Abstract
Alternative RNA processing of human calcitonin/CGRP pre-mRNA is regulated by an intronic enhancer element. Previous studies have demonstrated that multiple sequence motifs within the enhancer and a number of trans-acting factors play critical roles in the regulation. Here, we report the identification of TIAR as a novel player in the regulation of human calcitonin/CGRP alternative RNA processing. TIAR binds to the U tract sequence motif downstream of a pseudo 5′ splice site within the previously characterized intron enhancer element. Binding of TIAR promotes inclusion of the alternative 3′-terminal exon located more than 200 nucleotides upstream from the U tract. In cells that preferentially include this exon, overexpression of a mutant TIAR that lacks the RNA binding domains suppressed inclusion of this exon. In this report, we also demonstrate an unusual novel interaction between U6 snRNA and the pseudo 5′ splice site, which was shown previously to bind U1 snRNA. Interestingly, TIAR binding to the U tract sequence depends on the interaction of not only U1 but also U6 snRNA with the pseudo 5′ splice site. Conversely, TIAR binding promotes U6 snRNA binding to its target. The synergistic relationship between TIAR and U6 snRNA strongly suggests a novel role of U6 snRNP in regulated alternative RNA processing.
Alternative RNA processing is an important means of increasing proteomic complexity in eukaryotic organisms. Through this process, the majority of human pre-mRNAs generate more than one mRNA molecule, which often leads to production of multiple polypeptides from a single gene (5, 20, 42). Tissue-specific alternative RNA processing plays an important role in regulating gene expression. The human calcitonin/calcitonin gene-related peptide (CGRP) gene is a good example of such regulation (1, 46). This gene is predominantly expressed in two tissues, thyroid C cells and a subset of neurons. However, the pre-mRNA for calcitonin/CGRP is differentially processed in these two tissues, leading to production of calcitonin, a calcium-regulating peptide hormone, in thyroid C cells and CGRP, a neurotransmitter, in neuronal cells (Fig. 1A).
FIG. 1.
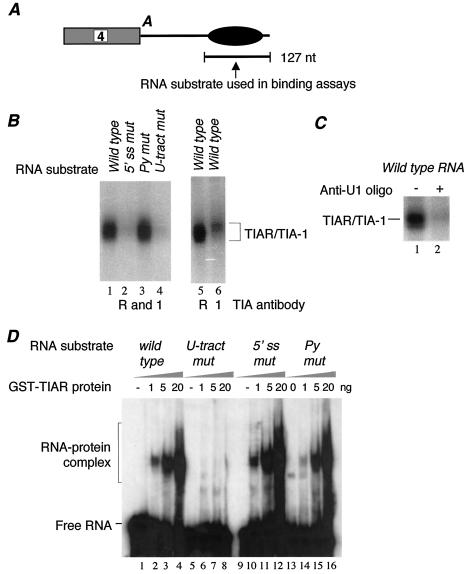
The U tract sequence in the calcitonin/CGRP gene intron 4 enhancer element is required for exon 4 inclusion. (A) Schematic diagram of the calcitonin/CGRP gene and its alternative RNA processing in thyroid and neuronal cells. A, polyadenylation signal. (B) Diagram showing the calcitonin/CGRP reporter gene and its cell-specific RNA processing patterns. The black oval represents the intron enhancer element. RSV, Rous sarcoma virus promoter. (C) Wild-type and mutant sequences of the three important positively acting motifs within the intron enhancer element. Py mut, pyrimidine mutant; 5′ ss mut, 5′ splice site mutant; U-tract mut, U tract mutant. (D) Results of RT-PCR assay of total RNA from HeLa cells transfected with the diagramed calcitonin/CGRP reporter gene with a wild-type enhancer (lane 1), an enhancer in which the pseudo 5′ splice site was mutated (lane 2), or an enhancer in which the U tract was mutated (lane 3). Amplification bands resulting from exon 4 inclusion or exclusion are indicated. The percent inclusion of exon 4 is indicated below each lane. Higher-molecular-weight products result from precursor RNA or activation of cryptic splicing. The latter product has been characterized previously and results from usage of a cryptic 5′ splice site within exon 4 (36). M, molecular weight markers.
Alternative RNA processing is subject to complex control, often involving multiple sequence elements and protein factors. Studies to date have identified a large number of cis-acting regulatory elements located in both exons and introns. In contrast, our understanding of what trans-acting factors regulate alternative RNA processing and how they do so is very limited. Only a small number of factors have been identified and characterized (4, 37). Studies on alternative processing factors have indicated that they are often RNA binding proteins capable of binding to specific cis-acting RNA elements and that they affect RNA processing choice through interactions with the basal splicing or polyadenylation machinery (4, 37). It has been estimated that the human genome may encode as many as 2,560 RNA binding proteins (25). There are at least 300 human genes that encode one or more RNA recognition motifs (RRM) (28). We are only beginning to uncover the functions of these proteins.
We use the human calcitonin/CGRP gene as a model to study the mechanism controlling tissue-specific alternative RNA processing. Similar to that of many other alternatively processed pre-mRNAs, alternative processing of calcitonin/CGRP pre-mRNA undergoes complex control involving multiple sequence elements and protein factors (32). One important sequence element is located in the intron downstream of exon 4 (Fig. 1B). This element is highly conserved between human, mouse, and rat and is required for exon 4 inclusion (36). One role of the intron element is to promote polyadenylation at the end of exon 4, thereby enhancing exon 4 inclusion (33, 35).
Two interesting features make this enhancer element an intriguing target to study. First, the intron element reflects the complex nature of RNA elements regulating alternative RNA processing by containing multiple sequence motifs. In particular, both positive and negative motifs that regulate exon 4 inclusion exist within the element. Second, some of the motifs bear a striking resemblance to the consensus sequences of splicing signals yet are not recognized as authentic splice sites. Specifically, the major positive motifs include a pseudo 5′ splice site preceded by a pyrimidine-rich sequence and followed by a U tract (Fig. 1C). Mutation of any of the three motifs drastically reduces exon 4 inclusion in cell types normally expressing RNA containing this exon (36). The major negative sequence motif is located 23 nucleotides (nt) upstream of these motifs and contains an additional 5′ splice site sequence. In contrast to that of the downstream 5′ splice site sequence, mutation of this upstream 5′ splice site sequence increased exon 4 inclusion significantly (36). It has been subsequently demonstrated that the serine- and arginine-rich protein SRp20 and the U1 small nuclear ribonucleoprotein particle (U1 snRNP) interact with the pyrimidine-rich sequence and the downstream pseudo 5′ splice site, respectively, thereby promoting polyadenylation of exon 4 (33, 35). The polypyrimidine tract binding protein (PTB) also interacts with the pyrimidine sequence and plays a positive role in regulating exon 4 inclusion (34), but the precise role of PTB in this regulation remains unclear. Although we have learned a great deal about the regulation of calcitonin/CGRP alternative RNA processing, it is clear that many questions remain to be answered. This report addresses two of them: what factors interact with the two pseudo 5′ splice sites and what factors interact with the U tract sequence following the downstream site.
To fully understand the activity of the enhancer element, we set out to determine why the two 5′ splice sites function differently by characterizing the factors that interact with each. In this report, we demonstrate the interactions of two novel factors with the sequences at or close to the downstream pseudo 5′ splice site. In particular, we found that TIAR, a recently characterized splicing regulator (30), specifically interacts with the U tract sequence that follows the downstream pseudo 5′ splice site in the intron enhancer element. In addition, psoralen cross-linking assays indicate an expected interaction between U1 snRNA and both of the pseudo 5′ splice sites as well as an unexpected interaction between U6 snRNA and the positively acting downstream site. The U6 snRNP interaction depends on the downstream pseudo 5′ splice site sequence but not on the upstream 5′ splice site. We provide evidence to support the idea that the TIAR protein plays an important role in regulating exon 4 inclusion. Furthermore, our studies uncovered a novel function of TIAR protein as an alternative splicing regulator. Specifically, TIAR appears to have little effect on the interaction between U1 snRNP and the pseudo 5′ splice site in the intron enhancer. Instead, TIAR promotes an interaction between U6 snRNP and the pseudo 5′ splice site. Moreover, binding of TIAR protein and U6 snRNP to their RNA targets showed a synergistic relationship. These studies have demonstrated the versatile activities of TIAR protein as a regulator of alternative RNA processing. Additionally, they strongly suggest a novel role of U6 snRNP in regulated alternative RNA processing. Finally, involvement of TIAR and U6 snRNP in the intron enhancer function predicts a different and novel role for this enhancer element in addition to enhancement of polyadenylation.
MATERIALS AND METHODS
Plasmids.
The human calcitonin/CGRP reporter constructs used in transfection experiments consist of calcitonin/CGRP gene exons 4 to 6 fused to a heterologous first exon from adenovirus (36). The mutant reporter constructs were generated by PCR-directed mutagenesis and contain mutations at the +1 to +3 positions of the 5′ splice site sequence (CAGGTAAGAC to CAGCATAGAC) of the downstream pseudo 5′ splice site located 215 nt downstream of the exon 4 poly(A) site or in the U tract sequence (UUUUUUAUUUU to GUGUUGAUGGU). Mutated nucleotides are underlined.
Plasmids used to generate in vitro-transcribed RNA substrates for psoralen cross-linking assays were constructed by PCR amplification from the various calcitonin/CGRP reporter constructs (wild type and mutants) by using primers flanking the intron enhancer sequence located in intron 4 (5′ primer, GCCCCGGGTCCCAGGCTTCATAG; 3′ primer, GCGATATCCACCCCAGTCACGGG). The PCR products that contain 238 nt of the intron 4 sequence extending 37 nt from the exon 4 poly(A) site were digested with SmaI and EcoRV and cloned into the SmaI site in the pGEM3Zf+ vector (Promega). Plasmids used to generate in vitro-transcribed RNA substrates for UV cross-linking and gel shift assays were previously described (35). These RNA substrates contain 127 nt of the intron 4 sequence extending 152 nt from the exon 4 poly(A) site and include all of the important sequence motifs.
