Abstract
Purpose
It is well known that lensectomy surgery during the first year of life increases the risk of aphakic glaucoma. However, it is controversial whether there is a specific time period during the first year of life after which performing lensectomy surgery has a lower risk of aphakic glaucoma development.
Methods
A retrospective chart review was done of all patients seen by a pediatric glaucoma specialist (D.S.W.) from 1970 to 2003. Patients were included if they had congenital cataract surgery performed by either D.S.W. or a referring ophthalmologist. Cataracts were defined as congenital if they were identified within the first 6 months of life, were dominantly inherited, or were of the lamellar type. Aphakic glaucoma was defined as having repeated intraocular pressures greater than 25 mm Hg after congenital cataract surgery. Patients were excluded if they had any conditions that independently are associated with glaucoma. Statistical analysis for risk factors for aphakic glaucoma development was done using SAS software.
Results
Three hundred sixty-eight eyes of 258 patients were included in the study. Two hundred sixteen eyes (58.7%) of 150 patients developed aphakic glaucoma. Risk factors of greatest significance (P < .0001) included having lensectomy within the first year of life and the development of postoperative complications.
Conclusions
No specific age for lensectomy during that first year of life was associated with a decreased risk for aphakic glaucoma development. Surgery for congenital cataracts should not be delayed if the only reason for delay is to prevent the development of aphakic glaucoma.
INTRODUCTION
Aphakic glaucoma is the most common complication of congenital cataract surgery, and the incidence of this complication ranges from 0% to 32%.1–14 Aphakic glaucoma is associated with a worse visual prognosis after lensectomy.15
Many of the following factors have been implicated in the development of aphakic glaucoma: surgery within the first year of life,2,16,17 corneal diameters less than 10 mm,2,7,17,18 the presence of other ocular abnormalities,3,19 certain cataract types (ie, complete, nuclear, or persistent hyperplastic vitreous),2,17,20 the aphakic state per se,1,16,21,22 retained lens proteins,16,20,23,24 and a history of secondary surgery.3,23,25 However, the timing of the cataract surgery is the only factor over which the surgeon has complete control.
Therefore, this study will evaluate which factors are significantly associated with an increased risk of developing aphakic glaucoma after lensectomy surgery. Specifically, the study will evaluate whether there is a time during the first year of life after which the risk for developing aphakic glaucoma is significantly less.
METHODS
Massachusetts Eye and Ear Infirmary Institutional Review Board approval was obtained. A retrospective chart review was done of all patients seen by the pediatric ophthalmology and glaucoma services of the Massachusetts Eye and Ear Infirmary from 1970 to 2003.
Patients were included if they had lensectomy surgery prior to 20 years of age. Cataracts were defined as congenital if they were identified within the first 6 months of life, were dominantly inherited, or were of the lamellar type. Aphakic glaucoma was defined as having repeated intraocular pressures (IOPs) greater than 25 mm Hg after congenital cataract surgery. Goldmann or Perkins applanation tonometry was used to determine eye pressures.
Patients were excluded if they had any conditions that independently are associated with glaucoma. Patients were also excluded if they had a history of trauma, intraocular neoplasm, radiation therapy, anterior uveitis, anterior segment dysgenesis, Stickler syndrome, Lowe syndrome, maternal rubella syndrome, or trisomy 13. Patients with a history of corticosteroid use prior to lensectomy or with a history of maternal corticosteroid use were also excluded. Patients with retinal detachment or vitreous hemorrhage prior to lensectomy were excluded.
Recorded information included age, sex, birth weight, birth history, associated ocular disease, systemic disease, family history, and cataract type. Preoperative and postoperative eye examinations included visual acuity, slit-lamp examination, corneal diameters, IOP, gonioscopy, direct and indirect ophthalmoscopy, and ultrasonography. Surgery-related information included age at lensectomy, surgical technique, surgeon, intraoperative and postoperative complications, postoperative medications, treatment of complications, secondary operations, and age at last follow-up.
Statistical analysis was performed using SAS software by one of the authors (E.F.H.) at the Massachusetts General Hospital Institute for Technology Assessment.
RESULTS
Two hundred sixteen eyes of 150 patients who developed aphakic glaucoma were compared to 152 eyes of 108 patients who did not develop aphakic glaucoma. Of the patients who developed aphakic glaucoma, 74 were male and 76 were female. Of the patients who did not develop aphakic glaucoma, 50 were male and 58 were female.
Ninety-eight of the total 368 eyes (26.6%) had a family history of congenital cataracts in a first-degree relative. Forty of these patients had bilateral cataracts.
Of the 368 eyes in the study, 218 eyes (59.2%) had cataract removal by one surgeon (D.S.W.). One hundred fifty eyes (40.8%) were operated on by other ophthalmologists. Of the 310 eyes in which the operating technique was known, 286 eyes were operated on with modern vitrectomy instruments, 23 underwent older techniques (ie, needling, aspiration, linear extraction), and one had extracapsular cataract extraction with a posterior chamber intraocular lens at 1 month of age. Of the 286 eyes in which modern vitrectomy instruments were used, 206 eyes had primary posterior capsulotomy and anterior vitrectomy, whereas the other 80 eyes had intact posterior capsules at the conclusion of the lensectomy surgery. In 58 eyes, the surgical technique was not known.
The average age at lensectomy was 22.0 months ± 36.2 (range, 1 week to 20 years). Of those eyes that developed aphakic glaucoma, the average age at time of lensectomy was 8.2 months ± 16.9 (range, 2 weeks to 120 months) compared to an average age of 37.7 months ± 44.9 (range, 1 week to 20 years) in eyes that did not develop aphakic glaucoma.
