Abstract
In bacteria, polynucleotide phosphorylase (PNPase) is one of the main exonucleolytic activities involved in RNA turnover and is widely conserved. In spite of this, PNPase does not seem to be essential for growth if the organisms are not subjected to special conditions, such as low temperature. We identified the PNPase-encoding gene (pnp) of Pseudomonas putida and constructed deletion mutants that did not exhibit cold sensitivity. In addition, we found that the transcription pattern of pnp upon cold shock in P. putida was markedly different from that in Escherichia coli. It thus appears that pnp expression control and the physiological roles in the cold may be different in different bacterial species.
Polynucleotide phosphorylase (PNPase), which is encoded by pnp, is widely conserved among bacteria, with the notable exception of the Mycoplasmataceae, a family that includes the smallest free-living bacteria bearing a minimal set of genetic information. Homologous genes are found not only in plants, where the protein localizes in the chloroplasts (22), but also in Caenorhabditis elegans, Drosophila melanogaster, mice, and humans, as assessed by BLAST (1) analysis. In human cells PNPase has been localized in mitochondria and seems to be involved in cellular senescence and terminal differentiation (30, 35a).
PNPase degrades RNA phosphorolytically and processively in a 3′-to-5′ direction and plays a central role in bacterial RNA degradation. In Escherichia coli PNPase may be found as part of a multiprotein machine, the degradosome, together with RNase E, the RNA helicase RhlB, and enolase (reference 11 and references therein). Despite its evolutionary conservation and the physiological relevance of RNA degradation, PNPase does not seem to be essential for survival of the bacterial species tested so far, possibly because of functional redundancy in RNA turnover and quality control pathways (8, 9, 14, 37, 38). Nevertheless, PNPase is essential for growth at low temperatures in the psychrotrophic mesophiles E. coli, Bacillus subtilis, and Yersinia enterocolitica (20, 31). A less severe growth defect at low temperatures has been observed in pnp mutants of Salmonella enterica (10). Additional phenotypes associated with pnp mutations include defective transformation competence, increased tetracycline sensitivity, and cell filamentation in B. subtilis (31, 42) and increased virulence in S. enterica (10). The pleiotropic effects of pnp mutations suggest that several functions are indirectly controlled by PNPase, possibly via regulation of mRNA processing and/or decay of a variety of genes. To detect common traits in the physiological roles of this enzyme in bacteria, we identified, cloned, and mutagenized the pnp gene of the gram-negative soil bacterium Pseudomonasputida and analyzed its expression profile in different growth phases and after cold shock.
Identification and cloning of the P. putida pnp gene.
The presence of a pnp homologue in Pseudomonas species was assessed by Southern blot hybridization of genomic DNA from P. putida TMB, Pseudomonas fluorescens N3, and Pseudomonas aeruginosa PAO11 (Table 1) digested with different restriction enzymes by using E. coli pnp DNA as a probe at a low stringency. In each species DNA fragments that hybridized with the heterologous probe were found (data not shown).
TABLE 1.