The mutant U1 gene containing mutations at positions 6 to 8 (UAC to AUG) was generated by PCR-directed mutagenesis.
Cell transfection and RNA and protein analysis.
HeLa cells were transfected with calcitonin/CGRP reporter constructs as previously described (34, 35). Cotransfections used 2 μg of the calcitonin/CGRP reporter plasmid and either 2 μg of U1 snRNA plasmids, 0.05 to 0.5 μg of full-length TIA-1/TIAR plasmids, or 0.5 to 1 μg of mutant TIA-1/TIAR plasmids. Procedures for total RNA and protein isolation and reverse transcription (RT)-PCR analysis were described previously (35). Use of low-cycle (16-20) PCR permitted determination of the relative abundance of individual RNA species. Quantification of exon inclusion was determined by using a phosphorimager. The results shown are representative of results from at least three independent transfections for each experiment. Western blot analysis using the proteins isolated from the transfected cells was carried out with antihemagglutinin (anti-HA) antibody (Covance) or anti-TIAR antibody (Santa Cruz Biotechnology, Inc.).
In vitro assays.
UV cross-linking reactions were carried out in a volume of 50 μl containing 44% (vol/vol) HeLa cell nuclear extract, 2 mM ATP, 20 mM creatine phosphate, 0.6 mM MgCl2, 1.5% polyethylene glycol, 0.15 mM dithiothreitol, and 5 × 105 cpm of 32P-labeled RNA. Reaction mixtures were incubated at 30°C for 10 min, and heparin was added to a final concentration of 2 μg/μl, followed by UV irradiation (254 nm) at 4°C for 10 min. Reaction mixtures were subsequently treated with 30 μg of RNase A at 37°C for 30 min. Cross-linked polypeptides were immunoprecipitated by using monoclonal antibodies against TIA-1 (ML29), TIAR (6E3), or both TIA-1 and TIAR (3E6). Immunoprecipitated proteins were separated on sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gels.
Psoralen cross-linking reactions were carried out in a volume of 25 μl containing 44% (vol/vol) HeLa cell nuclear extract, 2 mM ATP, 20 mM creatine phosphate, 0.6 mM MgCl2, 1.5% polyethylene glycol, 0.15 mM dithiothreitol, 20 μg of 4′-aminomethyl-4,5′,8-trimethyl psoralen (HRI Associates), and 2.5 × 105 cpm of 32P-labeled RNA. The reaction mixtures were incubated at 30°C for 10 min, heparin was added to a final concentration of 2 μg/μl, and UV irradiation (365 nm) at 4°C for 10 min was used to generate RNA-RNA cross-links. The reaction mixtures were deproteinized by incubation with 0.5 μg of proteinase K in 1× proteinase K buffer (10× proteinase K buffer is 0.1 M Tris [pH 8.0], 0.05 M EDTA, and 5% sodium dodecyl sulfate) at 42°C for 20 min followed by phenol-chloroform (1:1) extraction and ethanol precipitation. The cross-linked RNAs were analyzed on 5% polyacrylamide gels containing 8.3 M urea. To identify the snRNA involved in potential RNA-RNA cross-links, the RNAs isolated after psoralen cross-linking were incubated with 2 U of RNase H and 2 μg of snRNA-specific DNA oligonucleotides. After RNase H treatment, the RNAs in the reaction mixture were purified by phenol-chloroform (1:1) extraction and analyzed on denaturing acrylamide gels. To block U1 or U6 snRNA from interacting with the RNA probes, 2′-O-methyl RNA oligonucleotides complementary to the 5′ end of U1 snRNA, nt 77 to 97 of U6 (U6b), or nt 33 to 47 of U6 (U6c) were included in the reaction mixture before psoralen cross-linking.
Gel shift assays were performed by using recombinant glutathione S-transferase (GST)-TIAR prepared from bacteria and in vitro-transcribed RNA substrates. The reactions were carried out in 25 μl containing 50% Roeder D (14), 20 mM creatine phosphate, 2 mM ATP, 2 mg of heparin/ml, 1 mg of bovine serum albumin/ml, 1 to 20 ng of recombinant protein, and 125,000 cpm of 32P-labeled RNA. The reactions were stopped after 10 min of incubation at 30°C by addition of loading buffer containing 50% glycerol and 1% dye, and the complex was separated on a 4% nondenaturing polyacrylamide gel in 1× TG buffer (10× TG buffer is 0.5 M Tris and 0.5 M glycine).
Nuclear extract preparation from transfected HeLa cells.
HeLa cells in 50 100-mm-diameter dishes were transiently transfected with wild-type or mutant U1 expression plasmid. Transfection efficiencies for this cell line routinely exceed 90%. Nuclear extracts were prepared by using standard techniques 2 days posttransfection (14).
For the UV cross-linking experiments, the same amount of either extract (96 μg of protein) supplemented with 3 μl of standard HeLa extract (8 mg/ml) was used in each reaction.
RESULTS
The U tract within the intron enhancer of the calcitonin/CGRP pre-mRNA is required for exon 4 inclusion.
It has previously been demonstrated that the U tract sequence is required for exon 4 inclusion in vivo in HeLa cells transfected with the minigene reporter in which the calcitonin gene exon 4 with its flanking intron sequences was inserted into the first intron of the three-exon human MT2A gene (36). When the U tract sequence was mutated in this context, exon 4 inclusion was reduced significantly. To confirm the biological relevance of this sequence, we introduced the same point mutations into a more natural calcitonin/CGRP reporter that contains a fragment of the human calcitonin/CGRP gene extending from the middle of intron 3 to the 3′ end of the gene fused with a first exon and half of an intron from the major late transcription unit of adenovirus (Fig. 1B). The pre-mRNA of this reporter construct is processed differentially in cultured cells. In HeLa cells, exon 4 is predominantly included and in T98G cells, a human glioblastoma cell line, exon 4 is predominantly skipped (34-36). As shown in Fig. 1D, when HeLa cells were transfected with the reporter construct that contains U-to-G point mutations in the U tract sequence, exon 4 inclusion decreased dramatically from 71 to 30%. This result is consistent with that obtained with the previous minigene reporter, thus confirming the importance of the U tract sequence motif in regulating the use of exon 4. Note that the absolute levels of exon 4 inclusion vary from transfection to transfection (between 63 and 71%) but that relative levels of exon 4 inclusion between constructs containing a wild-type or mutant enhancer remain the same.
TIAR protein interacts with the U tract sequence.
TIA-1 and TIAR are two closely related proteins with 80% amino acid identity (23). Recent studies have revealed a role for these proteins as splicing regulators. Both TIA-1 and TIAR have been shown to function as alternative splicing regulators (12, 15, 16, 30). Specifically, they bind to uridine-rich sequences located immediately downstream of suboptimal 5′ splice sites and promote usage of these alternative 5′ splice sites. These 5′ splice sites reside at the exon-intron boundaries in the pre-mRNA for the fibroblast growth factor receptor 2 (K-SAM exon), the Drosophila melanogaster male-specific lethal 2 (alternatively removed intron in the 5′ untranslated region of msl-2), and TIA-1 and TIAR (exons 5A, 6A, 6B, 8A, and 11A) (12, 15, 16, 30). Although the precise role of TIA-1 and TIAR in regulating usage of 5′ splice sites is not clear, it has been demonstrated that they promote interaction of U1 snRNP with these sites in a manner analogous to that proposed for their putative yeast homolog Nam8, which is an integral constituent of the yeast U1 snRNP (12, 16).
We wished to determine whether TIA proteins could bind to the U tract sequence motif in the calcitonin/CGRP RNA intron enhancer, since the sequence is located 7 nt downstream of a pseudo 5′ splice site. As shown in Fig. 2A, the RNA substrate used in this assay contains 127 nt of the intron 4 sequence extending 152 nt from the exon 4 polyadenylation site and includes all of the previously characterized important sequence motifs. Our results indicate that, in HeLa cell nuclear extract, TIA proteins strongly interact with this RNA substrate in a UV cross-linking-immunoprecipitation (IP) assay using an antibody that recognizes both TIA-1 and TIAR (Fig. 2B, lane 1). This interaction is dependent on two sequence motifs: the pseudo 5′ splice site and the U tract sequence. Disruption of either sequence nearly abolished TIA binding, while mutation of another important sequence motif, the pyrimidine-rich motif, had no effect (Fig. 2B, lanes 2 to 4). To determine whether both TIA-1 and TIAR bind to the enhancer element, we carried out similar UV cross-linking-IP assays using either TIA-1- or TIAR-specific antibody. We observed a much stronger signal with the anti-TIAR antibody (Fig. 2B, lanes 5 and 6), a result that likely reflects the higher protein level of TIAR than of TIA-1 in HeLa cells.
FIG. 2.