In the 255 eyes in which the cataract type was known, complete and nuclear cataracts were the most common (Table 1). Mean corneal diameters prior to lensectomy were 10.2 mm ± 1.3 (range, 6 to 13 mm). Mean axial length by ultrasound prior to lensectomy was 15.5 mm ± 2.5 (range, 11.5 to 23 mm). Birth weight was 3.2 kg ± 0.8 (range, 0.6 to 4.5 kg).
TABLE 1.
DISTRIBUTION OF CATARACT TYPES IN EYES THAT HAD CONGENITAL CATARACT SURGERY
| TYPE OF CATARACT* | NUMBER OF EYES | PERCENTAGE |
|---|---|---|
| Nuclear | 72 | 28.2 |
| Lamellar | 30 | 11.8 |
| Persistent hyperplastic primary vitreous | 28 | 11.0 |
| Membranous | 16 | 6.3 |
| Cortical | 15 | 5.9 |
| Sutural | 6 | 2.4 |
| Anterior polar | 6 | 2.4 |
| Posterior lentiglobus/lenticonus | 3 | 1.2 |
| Posterior subcapsular | 3 | 1.2 |
| Posterior polar | 2 | 0.8 |
| Posterior cortical | 1 | 0.4 |
| Total | 255 |
Cataract type was known in only 255 of the 368 eyes that had congenital cataract surgery.
Other ocular abnormalities were noted in 127 of 368 eyes (34.5%), and strabismus and microphthalmos were the most common (Table 2). Sixty-nine of 258 patients (26.7%) had strabismus. Of the 69 patients with systemic abnormalities, cardiac abnormalities and developmental delay were the most common (Table 3).
TABLE 2.
OCULAR ABNORMALITIES NOTED IN EYES THAT HAD CONGENITAL CATARACT SURGERY
| OCULAR ANOMALY | NUMBER OF EYES* | PERCENTAGE |
|---|---|---|
| Strabismus | 69 | 48.3 |
| Microphthalmos | 62 | 43.4 |
| Persistent hyperplastic primary vitreous | 28 | 19.6 |
| Nasolacrimal duct obstruction | 16 | 11.2 |
| Myopia | 12 | 8.4 |
| Microcornea | 6 | 4.2 |
| Retinopathy of prematurity | 2 | 1.4 |
| Macular hypoplasia | 1 | 0.7 |
| Total | 127 |
Other ocular abnormalities were seen in only 127 of the 368 eyes that had congenital cataract surgery. Some eyes had more than one ocular abnormality.
TABLE 3.
SYSTEMIC ABNORMALITIES THAT WERE NOTED IN PATIENTS WHO HAD CONGENITAL CATARACT SURGERY*
| SYSTEMIC ABNORMALITIES | NUMBER OF PATIENTS |
|---|---|
| Cardiac anomaly | 12 |
| Developmental delay | 12 |
| Prematurity | 8 |
| Intrauterine infections | |
| Human immunodeficiency virus | 2 |
| Herpes simplex | 1 |
| Unknown viral syndrome | 1 |
| Metabolic diseases | |
| Hyperbilirubinemia | 6 |
| Hypoglycemia | 5 |
| Glucose-6-phosphate dehydrogenase deficiency | 1 |
| Hematologic disorders | |
| Acute lymphocytic leukemia | 1 |
| Leukopenia | 2 |
| Anemia | 1 |
| Thrombocytopenia | 1 |
| Idiopathic thrombocytopenic purpura | 1 |
| Exchange transfusion at birth | 1 |
| Central nervous system disorders | |
| Hydrocephalus | 2 |
| Seizures | 2 |
| Cranial nerve palsy | 1 |
| Atopy | |
| Asthma | 8 |
| Allergies | 3 |
| Renal anomaly | 5 |
| Musculoskeletal disorders | |
| Skeletal abnormality | 3 |
| Spasticity | 1 |
| Cutis marmorata telangiectasia, congenital | 2 |
| Gastrointestinal disorders | |
| Malrotation of intestines | 1 |
| Gastroesophageal reflux disease | 1 |
| Pyloric stenosis | 1 |
| Umbilical hernia | 1 |
| Imperforate anus | 1 |
| Peroxisomal disorders | |
| Rhizomelic chondrodysplasia Punctata | 1 |
| Marden Walker syndrome | 1 |
| Maternal conditions in utero | |
| Gestational diabetes | 4 |
| Toxemia of pregnancy | 2 |
| Multiple drug use | 3 |
| Cleft palate | 1 |
Systemic abnormalities were seen in only 69 of the 258 patients that had congenital cataract surgery. Some patients had more than one abnormality.
There were no reported major intraoperative complications. After lensectomy, eyes were usually treated with corticosteroids, antibiotics, or both for 1 to 2 months. One hundred thirty-four of the 368 eyes were treated with cycloplegics postoperatively. Postoperative complications occurred in 229 (62.2%) of 368 eyes (Table 4). There were no known cases of corneal decompensation or endophthalmitis. Non-pressure-related postoperative complications had occurred more commonly in eyes that developed aphakic glaucoma (111 of 216 eyes, or 51.4%) compared to eyes that never developed aphakic glaucoma (47 of 152 eyes, or 30.9%).
TABLE 4.
POSTOPERATIVE COMPLICATIONS NOTED IN EYES THAT HAD CONGENITAL CATARACT SURGERY*
| COMPLICATION | NUMBER OF EYES | PERCENTAGE OF TOTAL |
|---|---|---|
| Aphakic glaucoma | 209 | 56.8 |
| Pupilllary membrane | 85 | 23.1 |
| Residual lens material | 25 | 6.8 |
| Posterior capsule opacification | 19 | 5.2 |
| Pupillary block | 9 | 2.4 |
| Vitreous in the anterior chamber | 7 | 1.9 |
| Vitreous hemorrhage | 6 | 1.6 |
| Severe inflammation | 5 | 1.4 |
| Retinal detachment | 4 | 1.1 |
| Malignant glaucoma | 2 | 0.5 |
| Hyphema | 1 | 0.3 |
| With complications | 229 | 62.2 |
| No complications | 139 | 37.8 |
| Total | 368 | 100 |
Some eyes had more than one complication.
