2003.12.048. [DOI] [PubMed] [Google Scholar]