Bacterial strains and plasmids used
| Bacterial strains or plasmids | Relevant characteristicsa | Reference or source |
|---|---|---|
| Bacterial strains | ||
| E. coli C-5641 | Prototrophic, pnp::Tn5-Km | 35 |
| P. fluorescens N3 | Naf+ Sal+/pN3 | 5 |
| P. aeruginosa PAO11 | tfp-54 nal-19 fon-1 | B. W. Holloway Collection |
| P. putida TMB | Tmb+ Tol+/pGB | 3 |
| P. putida KT2440 | hsdR hdsM+ Ben+ Rifs | 2 |
| PPM101 | P. putida TMB pnp-1 (INS pKRF7A), Kanr Strr Sucs | This study |
| PPM103 | P. putida KT2440 pnp-1 (INS pKRF7A), Kanr Strr Sucs | This study |
| Plasmids | ||
| pGEM-4Z | Cloning vector | Promega |
| pGEM-4Z-Km1 | pHP45Ω-Km 2.2-kb BamHI fragment containing the Ω-Km interposon cloned in BamHI-digested pGEM-4Z | This study |
| pGeRF1 | P. putida pnp EcoRI (698)/XhoI (1268) fragment from pRF17 cloned in EcoRI/SalI-digested pGEM-4Z, 5′ end of pnp | This study |
| pGZ119EH (HE) | Cloning vector, Cmr | 29 |
| pHP45Ω-Km | pBR322 derivative carrying the Ω-Kmr interposon | 15 |
| pJB3Pnp2 | 2.5-kb SphI/SacI fragment from pRF17, containing the P. putida pnp gene, cloned in SphI/SacI-digested pJB3Tc19 | This study |
| pJB3Tc19 | Broad-host-range cloning vector, Apr/Tcr, RK2 oriT oriV | 6 |
| pKNG101 | Suicide cloning vector, R6K ori, RK2 (RP4) mob, strA strB (Srr) sacB (sucrose sensitivity) | 27 |
| pKRF7A | pMA9 3.8-kb BamHI/HindIII fragment, carrying the central part of P. putida pnp gene interrupted by Ω-Km interposon, cloned in SmaI-digested pKNG101 | This study |
| pMA5 | P. putida pnp fragment 889-2452 obtained by PCR with P. putida genomic DNA by using the FG218 and FG219 primers, cloned in HincII-digested pGEM-4Z | This study |
| pMA9 | Klenow-fragment-treated HindIII fragment of pGEM-4Z-Km1 containing the Ω-Km interposon cloned in HincII fragment of pMA5 interrupting the central fragment of pnp | This study |
| pRF14 | P. putida pnp fragment 1089-2888 obtained by PCR with P. putida genomic DNA by using the FG231 and FG393 primers, cloned in SmaI-digested pGZ119-EH; contains the 3′ end of pnp | This study |
| pRF16 | P. putida pnp fragment 396-2243 obtained by PCR with P. putida genomic DNA by using the FG390 and FG172 primers, cloned in SmaI-digested pGZ119-EH; contains the 5′ end of pnp | This study |
| pRF17 | 4.8-kb HindIII-EcoRI (partial digestion) fragment of pRF16 ligated to the 1.5-kb EcoRI-HindIII fragment of pRF14; contains the P. putida pnp gene (396-2857) reconstructed in pGZ119EH | This study |
| pURF10 | 215-bp P. putida pnp fragment amplified with degenerate primers FG143 and FG144, cloned in pUC18 SmaI | This study |
Numbers are coordinates. See Table 2 for oligonucleotides.
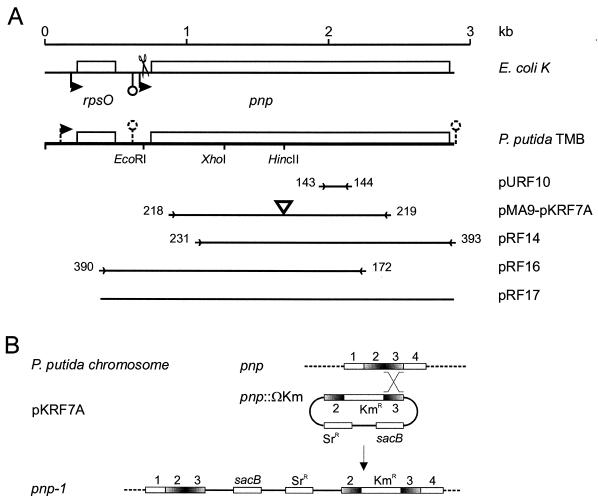
Attempts to clone the pnp gene by screening a P. putida TMB genomic bank in E. coli by using the specific P. putida probe cloned in pURF10 (Table 1 and Fig. 1) failed. We speculated that P. putida pnp or another nearby gene could be toxic to E. coli. We thus cloned and sequenced the locus by performing inverse PCR multiple times (23; data not shown). Within the 2.8-kb region sequenced (GenBank accession number Y18132), which is 98% identical to the equivalent region of the recently released P. putida KT2442 complete DNA sequence (accession number NC_002947), we found two open reading frames (ORFs) (Fig. 1A). The product of the upstream 89-codon ORF exhibited more than 80% similarity with the E. coli ribosomal protein S15. The product of the downstream 701-codon ORF exhibited 78.5% similarity with E. coli PNPase. Plasmid pRF17, which can express the putative P . putida pnp gene from the plac promoter, was able to complement the E. coli pnp mutant C-5641 (Table 1) for the growth defect at 15°C, although it was less efficient (i.e., smaller colonies were formed) than a plasmid carrying the E. coli gene (data not shown), thus indicating that the plasmid expressed a functional PNPase. We tentatively propose that GUG is the initiation codon, as suggested by the alignment with the E. coli protein.