The mean follow-up time for all patients was 124.5 months ± 83.5 (range, 3 months to 29 years).
Sixty-six patients had bilateral lensectomy and developed aphakic glaucoma in both eyes. Forty-five patients had unilateral lensectomy and developed aphakic glaucoma in only that operated eye. Thirty-nine patients had bilateral lensectomy and developed aphakic glaucoma in only one eye at last follow-up.
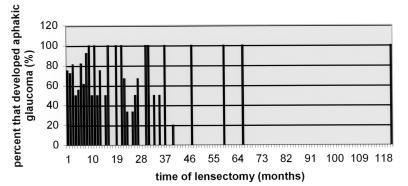
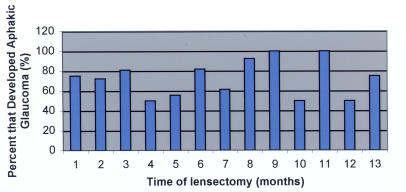
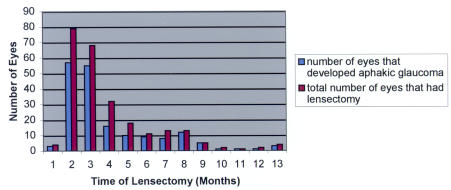
Although having lensectomy surgery in the first year of life is more commonly associated with the development of aphakic glaucoma (Table 5), there is no specific time in the first year of life when the incidence of aphakic glaucoma after lensectomy decreases significantly (Figures 1 and 2). By the ninth month (Figure 3), the number of eyes that had lensectomy decreased. For each time interval after the ninth month, there were no time intervals where there were more than five eyes that had lensectomy. This decreases the power of the study for eyes that had lensectomy surgery after 9 months of age.
TABLE 5.
POTENTIAL RISK FACTORS FOR APHAKIC GLAUCOMA AFTER CONGENITAL CATARACT SURGERY
| POTENTIAL RISK FACTOR | PVALUE (<.05) |
|---|---|
| Age (less than 1 year) | <.0001 |
| Postoperative complications | <.0001 |
| Postoperative cycloplegic use | <.0001 |
| Small corneal diameter | .0002 |
| Cataract type (nuclear/total) | .04 |
Multivariate analysis of 216 eyes of 150 patients who developed aphakic glaucoma vs 152 eyes of 108 patients who did not develop aphakic glaucoma.
FIGURE 1.
Percentage of eyes that developed aphakic glaucoma by the last follow-up examination after lensectomy surgery for congenital cataracts. There were never more than five eyes that had lensectomy surgery at any given time interval after 9 months of age (x-axis).
FIGURE 2.
Percentage of eyes that developed aphakic glaucoma after congenital cataract surgery performed during the first 13 months of life.
FIGURE 3.
Number of eyes that had lensectomy surgery for congenital cataracts vs number of eyes that developed aphakic glaucoma.
Risk factors significantly associated with the development of aphakic glaucoma included the following: having lensectomy at less than 1 year of age, having postoperative complications, having a history of postoperative cycloplegic use, having a small corneal diameter (less than 10 mm), and having a nuclear or total cataract (Table 5).
DISCUSSION
The visual prognosis for children with congenital cataracts has improved dramatically since it was first recognized that lensectomy during infancy is critical for a good visual outcome.26 Several studies have shown that the critical period for developing the fixation reflex in both unilateral and bilateral visual deprivation disorders is between 2 and 4 months of age, and the onset of nystagmus in a bilateral visually deprived infant probably marks the end of the critical period.27 However, it is also well known that having lensectomy at an early age, especially during the first year of life, increases the risk for aphakic glaucoma (Table 5).2,16,25,28,29 Because aphakic glaucoma is associated with a worse visual prognosis,15 it is important to know when and if there is a time during the first year of life when the risk of aphakic glaucoma after lensectomy decreases. Finding a balance between early surgery for improved visual prognosis and delaying surgery to possibly avoid an increased risk of aphakic glaucoma is necessary.
Knowing the optimal timing for lensectomy surgery is also important, because the other risk factors for aphakic glaucoma are largely out of the surgeon’s control. Some of these suggested nonmodifiable risk factors include the following: anatomic or physiologic predisposition of the eye,3,18,20 microcornea,2,6,18 microphthalmos,5,20 type of cataract,16,18 poor pupillary dilation,18 and genetic predisposition.4,20 Other risk factors include retained cortex, residual lens particle and protein,16,18 uveitis,23 need for secondary surgery,3,16,20 poor vasculature or supporting structures of the optic nerve,2 corticosteroids,21 blockage of the angle by vitreous,1 and vitreous factors altering trabecular meshwork structure and maturation.22
It is controversial whether there is a specific time during the first year of life when having lensectomy is associated with a decreased risk for developing aphakic glaucoma. Rabiah29 felt that the risk of aphakic glaucoma decreases if the cataract surgery is performed after 9 months of age. Vishwanath and colleagues30 felt that the risk of aphakic glaucoma decreases if cataract surgery is performed after 1 month of age. Lundvall and Kugelberg31 felt that late-onset open-angle glaucoma occurred predominantly in children who underwent cataract extraction during the first week of life.