TIAR specifically interacts with the U tract in the enhancer element. (A) Diagram showing the RNA substrate used in UV cross-linking and gel mobility shift assays. A, polyadenylation signal. (B) Interaction of TIAR and TIA-1 with the intron enhancer element in HeLa nuclear extract depends on both the U tract and the pseudo 5′ splice site sequence. The 32P-labeled in vitro-transcribed RNA containing either wild-type or mutated enhancer sequence was UV cross-linked in HeLa cell nuclear extract and immunoprecipitated with antibodies specific to both TIAR and TIA-1 (R and 1; lanes 1 to 4), TIAR (R; lane 5), or TIA-1 (1; lane 6). Wild-type intron enhancer (lanes 1, 5, and 6) and enhancers in which either the pseudo 5′ splice site (5′ ss mut; lane 2), pyrimidine (Py mut; lane 3), or U tract (U-tract mut; lane 4) sequence was mutated (Fig. 1C) were used in this assay. (C) Interaction of TIAR with the intron enhancer element depends on the 5′ end of U1 snRNA. UV cross-linking-IP assays were carried out with the wild-type enhancer in the absence (lane 1) or presence (lane 2) of a 2′-O-methyl ribo-oligonucleotide complementary to the 5′ terminus of U1 RNA. (D) Recombinant GST-TIAR binds to the U tract sequence specifically. Gel shift analysis was carried out with increasing amounts of GST-TIAR and 32P-labeled RNA containing the wild-type enhancer (lanes 1 to 4) and enhancers in which the U tract (lanes 5 to 8), the pseudo 5′ splice site (lanes 9 to 12), or the pyrimidine (lanes 13 to 16) sequence was mutated.
The finding that the interaction of TIAR with the intron enhancer depends on the integrity of the pseudo 5′ splice site is very interesting because previous studies using recombinant TIA-1 and TIAR proteins indicated that these proteins selected U-rich sequences, not 5′ splice site sequences (13). Moreover, a UV cross-linking assay using a site-specifically labeled RNA substrate containing the Drosophila msl-2 5′ splice site region mapped the TIA-1 binding site to the U stretch (16). We therefore examined whether the base-pairing interaction between U1 snRNA and the pseudo 5′ splice site is important for TIAR binding. As expected, when the HeLa cell nuclear extract was incubated with an oligonucleotide complementary to the 5′ end of U1 snRNA to block U1 from annealing with those of the pseudo 5′ splice site sequence, TIA binding to the intron element was abolished (Fig. 2C). A control anti-U3 snRNA oligonucleotide had no effect (data not shown).
We then carried out an RNA gel shift assay to determine the RNA binding specificity of TIAR protein. As shown in Fig. 2D, the recombinant TIAR protein formed a complex on the wild-type calcitonin/CGRP RNA intron enhancer (lanes 1 to 4). In contrast to the results of the UV cross-linking assay, in the absence of nuclear extract, interaction of TIAR with the intron element was dependent only on the U tract sequence (lanes 5 to 8). The same mutation of the pseudo 5′ splice site that abolished TIAR binding in the nuclear extract did not affect TIAR binding in the gel shift assay (lanes 9 to 12). To test whether this was due to the use of a gel shift assay, we carried out a UV cross-linking assay using the recombinant TIAR and various mutant RNA substrates. Essentially the same result was obtained (data not shown). We conclude that TIAR binds to the U tract sequence in the intron element.
A suppressor U1 snRNA restores binding of TIAR to the intron element containing the pseudo 5′ splice site mutations.
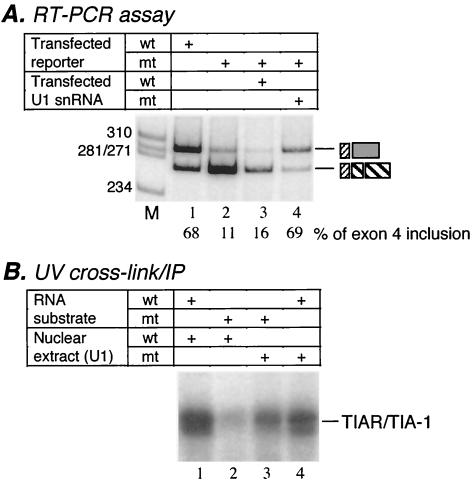
Previous experiments established the critical role of a base-pairing interaction between U1 snRNA and the pseudo 5′ splice site (35). In particular, an in vivo rescue experiment was performed that was similar to the experiments originally used to establish a requirement for U1 snRNA annealing in 5′ splice site recognition (56). In this assay, a calcitonin/CGRP reporter gene carrying a mutation at the +1 position within the pseudo 5′ splice site sequence (+1 G-to-C mutation) was transfected along with a U1 snRNA gene harboring a compensatory mutation (+8 C-to-G mutation) that restores the ability of U1 snRNA to anneal with those of the mutated pseudo 5′ splice site (35). The results indicated that the suppressor U1 only partially rescued exon 4 inclusion (35). Recently, we improved the system to achieve full rescue by mutating positions +1 to +3 of the pseudo 5′ splice site sequence and introducing compensatory mutations at positions +6 to +8 of U1 snRNA (Fig. 3A). We then used this suppressor system to determine whether TIAR binding at the U tract correlates with exon 4 inclusion. We reasoned that, if TIAR binding to the U tract sequence motif is required for exon 4 inclusion, its binding to the pseudo 5′ splice site mutant should also be restored by the suppressor U1 snRNA that can rescue the repressed exon 4 inclusion of the reporter carrying the same mutations.
FIG. 3.
TIAR binding depends on the base-pairing interaction between U1 snRNA and the pseudo 5′ splice site. (A) A suppressor U1 rescued exon 4 inclusion of the calcitonin/CGRP reporter that contains mutations at the +1 to +3 positions of the pseudo 5′ splice site. RT-PCR assays were carried out with total RNA isolated from HeLa cells transfected with the calcitonin/CGRP reporter with wild-type enhancer (wt; lane 1) or the enhancer that contains a mutated pseudo 5′ splice site (mt; lanes 2 to 4) in the absence (lane 2) or presence (lanes 3 and 4) of U1 plasmids (lane 3, wild-type U1; lane 4, mutant U1 containing the compensatory mutations). The percent inclusion of exon 4 is indicated below each lane, and products are identified as indicated in Fig. 1B. M, molecular weight markers. (B) The suppressor U1 restored TIAR binding to the enhancer that was mutated at the pseudo 5′ splice site. Nuclear extracts were prepared from HeLa cells transfected with wild-type U1 (lanes 1 and 2) or the suppressor U1 (lanes 3 and 4) plasmid and used in the UV cross-linking-IP assays. The UV cross-linking-IP reaction was carried out with the 32P-labeled RNA containing wild-type (lanes 1 and 4) or mutated (lanes 2 and 3) enhancer sequence. The RNA substrate indicated in Fig. 2A was used in this experiment.
To examine TIAR binding in the presence of the suppressor U1, we prepared nuclear extract from HeLa cells transfected with the suppressor U1 and carried out UV cross-linking-IP assays. Nuclear extract prepared from HeLa cells transfected with wild-type U1 snRNA was used as a control in this experiment. As shown in Fig. 3B, in the wild-type U1 extract, TIAR bound to the wild-type intron enhancer substrate very strongly and the binding was nearly abolished with a substrate containing the +1 to +3 GUA-to-CAU mutations (lanes 1 and 2). However, in the presence of the U1 suppressor snRNA that contained complementary mutations at positions +6 to +8 of U1, TIAR binding to the mutant substrate was increased to a level approaching that of the wild-type RNA in the same nuclear extract (Fig. 3B, compare lanes 3 and 4). This experiment provided a strong correlation between TIAR binding and exon 4 inclusion. In addition, this result confirmed that binding of TIAR to the U tract sequence does not depend on the sequence of the pseudo 5′ splice site per se but on the functional interaction between U1 and this site. The strong binding of TIAR to the wild-type RNA in the nuclear extract that contains mutant U1 can be explained by the presence of wild-type U1 snRNA in the same extract. Indeed, in a control experiment, when we cotransfected the wild-type calcitonin/CGRP reporter and the mutant U1, exon 4 was included to the same extent as that observed in the absence of mutant U1 (data not shown).
Mutant TIAR and TIA-1 proteins decrease exon 4 inclusion in HeLa cells.
To determine whether TIA proteins have a functional role in regulating exon 4 inclusion in vivo, we performed in vivo cotransfection assays with HeLa cells, which normally include exon 4 of the calcitonin/CGRP reporter minigene (Fig. 1B). These cells were cotransfected with cDNAs containing truncated TIAR or TIA-1 protein mutants in an attempt to depress exon 4 inclusion. The mutant proteins contain only the carboxy terminal glutamine-rich domain but lack the three RRM domains (Fig. 4A). The truncated TIA-1 mutant was previously shown to act as a dominant negative protein in a different context. Specifically, it blocks the recruitment of endogenous TIA-1 protein and poly(A)+ RNA to stress granules in response to arsenite (24).
FIG. 4.
Inclusion of exon 4 in HeLa cells is repressed by truncated TIAR lacking RRM domains. (A) Diagrams of wild-type TIAR (TIAR.FL) and a truncated form of TIAR (TIAR.Q) that contains the carboxy-terminal Q-rich domain but lacks the RRM domains. (B) HeLa cells that preferentially include exon 4 were cotransfected with a calcitonin/CGRP reporter and increasing amounts of either the TIAR.FL or the TIAR.Q plasmid. The left panel shows a Western blot produced by using tag-specific antibodies (anti-HA antibody) to document production of the desired TIAR forms following transfection (lanes 1 to 4). The middle panel shows Western blot analysis with anti-TIAR antibody to indicate the level of the overexpressed TIAR protein relative to the level of endogenous TIAR. The two bands represent the alternatively spliced isoforms of TIAR (3). The right panel shows results of an RT-PCR assay of total RNA from transfections of HeLa cells with the wild-type calcitonin/CGRP reporter gene and no TIAR (lane 1) or increasing amounts of full-length TIAR (FL; lanes 2 and 3) or truncated TIAR (Q; lanes 4 and 5). Products and percentages of inclusion are indicated as in Fig. 1B and D. (C) Results of RT-PCR assay of total RNA from HeLa cells transfected with the calcitonin/CGRP reporter carrying mutations at the pseudo 5′ splice site or with wild-type U1 snRNA (wt; lane 1) or suppressor U1 snRNA containing compensatory mutations (mt; lanes 2 to 4) and either no TIAR (lanes 1 and 2) or TIAR.Q (lane 3) or TIAR.FL (lane 4) plasmid.