FIG. 1.
(A) Map of P. putida TMB rpsO-pnp region. The E. coli K-12 region is included at the top for comparison. Relevant restriction sites and cloned regions are shown; the corresponding plasmids (designations on the right) are described in Table 1. Promoters and intrinsic terminators are indicated by bent arrows and lollipops, respectively (dashed lines indicate that such features are putative). The terminator downstream of pnp is outside the sequence established in this work and was inferred from the complete genomic sequence of P. putida KT2442 (accession number NC_002947). Scissors indicate the RNase III cut site in the E. coli mRNA. Primers (Table 2) are indicated by arrowheads flanked by the oligonucleotide numbers and delimit the regions amplified by PCR and cloned. The triangle in pMA9 and pKRF7A indicates the Ω-Km cassette cloned into the HincII site. The fragment cloned in pURF10 was obtained by PCR of P. putida TMB genomic DNA with degenerate primers FG143 and FG144 (Table 2) designed with two amino acid motifs (PK/GRREIGHG and KAA/PVAGIAMG) conserved in the PNPases of E. coli (SwissProt accession number P05055), Photorhabdus luminescens (P41121), Haemophilus influenzae (P44584), B. subtilis (P50849), and Streptomyces antibioticus (Q53597). (B) Mutagenesis of P. putida pnp gene. An internal fragment of pnp (regions 2 and 3) interrupted by a kanamycin resistance cassette and cloned in the suicide plasmid pKNG101 (giving pKRF7A) recombined with homologous region 3 of the chromosomal gene, producing a merodiploid with two mutant pnp alleles (pnp-1 mutation in PPM101 and PPM103).
The P. putida PNPase gene exhibits a high degree of similarity with the E. coli gene and other homologous bacterial genes and has the characteristic four-domain organization (two RNase PH domains that form the duplicated core and the two C-terminal RNA binding domains, KH and S1) (41; Conserved Domain Database at http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The linkage of pnp with the upstream gene rpsO is also a conserved feature (see the Clusters of Orthologous Groups database genomic context at http://www.ncbi.nlm.nih.gov/COG/). It is possible that expression of P. putida S15 ribosomal protein could be incompatible with the E. coli ribosome and could have been responsible for our failure to obtain clones carrying pnp in a genomic data bank. Comparison of the E. coli genomic map with the map deduced from the P. aeruginosa and P. putida KT2442 sequences also revealed that the homologous genomic organization extends to the upstream nusA operon, which in E. coli includes cold-induced genes (19, 26). This arrangement appears to be widely but not universally conserved among bacteria. In contrast, in P. putida deaD is not linked to pnp, whereas in E. coli this cold-induced gene (25) is located downstream of pnp and is cotranscribed with pnp during cold acclimation (43).