In contrast, Watts and coworkers32 suggested that postoperative complications in general are less common when lensectomy is performed within the first 2 weeks of life. He did also mention that aphakic glaucoma is more prevalent when the surgery was performed between 13.5 and 43 days of life.32 It is hard to interpret this data because the average follow-up time in their study was 2.85 years ± 1.9 (range, 0.5 to 8). With such a short follow-up time, it would be difficult to determine the true incidence of aphakic glaucoma, which usually occurs at an average of 3.4 years ± 3.715 to 4.0 years ± 4.625 after lensectomy surgery.
This study did not show a significant decrease in the incidence of aphakic glaucoma associated with surgery performed at any time point during the first year of life (Figures 1 and 2). There were few eyes that had lensectomy surgery after 9 months of age (Figure 3). This is in agreement with ophthalmologists’ recommendations to operate on congenital cataracts at an early age to maximize visual prognosis. One study determined that visual outcome was the same regardless of when the surgery was performed during the first 6 weeks of life.33 It has also been suggested that perhaps the critical period for treating children with bilateral cataracts may extend to 8 weeks of life.8,26,31 Based on the results of this study, we would not recommend delaying lensectomy surgery until after 6 weeks of life to minimize the risk of developing aphakic glaucoma.
The only study that specifically sought to determine risk factors for aphakic glaucoma compared a group of lensectomy patients who developed aphakic glaucoma to a group that did not.29 In the 118 of 570 eyes (21%) that developed aphakic glaucoma, risk factors included the following: surgery at 9 months of age or less, secondary membrane surgery, microcornea, and primary posterior capsulotomy/anterior vitrectomy.29 Similar risk factors were noted in this study, and some of these included (Table 5) having lensectomy surgery within the first year of life, having a small corneal diameter (less than 10 mm), and having postoperative complications (especially those requiring a secondary surgical procedure [Table 4]). Since Rabiah’s study excluded patients with less than 5 years of follow-up (ie, excluded 672 of 1,242 eyes), it is possible that results were subject to selection bias. Rabiah29 reported that 37% of lensectomy patients developed aphakic glaucoma when lensectomy was performed at the age of 9 months or younger, compared to 6% of children undergoing surgery thereafter. Similar to this study (Figure 3), Rabiah29 also noted a significant decrease in the number of eyes that had lensectomy surgery after 9 months of age (ie, nine eyes at 10 months, six at 11 months, and four at 12 months of age). However, Rabiah did not attempt to determine if there was a specific time during the first 9 months during which the incidence of aphakic glaucoma development decreased.
Rabiah29 also did not evaluate whether certain cataract types were associated with an increased risk of aphakic glaucoma development. We found that nuclear and complete cataracts, which were the most common types of cataracts in this study (Table 1), were associated with a higher risk of developing aphakic glaucoma (Table 5). This was suggested by Parks and colleagues,17 who proposed that certain cataract types were associated with worse visual prognosis and with complications such as aphakic glaucoma. These results may partly be related to the fact that complete and nuclear cataracts tend to have earlier lensectomy. We agree with others in that the immaturity of the developing infant’s angle may make it more susceptible to secondary surgical trauma.2,25 It also possible that these types of cataracts may be associated at birth with a more abnormal angle than other cataract types.
Rabiah also did not evaluate whether certain ocular or systemic abnormalities were associated with an increased risk of aphakic glaucoma development,29 whereas this study did (Tables 2 and 3). In this study, no specific ocular or systemic abnormality was found to be associated with an increased risk for aphakic glaucoma, except for those associated with a smaller corneal diameter (less than 10 mm) (Table 5). A smaller cornea may reflect an abnormal anterior segment and subtle filtration angle defects, which increase the risk of aphakic glaucoma.16,18,25 In response to an American Ophthalmological Society presentation, Albert W. Biglan, MD, observed that patients with corneal diameters less than 10 mm are usually left aphakic after lensectomy surgery.16 Paul Kaufman, MD, also suggested that taking out the lens during the first year of life without putting anything in its place may prevent normal meshwork development, which may require certain normal structural interactions between the native lens, zonules, ciliary body, and trabecular meshwork.16
Rabiah29 also mentioned that he did not evaluate whether poor pupillary dilation with cycloplegics was associated with an increased risk of aphakic glaucoma; this problem has been suggested by others to be a factor in the development of aphakic glaucoma.2,16,21 Although miosis and/or poor pupillary dilation could certainly make lensectomy surgery more difficult and lead to more postoperative complications, only postoperative cycloplegic use was found to be associated with an increased risk of developing aphakic glaucoma in this study (Table 5). It is possible that postoperative cycloplegic use is more common in complicated cases, especially those with either postoperative miosis or those with significant retained lens material. It is possible that the retained lens material can induce inflammation, which can then cause the pupil to start to scar down. In reaction to this, the ophthalmologist may then add cycloplegics in order to prevent the pupil from scarring down. Therefore, postoperative cycloplegic use may simply be associated with increased aphakic glaucoma development, but the cycloplegics per se may not cause the development of aphakic glaucoma. However, it is also well known that cycloplegic dilating drops can elevate IOPs in both normal and glaucomatous eyes with gonioscopically open angles.34–36 It is possible that prolonged cycloplegic use in an eye whose angle is still developing may lead to subtle changes in trabecular meshwork development or function.
This study confirmed what others have found or suggested, in that aphakic glaucoma is associated with early age at lensectomy surgery, increased postoperative complications, small corneal diameters, and the complete or nuclear cataract types (Table 5). Use of postoperative cycloplegics appears to also be associated with an increased risk of aphakic glaucoma development (Table 5). Although others have suggested delaying lensectomy surgery in order to minimize the risk of aphakic glaucoma, we do not suggest delaying lensectomy surgery to prevent aphakic glaucoma, because this study did not find that there was a clinically significant decrease in incidence of aphakic glaucoma at any point in the first year of life (Figures 1 and 2). Further studies need to be done to determine whether delaying lensectomy surgery may decrease the risk for other, less common postoperative complications, which could also affect the final visual prognosis. Until future studies show that delaying lensectomy surgery significantly decreases the risk for significant postoperative complications, the main determinant of lensectomy surgery timing should still be to optimize visual prognosis.