The expression of full-length and truncated HA-tagged TIAR proteins in HeLa cells after transfection is shown in Fig. 4B. By comparing the level of expressed proteins to that of endogenous TIA-1/TIAR using TIA-1/TIAR-specific antibody, we estimated that the transfected proteins were expressed at two- to fourfold higher levels than the endogenous proteins (Fig. 4B, lanes 6 to 8). In addition, the overexpressed proteins were detected at significant levels in both nuclear and cytoplasmic fractions of the transfected cells (data not shown). As shown in the RT-PCR analysis in Fig. 4B, expression of the truncated TIAR protein depressed exon 4 inclusion from 63 to 41% (compare lanes 1 to 4 and 5), while similar levels of full-length TIAR expression had no effect (lanes 2 and 3). To demonstrate the specificity of the TIAR mutant for influencing exon 4 inclusion, we examined splicing of a control single-intron RNA derived from the adenovirus major late transcription unit and containing two competing 5′ splice sites (35). The relative usage of the two 5′ splice sites remained the same in non-TIAR-transfected cells and cells transfected with either full-length or truncated TIAR protein (data not shown).
When HeLa cells were transfected with full-length or truncated TIA-1 protein, the results were similar to those obtained with full-length or truncated TIAR protein (data not shown). These data indicate that the Q-rich domain from either TIA-1 or TIAR is capable of interfering with the endogenous TIA-1 and TIAR proteins that are both present in HeLa cells (Fig. 2B and data not shown). Combined with the data from TIA cross-linking assays, these results indicate that both TIA-1 and TIAR can regulate exon 4 inclusion of the calcitonin/CGRP pre-mRNA.
In the second cotransfection experiment, we transfected HeLa cells with three plasmids: one with the calcitonin/CGRP reporter minigene carrying the mutations at positions +1 to +3 of the downstream pseudo 5′ splice site, one with the suppressor U1 snRNA carrying the compensatory mutations, and one encoding the truncated TIAR protein. As shown in Fig. 4C, expression of the truncated TIAR protein compromised the ability of the suppressor U1 to fully restore exon 4 inclusion. This result is consistent with those of the previous two-plasmid cotransfection experiments (Fig. 4B). Together, these two experiments provide strong support for a functional role for TIA proteins in the regulation of exon 4 inclusion.
TIAR promotes exon 4 inclusion from a reporter with a weakened TIAR binding site.
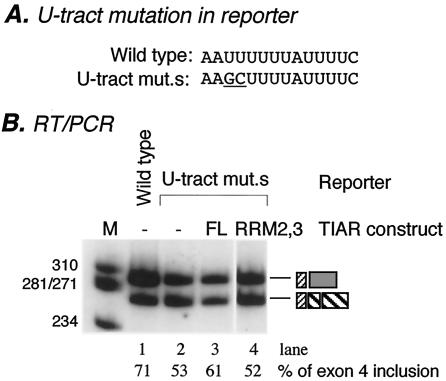
To provide stronger evidence for the functional role of TIAR in exon 4 inclusion, we took a more direct approach. We reasoned that, unlike the wild-type calcitonin/CGRP reporter (Fig. 4B), a reporter pre-mRNA containing a weakened TIAR binding site may respond to increasing levels of TIAR. By comparing 10 TIA-1/TIAR binding sites, Le Guiner et al. concluded that a good U-rich target for TIA-1 or TIAR should be at least 10 residues long. They also provided experimental evidence that a stretch of seven pyrimidines is not of sufficient length to activate a 5′ splice site but does retain some activity (30). Based on these observations, we generated a mutant reporter construct in which the 10 uridine residues were reduced to 8 (Fig. 5A). When this reporter was introduced into HeLa cells, exon 4 inclusion was reduced to approximately 53% from 71% (Fig. 5B, lanes 1 and 2). Note that when the U tract was completely disrupted, exon 4 inclusion was reduced to 30% (Fig. 1D). This experiment indicates that the mutant U tract retains partial activity in promoting exon 4 inclusion, presumably through binding to TIAR with a lower affinity. When this mutant reporter was cotransfected with TIAR, the results were as expected: exon 4 inclusion was increased to 61%, a modest yet consistent increase, while a mutant TIAR containing RRM2 and RRM3 had no effect (Fig. 5, lanes 3 and 4). The mutant TIAR protein had no effect on the wild-type reporter, either (data not shown). Expression of cotransfected proteins was verified by Western blot analysis (data not shown). Combined with data for the dominant negative TIAR mutant protein, these results provide strong evidence that TIAR plays a functional role in promoting exon 4 inclusion.
FIG. 5.
TIAR promotes exon 4 inclusion. (A) Sequences of the wild-type and mutant (mut.s) U tracts. Mutated nucleotides are underlined. (B) Results of RT-PCR assay of total RNA from HeLa cells transfected with calcitonin/CGRP reporter gene with a wild-type enhancer (lane 1), an enhancer in which the U tract was subtly mutated (lanes 2 to 4), and increasing amounts of the full-length TIAR (FL; lane 3) or the mutant TIAR containing RRM2 and RRM3 (RRM2,3; lane 4). Amplification bands resulting from exon 4 inclusion or exclusion are indicated. The percent inclusion of exon 4 is indicated below each lane. Products are identified as shown in Fig. 1B. M, molecular weight markers.
U6 snRNA interacts with the enhancer.
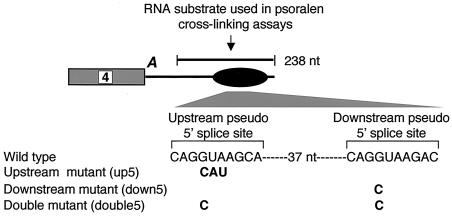
Our previous studies indicated that, in addition to the positively acting motifs shown in Fig. 1C, another pseudo 5′ splice site located upstream of these motifs functions negatively in the regulation of exon 4 inclusion (36). The two pseudo 5′ splice site sequences are separated by 37 nt (Fig. 6). Although their sequences are strikingly similar, the two pseudo 5′ splice sites appear to play opposite roles in regulating alternative inclusion of exon 4. In cells transfected with the calcitonin/CGRP minigene, disruption of the downstream site decreased exon 4 inclusion, while disruption of the upstream site increased exon 4 inclusion (36).
FIG. 6.
Diagram showing location of the intron enhancer (represented by a black oval) downstream of exon 4 and the two pseudo 5′ splice sites. The two splice sites are separated by 37 nt. Sequences of wild-type and mutant pseudo 5′ splice sites are shown. The black bar above the diagram indicates the RNA substrates used in all of the psoralen cross-linking assays mentioned in the legends to Fig. 7 and 8.
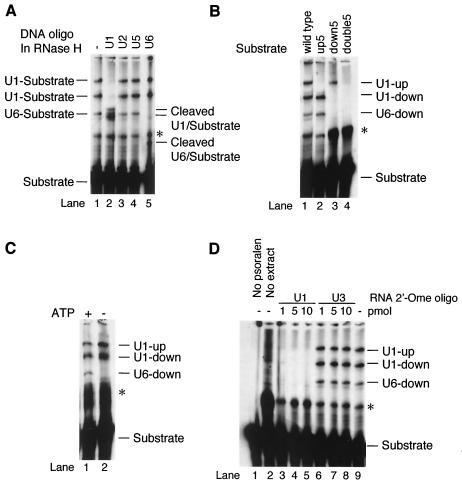
To understand the molecular mechanism underlying the functional difference between the two pseudo 5′ splice sites, we decided to identify nuclear factors that associate with both sites. Because splice sites are recognized, at least in part, via pairing of their bases with those of snRNAs, we employed a psoralen cross-linking assay to determine whether snRNAs interact with either one or both of these sites.
Initial experiments employed an in vitro-transcribed RNA substrate containing 238 nt of the calcitonin/CGRP RNA intron enhancer sequence, including all sequence motifs known to be important for regulation (Fig. 6). When an RNA substrate containing a wild-type enhancer element was incubated with HeLa cell nuclear extract and subjected to psoralen cross-linking, several RNA-RNA cross-linked species were observed that migrated more slowly than the free RNA on a denaturing polyacrylamide gel (Fig. 7A, lane 1). Formation of three of these cross-linked RNA species depended on both psoralen and nuclear extract (compare Fig. 7A, lane 1, to Fig. 7D, lanes 1 and 2), indicating that they are probably intermolecular cross-links between the RNA substrate and RNAs in the nuclear extract. The presence of pseudo splice sites within the substrate suggested snRNAs in the nuclear extract as the strongest candidates for RNAs interacting with the enhancer RNA. Indeed, RNase H digestion of the cross-linked RNAs by using specific anti-snRNA DNA oligonucleotides indicated that two of the cross-linked species contained U1 snRNA while one cross-linked species contained U6 snRNA (Fig. 7A, lanes 2 and 5). None of the cross-linked species contained U2 or U5 snRNAs (Fig. 7A, lanes 3 and 4).
FIG. 7.