PEER DISCUSSION
DR DAVID K. COATS
Is there an optimal time to perform cataract surgery during the first year of life that will balance the need to clear the visual axis during the most sensitive period of visual development with the risk of developing aphakic glaucoma, a condition that can be difficult to control and that itself is a cause of significant visual impairment? The authors of this paper have framed this dilemma exactly right. It is the all too common dichotomy faced by the pediatric ophthalmologic surgeon who is required to make important treatment recommendations that will have critical ramifications for the duration of a young child’s life, often with a paucity of good data upon which to base the decision. Few surgeons have sufficient experience with the surgical management of pediatric cataracts to be able to effectively explore this question. This study represents one in a series of studies on aphakic glaucoma published by Dr. Walton, a recognized expert in the field.
This retrospective study has some significant positive aspects. It has an ample sample size of 368 eyes in 258 children of which almost 60% developed glaucoma. It has extraordinarily long follow-up, averaging more than a decade; something that is almost unheard of in today’s mobile society. A potential drawback is the long time span of the study, encompassing the years from 1970 to 2003. During this period of time, substantial improvements in both the technology and techniques available to manage infantile and childhood cataracts have been achieved. The retrospective nature of the study adds potential bias such as loss to follow-up, variable length of follow-up and possibly other confounding factors. Nevertheless, this extraordinary group of patients, managed at a single center and followed for a long period of time can teach us a great deal about the risk of glaucoma in aphakic children.
The finding that the risk of glaucoma is the same, regardless of when cataract surgery is performed during the first year of life, is very important. Deferring cataract removal during the first year of life to reduce the risk of glaucoma would therefore seem to be unnecessary. Other factors, namely enhanced safety of general anesthesia in children beyond the first month of life, may still prompt ophthalmologic surgeons to defer surgery until during or after the second month of life.
An equally important finding in this study is the fact that postoperative complications such as the development of a pupillary membrane, retained lens material, pupillary block, and/or vitreous in the anterior chamber were associated with a higher rate of developing glaucoma. Many of these complications can be either prevented or minimized by meticulous surgical technique, including generous posterior capsulotomy, generous mechanized anterior vitrectomy, careful removal of all lens material, and peripheral iridotomy in susceptible eyes. Thus, this study has an important message for pediatric cataract surgeons, emphasizing the importance of technique and technology. It would be very interesting to know if modern techniques have reduced the occurrence of aphakic glaucoma and the authors may be able to provide insight on this topic by comparing the rate of glaucoma development for children operated with older versus newer techniques.
Another interesting finding was that postoperative use of cycloplegic agents was highly associated with development of glaucoma. The authors postulate that prolonged use of cycloplegic agents might have been more common in children who developed complications and that it is these complications, not the use of cycloplegic agents, which may have been the underlying issue. While the authors’ theory in this regard may be correct, an alternative explanation, as briefly alluded to by the authors, should be given greater consideration. In a study of 65 children with aphakic glaucoma published by Dr. Walton in 1995, the majority of eyes had developed progressive structural abnormalities of the anterior chamber filtration angle over time.1 It is reasonable to postulate that use of cycloplegic agents could amplify this process through prolonged distortion and compression of filtration angle structures. Thus, I believe that an important message of this paper is to exercise judicious use of cycloplegic agents in aphakic children, unless and until future studies nullify them as a contributing factor to the development of glaucoma.
In summary, I would like to congratulate the authors on a job well done. I agree with their conclusions and I hope that they will continue to study this important problem. I think that there are two very important take home messages in this study: (1) Delaying surgery in the first year of life does not appear to reduce the risk of developing aphakic glaucoma. (2) The surgeon may be able to influence a reduction in the risk of developing glaucoma by using meticulous surgical technique which avoids complications and/or by judicious use of cycloplegic agents in the postoperative period.
REFERENCE
- 1.Walton DS. Pediatric aphakic glaucoma: a study of 65 patients. Trans Am Ophthalmol Soc. 1995;93:403–413. [PMC free article] [PubMed] [Google Scholar]
DR MALCOLM R. ING
The advantage of a long-term study like this gives us a meaningful perspective. Is the angle involved in the development of aphakic glaucoma in children? We now say it is typically an open-angle, although the senior author at one time in the past advocated iridectomy. Is there a role for iridectomy or iridotomy in perhaps preventing some of the long-term complications? A question related to management -although it was not the subject of the paper, what is the current management of severe pediatric glaucoma?
DR EDWARD L. RAAB
There is an issue of whether you should be talking about numbers of eyes or numbers of patients. In a bilateral case, it would be unlikely that the cataracts were of different types or that the corneal diameters would be normal on one side and not normal on the other side. The latter is of course possible in a unilateral case. I wonder if dealing with patients, rather than eyes, would alter any of your numbers substantially. I would expect congruence of cataract types in bilateral cases. Obviously the systemic and the other risk factors would be substantially the same, and probably the handling by a single surgeon of both eyes of the bilateral case would be the same.
DR EVELYN A. PAYSSE
What was the earlier age of surgery and type of cataract versus later age of surgery and type of cataract? We typically operate nuclear cataracts at an earlier age and lamellar cataracts at a later age, as you did. The lamellar cataracts tend to be associated with a normal eye size whereas the nuclear cataract is usually present in small eyes. The definition of glaucoma was just based on intraocular pressure. Corneal thickness is becoming a factor in deciding whether the IOP measured is really valid. This is not a finding that you can do retrospectively in your study, but we may be overcalling high pressure in children who have thick corneas. We are currently looking at microphthalmic eyes in children who have cataract at our institution and are finding congenital cataract eyes tend to have thicker corneas. So again we may be overcalling glaucoma. I would respectfully suggest that other findings, such as optic nerve changes, be taken into account.