A psoralen cross-linking assay demonstrates that U1 and U6 snRNAs interact with the pseudo 5′ splice sites in the enhancer. Wild-type or mutant enhancer RNAs were subjected to psoralen cross-linking after a 10-min incubation in nuclear extract. (A) Identification of cross-linked RNAs. Cross-linked RNAs were displayed on denaturing acrylamide gels either without (lane 1) or with (lanes 2 to 5) subsequent cleavage with RNase H in the presence of oligonucleotides (oligo) complementary to U1 (lane 2), U2 (lane 3), U5 (lane 4), or U6 (lane 5) snRNA. Full-length cross-linked species are identified to the left of the gel; cleavage products are indicated to the right of the gel. (B) Identification of sequences within the enhancer required for U RNA cross-linking. Psoralen cross-linking reactions were performed with either wild-type or mutant (Fig. 6) RNAs. Bands indicative of cross-linking of a U RNA to one of the enhancer 5′ splice sites are labeled to the right of the figure. (C) Cross-linking of U6 RNA is ATP dependent. Cross-linking of wild-type RNA as in panel A was repeated in the presence (lane 1) or absence (lane 2) of ATP. (D) Cross-linking of wild-type enhancer RNA as in panel A was repeated in the presence (lanes 3 to 5) or absence (lanes 1, 2, and 6 to 9) of a 2′-O-methyl ribo-oligonucleotide (2′-Ome oligo) complementary to the 5′ terminus of U1 RNA. Lane 1, cross-linking in the absence of psoralen; lane 2, cross-linking in the absence of nuclear extract; lanes 3 to 5, cross-linking in the presence of increasing amounts of the antisense oligonucleotide of U1 snRNA; lanes 6 to 8, cross-linking in the presence of increasing amounts of a control antisense oligonucleotide directed against U3 snRNA; lane 9, control cross-linking in the absence of any ribo-oligonucleotide. In all panels, an asterisk indicates a major band resulting from an extract-independent intramolecular cross-link.
To determine which sequence motifs within the calcitonin/CGRP RNA enhancer were required for interaction with U1 or U6 snRNA, we repeated the psoralen cross-linking assays with enhancer mutants. Mutation of the upstream pseudo 5′ splice site at positions +1 to +3 abolished one of the two U1-containing cross-linking products, while mutation of the downstream site at position +1 abolished the other U1-containing cross-linking product as well as the U6-containing cross-linking product (Fig. 7B, lanes 1 to 3). This assay indicated that U1 snRNA interacts with both pseudo 5′ splice sites, whereas U6 snRNA interacts only with the downstream pseudo 5′ splice site. The result with the downstream pseudo 5′ splice site +1 mutant also suggests that the U6 interaction depends on an interaction between U1 and this site. As expected, simultaneous mutation of both sites abolished all snRNA-containing cross-linked species (Fig. 7B, lane 4).
Cross-linking between U6 snRNA and the enhancer was a surprising result because U6 is thought to function in catalysis of splicing yet the 5′ splice site sequences in the enhancer element are not used in transesterification reactions. Interestingly, the U6 interaction with the downstream site shows a striking resemblance to the one that occurs with a genuine 5′ splice site. First, this interaction depends on the presence of ATP (Fig. 7C). Second, an interaction between U1 snRNA and the downstream pseudo 5′ splice site was required for observation of U6 cross-linking (Fig. 7D). No U6 cross-linking was observed when the ability of U1 snRNA to anneal with those of the 5′ splice site was blocked by U1-directed antisense 2′-O-methyl RNA (Fig. 7D).
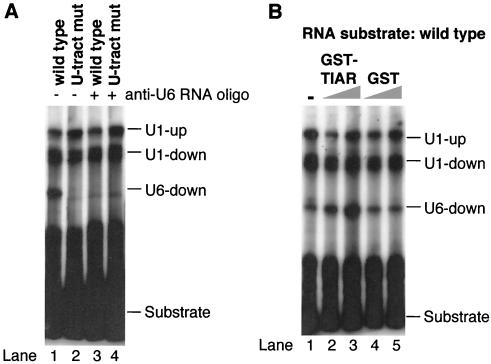
Mutation of the U tract sequence abolishes the interaction of U6 snRNA, but not that of U1 snRNA, with the intron element.
It was previously demonstrated that TIA-1 binding at U-rich sequences promotes U1 interaction with a number of 5′ splice sites located upstream from the U-rich sequence (12, 16). We therefore wished to determine whether the U tract within the calcitonin/CGRP RNA intron enhancer is required for U1 or U6 interaction with the pseudo 5′ splice site. To this end, we compared the psoralen cross-linking patterns of the wild-type enhancer and the U tract mutant that abolished the TIAR binding. Interestingly, the U tract mutation completely abolished the interaction of U6 with the intron enhancer element, while the interaction of U1 snRNA with the pseudo 5′ splice site was still observed in the U tract mutant (Fig. 8A, lanes 1 and 2). The latter observation is perhaps not surprising because Forch et al. demonstrated that the presence of a consensus G at position 5, as in the pseudo 5′ splice site, makes binding of U1 TIA-1 independent (16). When the region of U6 snRNA that interacts with authentic 5′ splice sites was blocked by an antisense oligonucleotide (see below), U6 cross-linking was abolished (Fig. 8A, lanes 3 and 4), while a different anti-U6 oligonucleotide had no effect (Fig. 9A).
FIG. 8.
The U tract sequence promoted U6 but not U1 binding to the enhancer element. (A) The 32P-labeled in vitro-transcribed RNA shown in Fig. 6 containing the wild-type enhancer (lanes 1 and 3) or enhancer with mutated U tract sequence (U-tract mut; lanes 2 and 4) was UV cross-linked in the presence of psoralen to nuclear RNAs. An anti-U6 2′-O-methyl oligonucleotide U6c (anti-U6 RNA oligo) (Fig. 9) was included in the mixtures for reactions with results shown in lanes 3 and 4. (B) The wild-type RNA substrate was cross-linked to nuclear RNAs in the absence (lane 1) or presence (lanes 2 to 5) of increasing amounts of GST-TIAR (lanes 2 and 3, 0.5 or 1.75 μg) or GST (lanes 4 and 5, 0.5 or 1.75 μg).
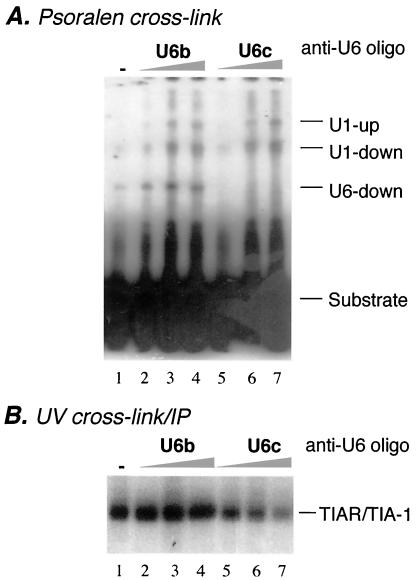
FIG. 9.
Binding of TIAR depends on U6 interaction with the element. (A) Results of psoralen cross-linking assay using wild-type enhancer (Fig. 6) and HeLa nuclear extract in the absence (lane 1) or presence (lanes 2 to 7) of increasing amounts (20, 100, and 200 ng) of anti-U6 2′-O-methyl ribo-oligonucleotide (anti-U6 oligo) U6b (lanes 2 to 4) or U6c (lanes 5 to 7). (B) A UV cross-linking-IP assay was carried out with the wild-type enhancer RNA substrate (Fig. 2A) in the absence (lane 1) or presence (lanes 2 to 7) of U6b (lanes 2 to 4) or U6c (lanes 5 to 7) oligonucleotides and an antibody specific for TIA-1 and TIAR.
This result indicates that U6 binding requires the U tract sequence. To determine whether TIAR promotes U6 binding, a psoralen cross-linking assay was carried out in the presence of increasing concentrations of recombinant GST-TIAR fusion protein. As shown in Fig. 8B, cross-linking of U6, but not that of U1, was increased under these conditions, while GST alone showed no effect.
Interaction of TIAR with the enhancer element depends on U6 snRNA.
The result described in the previous section prompted us to determine whether binding of TIAR to the U tract sequence requires an interaction between U6 snRNA and the intron element. To address this question, we took advantage of a pair of anti-U6 snRNA ribo-oligonucleotides (U6b and U6c) that anneal to different regions of U6 snRNA and differentially affect the interaction of U6 with 5′ splice sites (39). Although both oligonucleotides inhibit splicing in vitro of an adenovirus-based splicing substrate (data not shown), only U6c, which is complementary to the region that interacts with authentic 5′ splice sites, blocks the U6 interaction with the pseudo 5′ splice site (Fig. 9A). When increasing amounts of the U6c oligonucleotide were incubated with nuclear extract, TIAR binding was reduced gradually (Fig. 9B). These results indicate that binding of TIAR depends on the interaction of not only U1 but also U6 snRNA with the pseudo 5′ splice site. This U6-dependent binding of TIAR is an exciting, novel finding because it strongly suggests that the U6 interaction is functionally important and that its role in promoting exon 4 inclusion is mediated by TIAR.
DISCUSSION
The calcitonin/CGRP RNA intron enhancer is complex, containing multiple short sequence motifs that are required for full function in cell types that include or exclude exon 4. As a consequence, a number of trans-acting factors bind to the enhancer through their interaction with the individual motifs. Previously, functional interactions between the enhancer element and U1 snRNP, SRp20, and PTB have been identified (33-35). In this report, we have demonstrated the interaction of two additional factors, TIA-1/TIAR and U6 snRNA, with the intron element.
Novel activity of TIAR in regulating alternative splicing.