DR RICHARD P. MILLS
What interval would you recommend for monitoring intraocular pressure in the aphakic child? That question is particularly relevant during the years when they cannot cooperate for IOP measurements, but is an issue even later, when they can cooperate.
DR M. EDWARD WILSON
I had a question about including lamellar cataracts, dominantly inherited lamellar cataracts, as “congenital.” The definition of congenital we usually think of is a cataract that’s present conatally and it is often fetal nuclear. Lamellar cataracts may be developmental and not even be present at birth, so I am concerned about use of the term congenital. Concerning microcornea as a risk factor, eyes that present early with fetal nuclear cataracts, and microphthalmic eyes in general, are the eyes at risk. We looked at our aphakic and pseudophakic glaucoma patients. If you just look within the group that had surgery early, within the first six months of life, corneal diameter was not predictive of those who would develop glaucoma. Eyes at risk are eyes at risk, and corneas that are small tend to need surgery early. Within the at-risk group, we did not find that corneal diameter was a risk factor. In our recently published paper, we also found that, within that at-risk group, the intraocular lens patients and the aphakic patients had an equal incidence of glaucoma. So we found that an IOL was not a protective agent against glaucoma.
DR ALBERT W. BIGLAN
After the Beller and Hoyt’s article that was published in the early 1980’s, we tried to operate on children with congenital cataracts within the first week of life. We rehabilitated these eyes with contact lenses. With early intervention, in eyes with relatively small corneas and bilateral nuclear cataracts, these eyes respond quite differently. They are the ones with early surgery and aphakic glaucoma. We find that these eyes have an exuberant healing response. We have had episodes of angle closure glaucoma in some of these patients. We perform iridectomies in these eyes routinely. Other eyes develop the glaucoma later.
Have you looked for early spikes of intraocular pressure due to angle closure and compared them with the long-term late onset glaucoma eyes? Do you have any specific recommendations regarding management of eyes that are microphthalmic and have nuclear cataracts?
DR MALCOLM L. MAZOW
One of our recent departed members of the AOS, Dr Marshall Parks, felt that the incidence of glaucoma in aphakic children was exponentially increased in nanophthalmic eyes. If you’re considering microcornea as part of the nanophthalmic eye, it would be nice to separate out those cases that have glaucoma in a nanophthalmic eye, to see whether Dr Parks’ comments were correct.
DR THOMAS D. FRANCE
Do you have data as to the specific age that the glaucoma begins? A Monte Mills paper years ago showed that, over time, a greater percentage of these patients will develop glaucoma. Can you tell us how far out are we still going to see glaucoma developing in these patients?
DR TERESA C. CHEN
David Coats emphasizes the need for excellent surgical technique and that a possible risk factor for glaucoma is poor surgical technique. Retained lens material certainly that can lead to release of cytokines and secondary damage to the angle, and certainly if that retained lens material requires a second surgery, that might increase the risk of aphakic glaucoma. Meticulous surgical technique is very important.
Dr. Coats questions whether modern surgical techniques have changed the incidence of glaucoma compared to the older surgical lensectomy techniques. Although this particular study does not probably have the power to evaluate this problem since there were only 23 eyes that had the older surgical techniques, a review of the literature suggests that the incidence of glaucoma has not changed between the older and newer techniques. It does appear that the type of glaucoma is different. For the older techniques it appears that there is more commonly angle-closure pupillary block type of glaucoma while with the more modern surgical techniques there is more commonly the open angle type of glaucoma. As Dr. Walton has published in a previous Archives of Ophthalmology paper, probably 94 percent of patients with modern surgical techniques have open-angle glaucoma. But he emphasizes that these “open angles” are not normal and that about 96 percent of these open angle patients have some abnormality such as some circumferential forward positioning of the iris to the posterior or middle trabecular meshwork, some pigment deposits in the angle, or some crystalline type deposits which represent retained lens material.
Is the angle involved in this type of glaucoma? Is an iridotomy helpful in treating these patients? Certainly iridotomy is most helpful in those patients with a pupillary block type of glaucoma, where there might be posterior synechiae and subsequent angle closure. With more modern surgery techniques, it seems that open-angle glaucoma is a more common variant and iridotomy may not helpful in those instances.
Concerning the management of these more severe types of glaucoma, Dr. Walton has published a large series on aphakic glaucoma in the Archives of Ophthalmology recently and reported that angle surgery does not seem to be helpful, with a success rate being less than 20 percent. His report is a long-term follow-up over at least a decade. Angle surgery may not be helpful because there are some intrinsic angle abnormalities. For example, there may be some problem with Schlemm’s canal where angle surgery may not be helpful. In that report, he found that tube shunt surgery had a higher success rate of about 44 percent. Management of these patients certainly is different than the classic pediatric glaucoma patient.
A point was made about classifying by eyes versus classifying by number of patients. Certainly it is very possible that one eye may have high correlation with the other eye in the same patient, and this is obviously a problem with many studies. Our hope is that multivariate analysis did adjust for those problems. I’m also assuming that we have a high correlation coefficient between the two eyes, although we did not calculate that number. In the recent glaucoma literature there has been controversy about whether one needs to have a monocular drug trial with initial therapy as it is not clear how closely the two eyes are really related in terms of pressure development.
Are lamellar cataracts per se really associated with a decreased incidence of aphakic glaucoma as it has been suggested that lamellar cataracts are associated with a more normal size eye? Our hope is that our multi-variate analysis would adjust for that.