We found that TIAR as well as TIA-1 binds to the U tract sequence that follows the downstream pseudo 5′ splice site. This interaction is functionally important for the inclusion of exon 4, which is located more than 200 nt upstream of the U tract sequence. Introduction of a truncated TIAR or TIA-1 protein that contains only the carboxy-terminal Q-rich domain reduced exon 4 inclusion, presumably by dominantly sequestering TIA-interacting proteins (see below). Overexpression of the full-length TIAR protein increased exon 4 inclusion in a mutant calcitonin/CGRP reporter that contains fewer uridines in the U tract but not that in the wild-type reporter. The latter result can be explained by postulating that TIAR is not the limiting factor in HeLa cells in processing the wild-type reporter pre-mRNA to include exon 4.
Previous studies by two groups have demonstrated that TIA-1 protein functions to promote usage of a number of 5′ splice sites immediately followed by U-rich sequences (12, 16). In one study, overexpression of TIA-1 or TIAR cDNA activated inclusion of several alternative exons of the TIA-1/TIAR pre-mRNAs, a process leading to production of truncated TIA-1/TIAR proteins because of the presence of stop codons in these alternative exons (30). U-rich sequences have been identified immediately downstream of apparently weak 5′ splice sites of all of these alternative exons, and TIA-1/TIAR proteins were proposed to mediate the enhancement of U1 snRNP binding to these 5′ splice sites by binding to the U-rich sequences (30). Our studies using the calcitonin/CGRP system have revealed an activity of TIA-1/TIAR proteins that is novel in the following respects. First, the downstream pseudo 5′ splice site in the calcitonin/CGRP RNA enhancer element is a good match to the 5′ splice site consensus sequence, which perhaps explains why U1 snRNA interaction with this site does not depend on TIAR binding to the U tract. This is consistent with the findings of Forch et al., who demonstrated that binding of U1 snRNP was no longer enhanced by the U-rich sequence when the msl-2 5′ splice site was mutated to better match the consensus 5′ splice site sequence (16). Secondly, instead of promoting usage of the 5′ splice site immediately upstream of its binding site, TIAR protein promotes inclusion of a 3′-terminal exon, exon 4, which is located more than 200 nt upstream of the U tract sequence. Thirdly, although not required for U1 snRNA binding, TIAR protein promotes binding of U6 snRNA to the downstream pseudo 5′ splice site.
TIA-1 and TIAR proteins consist of three RRM domains and a Q-rich domain. It has been demonstrated in an in vitro binding assay using recombinant proteins that RRM2 and RRM3 are responsible for specific binding to U-rich sequences (13). Indeed, full-length TIAR and a mutant TIAR containing only RRM2 and RRM3 showed comparable binding affinities and specificities for the calcitonin/CGRP RNA intron enhancer element in a gel shift assay (data not shown). Interestingly, the result that binding of TIAR to the U tract sequence in nuclear extract depends on the interaction between U1 and the pseudo 5′ splice site suggests that TIAR binding in cells requires more of the protein than RRM2 and RRM3. This result also suggests the presence of an inhibitory activity in nuclear extract that prevents TIAR protein from binding to its target sequence in the absence of U1 snRNP binding. Consistent with this notion, when nuclear extract was diluted and used in the UV cross-linking-IP analysis, TIAR binding to the U tract sequence no longer discriminated between RNA substrates containing the wild-type and mutated pseudo 5′ splice sites, presumably due to decreased concentration of the inhibitory activity (data not shown).
It has been postulated that RRM1 and the Q-rich domain are involved in interactions with other proteins. In fact, a recent study by Forch et al. demonstrated that TIA-1 interacts with the U1 snRNP-specific C protein through RRM1 and the Q domain to perform its role in promoting U1 recruitment to 5′ splice sites (17). The interdependence of binding of TIA-1/TIAR and U1 and U6 snRNAs to their target RNA sites in the calcitonin/CGRP RNA intron enhancer element suggests that TIA-1/TIAR interacts with both the U1 and U6 snRNPs. It is possible that the Q-rich domain functions as a dominant negative mutant by sequestering U1 or U6 snRNP, thereby interfering with the normal function of the endogenous TIA-1/TIAR. When we introduced a truncated TIA-1 protein containing only RRM1 and the Q domain into HeLa cells, a similar dominant negative effect was observed (not shown), consistent with the notion that this mutant protein sequesters U1 and/or U6 snRNP.
TIA-1 and TIAR were originally identified as apoptosis-promoting factors that induce DNA fragmentation in digitonin-permeabilized thymocytes (53). Similar to other proteins that shuttle between the nucleus and cytoplasm, TIA proteins have diverse cellular functions (18). TIA proteins have been shown to bind to an AU-rich element in the 3′ untranslated region of tumor necrosis factor alpha mRNA and regulate the stability and translatability of this transcript (21, 44). Specifically, TIA-1 has been demonstrated to act as an inhibitor of tumor necrosis factor alpha mRNA translation (44). In addition to regulating mRNA stability and translation of specific mRNAs, TIA proteins also play an important role in general translational control in response to cellular stress (2). During stress, TIA-1 and TIAR are required to sequester the translationally arrested mRNAs to cytoplasmic foci termed stress granules, which are thought to serve as triage centers for sorting out the fates of these mRNAs (2). Interestingly, the dominant negative effect of the Q-rich domain was also observed in an assay that tested the function of TIA proteins in response to cellular stress (24). Two recent reports have further expanded the cellular function of TIAR, implicating a role of this protein in virus-induced apoptosis (22) and virus replication (31). A double knockout of TIA-1 and TIAR was generated in chicken DT40 cells, and it was demonstrated that DT40 cells require either TIA-1 or TIAR for viability (29)
The role of U6 snRNP in promoting exon 4 inclusion.
The U6 snRNA interaction with the downstream pseudo 5′ splice site is reminiscent of the interaction between U6 and a bona fide 5′ splice site in that it depends on ATP and the base-pairing interaction between U1 snRNP and the same 5′ splice site (Fig. 7). Although the exact sites of cross-linking between U6 snRNA and the enhancer remain to be determined, it seems likely that the cross-linking sites will be the same as those that occur when U6 snRNA binds to an authentic 5′ splice site. Indeed, psoralen cross-linking between U6 snRNA and the enhancer was abolished when nuclear extract was incubated with an anti-U6 RNA ribo-oligonucleotide complementary to the region of U6 that interacts with authentic 5′ splice sites (Fig. 8A and 9A).
The functional importance of U6 binding for the regulation of exon 4 inclusion and exclusion is not known and is currently under investigation. We hypothesize that the U6 interaction is functionally important for the following reasons. First, this interaction is not adventitious: U6 preferentially interacts with the downstream pseudo 5′ splice site but not with the upstream pseudo 5′ splice site nearby (Fig. 7). The fact that the downstream pseudo 5′ splice site plays a critical role in controlling exon 4 inclusion strongly suggests that binding of U6 snRNP to this site is functionally important in the regulation. Secondly, our studies demonstrating that TIA proteins are required for exon 4 inclusion and that binding of these proteins to the intron enhancer depends on the interaction between U6 and the downstream pseudo 5′ splice site provides indirect yet strong evidence for the functional role of U6 snRNP in regulating exon 4 usage. Future experiments will include a suppressor U6 rescue strategy, analogous to the one used to delineate the functional interaction between U1 snRNA and the pseudo 5′ splice site in the intronic enhancer element (Fig. 3A).
The U6 interaction with the downstream pseudo 5′ splice site is extremely intriguing in light of the established function of U6 snRNP. Results from recent biochemical and genetic experiments position U6 snRNP at the center of the spliceosome where the chemical reactions occur (7). U6 snRNA (U6atac for minor-class introns) has been shown to contact the 5′ splice site of an intron through Watson-Crick pairing of bases during splicing of both major- and minor-class introns (7). Moreover, it was recently shown in yeast that U6 snRNA also functionally interacts with the 3′ splice site of an intron (10). Two interesting studies on the influenza virus NS1 protein demonstrated that the inhibition of general splicing by NS1 targets U6 and U6atac snRNAs (45, 54). NS1 binds to specific regions on U6 (U6atac) snRNA to prevent U6-U2 (U12-U6atac) and U6-U4 interactions during splicing (45, 54). Although there is precedent for the involvement of snRNPs (U1 and U11 snRNPs) in alternative removal of an intron (19, 40), U6 snRNP has never been implicated in regulation of alternative RNA processing. Thus, the functional involvement of U6 snRNP in calcitonin/CGRP alternative RNA processing would represent a novel function for this snRNP.
How may TIAR and U6 snRNP interaction with the intron enhancer promote exon 4 inclusion?
In an initial attempt to address the issue of how TIAR and U6 snRNP interaction with the intron enhancer may promote exon 4 inclusion, we tested whether, like SRp20 and U1 snRNP, TIAR and U6 snRNP are involved in enhancing polyadenylation of exon 4 (33, 35). When an RNA substrate that contains the mutated U tract sequence was incubated with HeLa cell nuclear extract, polyadenylation cleavage of exon 4 was not affected (data not shown), even though TIAR binding was nearly abolished. In a separate experiment, the anti-U6 snRNA oligonucleotide capable of blocking the interaction between U6 snRNA and the enhancer element (U6c) (Fig. 8A and 9A) had no effect on exon 4 polyadenylation in an in vitro polyadenylation cleavage assay (data not shown). Taken together, these results suggest that the enhancer element plays a second role in regulation of exon 4 inclusion, in addition to polyadenylation enhancement, and that this novel role is mediated by TIAR and U6 snRNP.
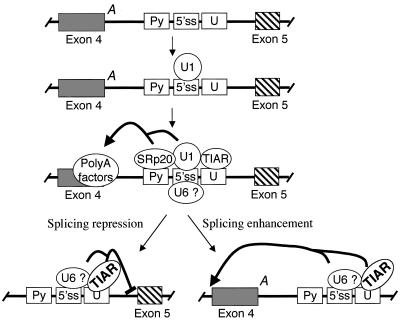
We propose that during transcription of calcitonin/CGRP pre-mRNA, U1 snRNP binds to the core pseudo 5′ splice site and nucleates binding of a number of factors, including SRp20, TIA, and U6 snRNP (Fig. 10). Of these factors, SRp20 and perhaps U1 snRNP function to enhance polyadenylation by directly interacting with polyadenylation factors, while TIA and/or U6 snRNP play a different role. TIA and U6 may inhibit recognition of the 3′ splice site of exon 5 or enhance recognition of the 3′ splice site of exon 4 (Fig. 10). There are precedents for either possibility. Intron elements have been shown to enhance inclusion of an upstream exon (8, 41, 47). A good example for the suppression model is splicing of the Rous sarcoma virus pre-mRNA. In that case, a complex containing U1 and U11 snRNPs assembles on a negative regulator of splicing element in the middle of an intron, and this complex has an inhibitory effect on splicing to the downstream 3′ splice site (11, 40).
FIG. 10.
Two alternative models for the roles of TIAR and U6 snRNP in the intron enhancer-mediated facilitation of exon 4 inclusion. A, polyadenylation signal; Py, pyrimidine sequence; 5′ ss, 5′ splice site; U, U tract.
Forch and colleagues demonstrated that TIA-1 promotes U1 snRNP binding to 5′ splice sites followed by U-rich sequences, indicating that TIA-1 is involved in early recognition of authentic 5′ splice sites (16). We are currently investigating how TIAR protein interacts with the basic splicing machinery to regulate exon 4 inclusion in the calcitonin/CGRP pre-mRNA.
Interplay of two pseudo 5′ splice sites within the enhancer element.
The two pseudo 5′ splice sites in the calcitonin/CGRP RNA enhancer element are separated by 37 nt. Although they are identical in sequence at 8 of 10 positions (upstream, CAGGUAAGCA; downstream, CAGGUAAGAC), they have opposite functions in regulating exon 4. In transfected cells, the downstream site is required for exon 4 inclusion while the upstream site suppresses exon 4 inclusion (36). The apparent competition between the two pseudo 5′ splice sites is reminiscent of the regulation of the Drosophila P element alternative splicing. Removal of intron 3 of the P element pre-mRNA occurs in germ cells but is suppressed in somatic tissue (50). In the soma, usage of the authentic 5′ splice site of intron 3 is suppressed by the presence of two pseudo 5′ splice sites located immediately upstream of the authentic site (48). The pseudo 5′ splice sites are bound by a KH type RNA binding protein, PSI (P element somatic inhibitor), which recruits U1 snRNP to the pseudo 5′ splice sites through an interaction with U1 70K, thereby preventing U1 snRNP from binding to the authentic site (26, 49).
We demonstrated in this report that the interaction of U6 snRNP with the intron enhancer element depends on the integrity of the downstream but not the upstream pseudo 5′ splice site. In addition, TIAR binds to the U tract sequence immediately following the downstream pseudo 5′ splice site. These observations may explain the different roles played by the two pseudo 5′ splice sites. We predict that a different set of protein factors as well as snRNPs interact specifically with each of the two pseudo 5′ splice sites.
Tissue-specific regulation of exon 4 inclusion.
U6 snRNP is an essential, universal splicing factor. TIAR has also been found in many tissues, although TIA-1 shows a much more restricted tissue distribution (3, 52). Indeed, Western blot analysis with the antibody that recognizes both TIAR and TIA-1 detected similar levels of these proteins in cell lines of either thyroid or neuron origin (data not shown). It is likely that in cells predominantly including exon 4, a positively acting enhancer complex consisting of U6 snRNP and TIAR as well as the previously identified factors, U1 snRNP, SRp20, and PTB, assembles on the intron enhancer. Upon binding, this complex interacts with the splicing and polyadenylation machineries to mediate exon 4 inclusion. What controls the neuron-specific, exon 4 exclusion processing pathway? There is precedent for the idea that the function of a complex formed in nonneural cells can be changed by inclusion of a neuron-enriched protein. In neurons, inclusion of the N1 exon of the c-src pre-mRNA is enhanced by the downstream intronic splicing enhancer DCS (downstream control sequence) that binds to a complex of several proteins. These proteins include hnRNP F and H, a neuron-enriched protein nPTB, and KSRP (KH type splicing regulator protein) (38). In nonneuronal cells, a similar but less stable complex forms on the DCS and contains the same proteins except that the nPTB is replaced by the ubiquitous form of PTB. Interestingly, the presence of “normal” PTB in the complex strongly represses inclusion of the N1 exon in the nonneuronal cells (38). In the case of the calcitonin/CGRP RNA enhancer, PTB is included in the complex formed in HeLa cells. It is possible that PTB is replaced by nPTB in neural cells, thereby altering the function of the complex. Additional neuron-specific proteins may also be present in the complex formed in neural cells. A number of neuron-specific RNA binding proteins have been isolated, including Nova1 and Nova2 (6, 55), CELF proteins (CUG binding and ETR3-like factor) (9, 27), and the mammalian homologs of Drosophila ELAV, known as Hu proteins (43, 51). It is highly likely that inclusion of such factors in the complex formed on the intron enhancer element in neurons will change the activity of the complex, thereby leading to exon 4 exclusion.
Acknowledgments
We thank Sue Berget for helpful advice and numerous reagents, including nuclear extracts (NIH grant GM58019). We thank Tim Nilsen for providing U6 oligonucleotides. We thank Andy McCullough, Helen Salz, and Jo Ann Wise for critical reading of the manuscript.
This work was supported by an ACS institutional grant to the Ireland Cancer Center at Case Western Reserve University (IRG-91-022-06). N.L.K. was supported by NIH grants to Paul Anderson (AI50167 and AI33600).
REFERENCES
- 1.Amara, S. G., V. Jonas, M. G. Rosenfeld, E. S. Ong, and R. M. Evans. 1982. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298:240-244. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227-3234. [DOI] [PubMed] [Google Scholar]
- 3.Beck, A. R., Q. G. Medley, S. O'Brien, P. Anderson, and M. Streuli. 1996. Structure, tissue distribution and genomic organization of the murine RRM-type RNA binding proteins TIA-1 and TIAR. Nucleic Acids Res. 24:3829-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, D. L. 2003. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72:291-336. [Online.] [DOI] [PubMed] [Google Scholar]
- 5.Black, D. L. 2000. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell 103:367-370. [DOI] [PubMed] [Google Scholar]
- 6.Buckanovich, R. J., J. B. Posner, and R. B. Darnell. 1993. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron 11:657-672. [DOI] [PubMed] [Google Scholar]
- 7.Burge, C. B., T. Tuschl, and P. A. Sharp. 1999. Splicing of precursors to mRNAs by the spliceosomes, p. 525-560. In R. F. Gesteland, T. R. Cech, and J. F. Atkins (ed.), The RNA world. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 8.Carlo, T., D. A. Sterner, and S. M. Berget. 1996. An intron splicing enhancer containing a G-rich repeat facilitates inclusion of a vertebrate micro-exon. RNA 2:342-353. [PMC free article] [PubMed] [Google Scholar]
- 9.Charlet, B. N., P. Logan, G. Singh, and T. A. Cooper. 2002. Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol. Cell 9:649-658. [DOI] [PubMed] [Google Scholar]
- 10.Collins, C. A., and C. Guthrie. 2001. Genetic interactions between the 5′ and 3′ splice site consensus sequences and U6 snRNA during the second catalytic step of pre-mRNA splicing. RNA 7:1845-1854. [PMC free article] [PubMed] [Google Scholar]
- 11.Cook, C. R., and M. T. McNally. 1999. Interaction between the negative regulator of splicing element and a 3′ splice site: requirement for U1 small nuclear ribonucleoprotein and the 3′ splice site branch point/pyrimidine tract. J. Virol. 73:2394-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Gatto-Konczak, F., C. F. Bourgeois, C. Le Guiner, L. Kister, M. C. Gesnel, J. Stevenin, and R. Breathnach. 2000. The RNA-binding protein TIA-1 is a novel mammalian splicing regulator acting through intron sequences adjacent to a 5′ splice site. Mol. Cell. Biol. 20:6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dember, L. M., N. D. Kim, K. Q. Liu, and P. Anderson. 1996. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J. Biol. Chem. 271:2783-2788. [DOI] [PubMed] [Google Scholar]
- 14.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forch, P., L. Merendino, C. Martinez, and J. Valcarcel. 2001. Modulation of msl-2 5′ splice site recognition by Sex-lethal. RNA 7:1185-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forch, P., O. Puig, N. Kedersha, C. Martinez, S. Granneman, B. Seraphin, P. Anderson, and J. Valcarcel. 2000. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol. Cell 6:1089-1098. [DOI] [PubMed] [Google Scholar]