Drs. Michael Kass and Jamie Brandt have been strong proponents of corneal thickness measurements in evaluating glaucoma in addition to pressure measurements. Patients who have aphakic glaucoma or certainly children who have lensectomy surgery and are aphakic or pseudophakic have a higher corneal thickness measurement. John Simon published a paper suggesting that the average corneal thickness is around 660 in these patients, and an ARVO poster with lead author Jody Piltz Seymour found the average corneal thickness was 651. So it is possible that the pressure measurements in these patients may have been artifactually elevated. This underscores the importance of defining glaucoma, not just by pressure, but also by other parameters such as cupping and visual field loss. Dr. Lichter has also published that the definition of glaucoma should emphasize more on the characteristic cupping and visual field loss.
What is a good interval for monitoring these patients? It is strongly suggested in the literature that life-long follow-up for these patients is important, because we cannot predict exactly when these patients will develop glaucoma and certainly early detection of glaucoma is a major focus of the ophthalmologist. The interval for follow up depends on the patient. If they have more risk factors, then certainly we need to watch them more closely. If they have less risk factors, then they don’t need to be followed as closely. The literature suggests that probably the average time for the development of glaucoma is around three to four years after lensectomy surgery. So it is reasonable to follow these patients perhaps every four to six months initially. If the patient does not have risk factors, then maybe every year is sufficient. Life-long follow-up is very important.
In terms of definitions and semantics about congenital cataracts, it was pointed out that lamellar cataracts in many cases are not truly “congenital.” Semantics are important as Dr. Walton has also emphasized that the term congenital glaucoma may not accurately describe babies who may not develop high pressures until they are 8 or 9 months of age. Dr. Walton has suggested that maybe “newborn glaucoma” would be a more appropriate term for the truly “congenital” cases, and that infantile glaucoma can be reserved for those patients who develop “congenital glaucoma” at a later age. Certainly the definition of many terms is not always accurate.
Dr. Wilson mentioned that corneal diameter was not a significant risk factor in his recent paper. He also reported that primary IOL placement does not seem to protect them from developing glaucoma. A prior retrospective study by Dr. Sanjay Asrani included 377 eyes with primary pseudophakia but unfortunately excluded patients who had surgery at an early age. The patients included in that study also mostly had normal corneal diameters. If you eliminate the patients at higher risk, as that study did, you are going to have a lower incidence of glaucoma. Therefore, that study also does not definitely prove whether an IOL is protective.
There was a comment about microcornea and other associated abnormalities such as microphthalmia and whether those different diagnoses, independently, can be risk factors for glaucoma. Based on our multivariate analysis, it seems that just microcornea is a risk factor. It is probable that with the smaller numbers of microphthalmic and nanophthalmic patients in our study, the power of our study would not be sufficient to more clearly evaluate whether those other separate diagnoses or ocular abnormalities are independently associated with aphakic glaucoma. Although the microcornea may not directly cause the glaucoma, the microcornea may be associated with anterior segment abnormalities of the iris and angle and that the microcornea may be just one sign of the abnormal anterior segment. Certainly an abnormal anterior segment has a higher risk for developing glaucoma after lensectomy surgery.
Footnotes
Originally published in the Journal of Pediatric Ophthalmology & Strabismus, 2006;43(5); 274-280
REFERENCES
- 1.Asrani SG, Wilensky JT. Glaucoma after congenital cataract surgery. Ophthalmology. 1995;102:863–867. doi: 10.1016/s0161-6420(95)30942-6. [DOI] [PubMed] [Google Scholar]
- 2.Mills MD, Robb RM. Glaucoma following childhood cataract surgery. J Pediatr Ophthalmol Strabismus. 1994;31:355–360. doi: 10.3928/0191-3913-19941101-03. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GA, Parks MM, O’Neill JF. Incidence of chronic glaucoma, retinal detachment and secondary membrane surgery in pediatric aphakic patients. Ophthalmology. 1984;91:1238–1241. doi: 10.1016/s0161-6420(84)34161-6. [DOI] [PubMed] [Google Scholar]
- 4.Keech RV, Tongue AC, Scott WE. Complications after surgery for congenital and infantile cataracts. Am J Ophthalmol. 1989;108:136–141. doi: 10.1016/0002-9394(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 5.Simon JW, Mehta N, Simmons ST, et al. Glaucoma after pediatric lensectomy/vitrectomy. Ophthalmology. 1991;98:670–674. doi: 10.1016/s0161-6420(91)32235-8. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CP, Keech RV. Prevalence of glaucoma after surgery for PHPV and infantile cataract. J Pediatr Ophthalmol Strabismus. 1996;33:14–17. doi: 10.3928/0191-3913-19960101-05. [DOI] [PubMed] [Google Scholar]
- 7.Robb RM, Peterson RA. Outcome of treatment for congenital cataracts. Ophthalmic Surg. 1992;23:650–656. [PubMed] [Google Scholar]