- 17.Forch, P., O. Puig, C. Martinez, B. Seraphin, and J. Valcarcel. 2002. The splicing regulator TIA-1 interacts with U1-C to promote U1 snRNP recruitment to 5′ splice sites. EMBO J. 21:6882-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forch, P., and J. Valcarcel. 2001. Molecular mechanisms of gene expression regulation by the apoptosis-promoting protein TIA-1. Apoptosis 6:463-468. [DOI] [PubMed] [Google Scholar]
- 19.Gontarek, R. R., M. T. McNally, and K. Beemon. 1993. Mutation of an RSV intronic element abolishes both U11/U12 snRNP binding and negative regulation of splicing. Genes Dev. 7:1926-1936. [DOI] [PubMed] [Google Scholar]
- 20.Graveley, B. R. 2001. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 17:100-107. [DOI] [PubMed] [Google Scholar]
- 21.Gueydan, C., L. Droogmans, P. Chalon, G. Huez, D. Caput, and V. Kruys. 1999. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J. Biol. Chem. 274:2322-2326. [DOI] [PubMed] [Google Scholar]
- 22.Iseni, F., D. Garcin, M. Nishio, N. Kedersha, P. Anderson, and D. Kolakofsky. 2002. Sendai virus trailer RNA binds TIAR, a cellular protein involved in virus-induced apoptosis. EMBO J. 21:5141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami, A., Q. Tian, X. Duan, M. Streuli, S. F. Schlossman, and P. Anderson. 1992. Identification and functional characterization of a TIA-1-related nucleolysin. Proc. Natl. Acad. Sci. USA 89:8681-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kedersha, N. L., M. Gupta, W. Li, I. Miller, and P. Anderson. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147:1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keene, J. D. 2001. Ribonucleoprotein infrastructure regulating the flow of genetic information between the genome and the proteome. Proc. Natl. Acad. Sci. USA 98:7018-7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labourier, E., M. D. Adams, and D. C. Rio. 2001. Modulation of P-element pre-mRNA splicing by a direct interaction between PSI and U1 snRNP 70K protein. Mol. Cell 8:363-373. [DOI] [PubMed] [Google Scholar]
- 27.Ladd, A. N., N. Charlet, and T. A. Cooper. 2001. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol. Cell. Biol. 21:1285-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lander, E. S., L. M. Linton, B. Birren, C. Nusbaum, M. C. Zody, J. Baldwin, K. Devon, K. Dewar, M. Doyle, W. FitzHugh, R. Funke, D. Gage, K. Harris, A. Heaford, J. Howland, L. Kann, J. Lehoczky, R. LeVine, P. McEwan, K. McKernan, J. Meldrim, J. P. Mesirov, C. Miranda, W. Morris, J. Naylor, C. Raymond, M. Rosetti, R. Santos, A. Sheridan, C. Sougnez, N. Stange-Thomann, N. Stojanovic, A. Subramanian, D. Wyman, J. Rogers, J. Sulston, R. Ainscough, S. Beck, D. Bentley, J. Burton, C. Clee, N. Carter, A. Coulson, R. Deadman, P. Deloukas, A. Dunham, I. Dunham, R. Durbin, L. French, D. Grafham, S. Gregory, T. Hubbard, S. Humphray, A. Hunt, M. Jones, C. Lloyd, A. McMurray, L. Matthews, S. Mercer, S. Milne, J. C. Mullikin, A. Mungall, R. Plumb, M. Ross, R. Shownkeen, S. Sims, R. H. Waterston, R. K. Wilson, L. W. Hillier, J. D. McPherson, M. A. Marra, E. R. Mardis, L. A. Fulton, A. T. Chinwalla, K. H. Pepin, W. S. Gish, S. L. Chissoe, M. C. Wendl, K. D. Delehaunty, T. L. Miner, A. Delehaunty, J. B. Kramer, L. L. Cook, R. S. Fulton, D. L. Johnson, P. J. Minx, S. W. Clifton, T. Hawkins, E. Branscomb, P. Predki, P. Richardson, S. Wenning, T. Slezak, N. Doggett, J. F. Cheng, A. Olsen, S. Lucas, C. Elkin, E. Uberbacher, M. Frazier, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409:860-921. [DOI] [PubMed] [Google Scholar]
- 29.Le Guiner, C., M. C. Gesnel, and R. Breathnach. 2003. TIA-1 or TIAR is required for DT40 cell viability. J. Biol. Chem. 278:10465-10476. [DOI] [PubMed] [Google Scholar]
- 30.Le Guiner, C., F. Lejeune, D. Galiana, L. Kister, R. Breathnach, J. Stevenin, and F. Del Gatto-Konczak. 2001. TIA-1 and TIAR activate splicing of alternative exons with weak 5′ splice sites followed by a U-rich stretch on their own pre-mRNAs. J. Biol. Chem. 276:40638-40646. [DOI] [PubMed] [Google Scholar]
- 31.Li, W., Y. Li, N. Kedersha, P. Anderson, M. Emara, K. M. Swiderek, G. T. Moreno, and M. A. Brinton. 2002. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J. Virol. 76:11989-12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lou, H., and R. F. Gagel. 2001. Alternative ribonucleic acid processing in endocrine systems. Endocr. Rev. 22:205-225. [DOI] [PubMed] [Google Scholar]
- 33.Lou, H., R. F. Gagel, and S. M. Berget. 1996. An intron enhancer recognized by splicing factors activates polyadenylation. Genes Dev. 10:208-219. [DOI] [PubMed] [Google Scholar]
- 34.Lou, H., D. M. Helfman, R. F. Gagel, and S. M. Berget. 1999. Polypyrimidine tract-binding protein positively regulates inclusion of an alternative 3′-terminal exon. Mol. Cell. Biol. 19:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou, H., K. M. Neugebauer, R. F. Gagel, and S. M. Berget. 1998. Regulation of alternative polyadenylation by U1 snRNPs and SRp20. Mol. Cell. Biol. 18:4977-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lou, H., Y. Yang, G. J. Cote, S. M. Berget, and R. F. Gagel. 1995. An intron enhancer containing a 5′ splice site sequence in the human calcitonin/calcitonin gene-related peptide gene. Mol. Cell. Biol. 15:7135-7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maniatis, T., and B. Tasic. 2002. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature 418:236-243. [DOI] [PubMed] [Google Scholar]
- 38.Markovtsov, V., J. M. Nikolic, J. A. Goldman, C. W. Turck, M. Y. Chou, and D. L. Black. 2000. Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Mol. Cell. Biol. 20:7463-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maroney, P. A., C. M. Romfo, and T. W. Nilsen. 2000. Functional recognition of 5′ splice site by U4/U6. U5 tri-snRNP defines a novel ATP-dependent step in early spliceosome assembly. Mol. Cell 6:317-328. [DOI] [PubMed] [Google Scholar]
- 40.McNally, L. M., and M. T. McNally. 1999. U1 small nuclear ribonucleoprotein and splicing inhibition by the Rous sarcoma virus negative regulator of splicing element. J. Virol. 73:2385-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modafferi, E. F., and D. L. Black. 1997. A complex intronic splicing enhancer from the c-src pre-mRNA activates inclusion of a heterologous exon. Mol. Cell. Biol. 17:6537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modrek, B., and C. Lee. 2001. A genomic view of alternative splicing. Nat. Genet. 30:13-19. [DOI] [PubMed] [Google Scholar]
- 43.Okano, H. J., and R. B. Darnell. 1997. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci. 17:3024-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piecyk, M., S. Wax, A. R. Beck, N. Kedersha, M. Gupta, B. Maritim, S. Chen, C. Gueydan, V. Kruys, M. Streuli, and P. Anderson. 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 19:4154-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu, Y., M. Nemeroff, and R. M. Krug. 1995. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6-U2 and U6-U4 snRNA interactions during splicing. RNA 1:304-316. [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenfeld, M. G., S. G. Amara, and R. M. Evans. 1984. Alternative RNA processing: determining neuronal phenotype. Science 225:1315-1320. [DOI] [PubMed] [Google Scholar]
- 47.Ryan, K. J., and T. A. Cooper. 1996. Muscle-specific splicing enhancers regulate inclusion of the cardiac troponin T alternative exon in embryonic skeletal muscle. Mol. Cell. Biol. 16:4014-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siebel, C. W., L. D. Fresco, and D. C. Rio. 1992. The mechanism of somatic inhibition of Drosophila P-element pre-mRNA splicing: multiprotein complexes at an exon pseudo-5′ splice site control U1 snRNP binding. Genes Dev. 6:1386-1401. [DOI] [PubMed] [Google Scholar]
- 49.Siebel, C. W., R. Kanaar, and D. C. Rio. 1994. Regulation of tissue-specific P-element pre-mRNA splicing requires the RNA-binding protein PSI. Genes Dev. 8:1713-1725. [DOI] [PubMed] [Google Scholar]
- 50.Siebel, C. W., and D. C. Rio. 1990. Regulated splicing of the Drosophila P transposable element third intron in vitro: somatic repression. Science 248:1200-1208. [DOI] [PubMed] [Google Scholar]
- 51.Szabo, A., J. Dalmau, G. Manley, M. Rosenfeld, E. Wong, J. Henson, J. B. Posner, and H. M. Furneaux. 1991. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell 67:325-333. [DOI] [PubMed] [Google Scholar]
- 52.Taupin, J. L., Q. Tian, N. Kedersha, M. Robertson, and P. Anderson. 1995. The RNA-binding protein TIAR is translocated from the nucleus to the cytoplasm during Fas-mediated apoptotic cell death. Proc. Natl. Acad. Sci. USA 92:1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian, Q., M. Streuli, H. Saito, S. F. Schlossman, and P. Anderson. 1991. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 67:629-639. [DOI] [PubMed] [Google Scholar]
- 54.Wang, W., and R. M. Krug. 1998. U6atac snRNA, the highly divergent counterpart of U6 snRNA, is the specific target that mediates inhibition of AT-AC splicing by the influenza virus NS1 protein. RNA 4:55-64. [PMC free article] [PubMed] [Google Scholar]
- 55.Yang, Y. Y., G. L. Yin, and R. B. Darnell. 1998. The neuronal RNA-binding protein Nova-2 is implicated as the autoantigen targeted in POMA patients with dementia. Proc. Natl. Acad. Sci. USA 95:13254-13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhuang, Y., and A. M. Weiner. 1986. A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell 46:827-835. [DOI] [PubMed] [Google Scholar]