- 8.Magnusson G, Abrahamsson M, Sjostrand J. Glaucoma following congenital cataract surgery: an 18-year longitudinal follow-up. Acta Ophthalmol Scand. 2000;78:65–70. doi: 10.1034/j.1600-0420.2000.078001065.x. [DOI] [PubMed] [Google Scholar]
- 9.Pearson RV, Aylward GW, Marsh RJ. Ocutome lensectomy: results and complications. Br J Ophthalmol. 1991;75:482–486. doi: 10.1136/bjo.75.8.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonkers GH. Secondary glaucoma after lens extraction. Ophthalmologica. 1975;171:255–257. doi: 10.1159/000307520. [DOI] [PubMed] [Google Scholar]
- 11.Green BF, Morin JD, Brent HP. Pars plicata lensectomy/vitrectomy for developmental cataract extraction: surgical results. J Pediatr Ophthalmol Strabismus. 1970;27:229–232. doi: 10.3928/0191-3913-19900901-03. [DOI] [PubMed] [Google Scholar]
- 12.Francois J. Late results of congenital cataract surgery. Ophthalmology. 1979;86:1586–1598. doi: 10.1016/s0161-6420(79)35360-x. [DOI] [PubMed] [Google Scholar]
- 13.Owens WC, Hughes WF., Jr Results of surgical treatment of congenital cataract. Arch Ophthalmol. 1948;39:339–350. doi: 10.1001/archopht.1948.00900020346009. [DOI] [PubMed] [Google Scholar]
- 14.Bradford GM, Keech RV, Scott WE. Factors affecting outcome after surgery for bilateral congenital cataracts. Am J Ophthalmol. 1994;117:58–64. doi: 10.1016/s0002-9394(14)73015-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen TC, Bhatia LS, Walton DS. Complications of pediatric lensectomy in 193 eyes. Ophthalmic Surg Lasers Imaging. 2005;36:6–13. [PubMed] [Google Scholar]
- 16.Walton DS. Pediatric aphakic glaucoma—a study of 65 patients. Trans Am Ophthalmol Soc. 1995;93:403–413. [PMC free article] [PubMed] [Google Scholar]
- 17.Parks MM, Johnson DA, Read GW. Long term visual results and complications in children with aphakia: a function of cataract type. Ophthalmology. 1993;100:826–840. doi: 10.1016/s0161-6420(93)31566-6. [DOI] [PubMed] [Google Scholar]
- 18.Wallace DK, Plager DA. Corneal diameter in childhood aphakic glaucoma. J Pediatr Ophthalmol Strabismus. 1996;33:230–234. doi: 10.3928/0191-3913-19960901-06. [DOI] [PubMed] [Google Scholar]
- 19.Cordes FC. Evaluation of the surgery of congenital cataracts. Arch Ophthalmol. 1954;46:132–144. doi: 10.1001/archopht.1951.01700020138002. [DOI] [PubMed] [Google Scholar]
- 20.Phelps CD, Arafat NI. Open angle glaucoma following surgery for congenital cataracts. Arch Ophthalmol. 1977;95:1985–1987. doi: 10.1001/archopht.1977.04450110079005. [DOI] [PubMed] [Google Scholar]
- 21.Russell-Eggitt I, Zamiri P. Review of aphakic glaucoma after surgery for congenital cataract. J Cataract Refract Surg. 1997;23:664–668. doi: 10.1016/s0886-3350(97)80051-x. [DOI] [PubMed] [Google Scholar]
- 22.Asrani S, Freedman S, Hasselblad V, et al. Does primary intraocular lens implantation prevent “aphakic” glaucoma in children? J AAPOS. 1999;3:33–39. doi: 10.1016/s1091-8531(00)90009-0. [DOI] [PubMed] [Google Scholar]
- 23.Walton DS. Unusual pediatric glaucomas. In: Epstein DL, ed. Chandler and Grant’s Glaucoma Philadelphia: Williams & Wilkins; 1996:623–638.
- 24.Pressman SH, Crouch ER., Jr Pediatric aphakic glaucoma. Ann Ophthalmol. 1983;15:568–573. [PubMed] [Google Scholar]
- 25.Chen TC, Walton DS, Bhatia LS. Aphakic glaucoma after congenital cataract surgery. Arch Ophthalmol. 2004;122:1819–1825. doi: 10.1001/archopht.122.12.1819. [DOI] [PubMed] [Google Scholar]
- 26.Lambert SR. Treatment of congenital cataract: it may all come down to timing. Br J Ophthalmol. 2004;88:854–855. doi: 10.1136/bjo.2004.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parks MM. Visual results in aphakic children. Am J Ophthalmol. 1982;94:441–449. doi: 10.1016/0002-9394(82)90237-9. [DOI] [PubMed] [Google Scholar]
- 28.Chandler PA, Grant WM. Glaucoma secondary to operation for congenital cataract. In: Lectures on Glaucoma Philadelphia: Lea & Febiger; 1965:367–375.
- 29.Rabiah PK. Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol. 2004;137:30–37. doi: 10.1016/s0002-9394(03)00871-7. [DOI] [PubMed] [Google Scholar]
- 30.Vishwanath M, Cheong-Leen R, Taylor D, et al. Is early surgery for congenital cataract a risk factor for glaucoma? Br J Ophthalmol. 2004;88:905–910. doi: 10.1136/bjo.2003.040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundvall A, Kugelberg U. Outcome after treatment of congenital bilateral cataract. Acta Ophthalmol Scand. 2002;80:593–597. doi: 10.1034/j.1600-0420.2002.800607.x. [DOI] [PubMed] [Google Scholar]
- 32.Watts P, Abdolell M, Levin AV. Complications in infants undergoing surgery for congenital cataract in the first 12 weeks of life: Is early surgery better? J AAPOS. 2003;7:81–85. doi: 10.1016/mpa.2003.S1091853102420095. [DOI] [PubMed] [Google Scholar]
- 33.Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37:1532–1538. [PubMed] [Google Scholar]
- 34.Harris LS. Cycloplegic-induced intraocular pressure elevations. A study of normal and open-angle glaucomatous eyes. Arch Ophthalmol. 1968;79:242–246. doi: 10.1001/archopht.1968.03850040244004. [DOI] [PubMed] [Google Scholar]
- 35.Hill RA, Minckler DS, Lee M, et al. Apraclonidine prophylaxis for postcycloplegic intraocular pressure spikes. Ophthalmology. 1991;98:1083–1086. doi: 10.1016/s0161-6420(91)32188-2. [DOI] [PubMed] [Google Scholar]
- 36.Chen TC. Risk factors for intraocular pressure elevations after pupillary dilation in patients with open angles. Ann Ophthalmol. 2005;37:69–76. [Google Scholar]