A single promoter upstream of rpsO (at positions 185 to 230, with a putative transcription start point at position 220) was predicted by the Neural Network Promoter Prediction program (http://www.fruitfly.org/seq_tools/promoter.html) with a score of 0.95. The 250-nucleotide (nt) rpsO-pnp intergenic region of P. putida TMB did not exhibit any significant similarity with the equivalent E. coli region that was the same length, nor were similar sequences found by BLAST analysis of 228 bacterial genome sequences available at the ENTREZ search andretrieval system (http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/genom_table_cgi); exceptions were the homologous region in P. putida KT2440 (100% identity) and a ca. 100-nt sequence upstream of the Pseudomonas syringae and P. fluorescens pnp putative genes. Two large hairpin structures within the first two-thirds of the intergenic region, which are potential RNase III cut sites, were predicted by MFOLD analysis performed at http://bioinfo.math.rpi.edu/∼zukerm (32, 44). Computer analysis (TERMINATOR, Wisconsin GCG package) revealed a putative intrinsic transcription terminator in the middle of the rpsO-pnp intergenic region, ending 125 nt downstream of rpsO. Another terminator ended 47 nt downstream of the pnp coding region in P. putida KT2442 (Fig. 1), immediately outside the strain TMB region sequenced.
Isolation and characterization of P. putida pnp mutants.
The procedure used to create pnp mutants by allelic substitution is shown in Fig. 1B. The internal fragment of the pnp gene from nt 889 to 2,473 interrupted by the Ω-Km interposon (15) in the HincII site (nt 1682) was cloned in the suicide plasmid vector pKNG101, which can replicate in E. coli but not in Pseudomonas (27) (Table 1). The resulting plasmid, pKRF7A, was transferred by conjugation, essentially as described previously (28), into both P. putida KT2440 and TMB. P. putida exconjugants, selected for the ability to use benzoate as a sole carbon and energy source and for kanamycin resistance, were also streptomycin resistant and sucrose sensitive, as expected after a single crossover in one homology region on either side of the Kmr cassette. In all 15 clones analyzed recombination occurred within the 3′ region of pnp (region 3 in Fig. 1B), as assessed both by Southern blotting and by PCR analysis (data not shown). We attempted to obtain second-site recombinants to get rid of the integrated plasmid vector by selecting for sucrose resistance on LD agar plates (17) containing 4% NaCl, 6% sucrose, and 50 μg of kanamycin per ml. However, all clones obtained in several independent experiments retained the vector Srr marker, indicating that the loss of sucrose sensitivity was not due to excision of the inserted plasmid via homologous recombination. It should be noted that in the single-crossover clones the efficiency of plating on sucrose was 10−2 to10−3, and thus sucrose tolerance selection was not very effective in this system.
Insertion of plasmid pKRF7A by recombination in region 3 generated two mutant pnp alleles, one truncated at the C terminus and the other missing the N terminal region; therefore, the recombinants were pnp mutants. However, the allele truncated at the C terminus was predicted to encode a 578-amino-acid peptide missing the KH and S1 RNA binding domains but retaining the putative catalytic core. Our failure to obtain simple allelic substitutions suggests that pnp is an essential gene in Pseudomonas and that the 3′-truncated allele expresses the essential function(s). One thing that supports this hypothesis is that in E. coli PNPase-RNase II double mutants are not viable (14), and in P. putida RNase II seems to be missing. (However, the same situation occurs in B. subtilis [13], in which pnp is not essential [31].) We therefore tested two single-crossover strains, PPM101 and PPM103 (TMB and KT2440 derivatives, respectively) for PNPase activity. The phosphorolytic activity in crude extracts was below the level of detection for both mutants, as measured by the photometric assay described by Fontanella et al. (16) (in comparison, the activity for both wild-type P. putida strains was 0.05 μM min−2 and in E. coli it was 0.2 μM min−2). P. putida PNPase readily reacted with anti-E. coli PNPase polyclonal antibodies in Western blotting assays; however, no truncated polypeptide could be detected in the pnp mutants (Fig. 2A). These results confirm that PPM101 and PPM103 are PNPase-deficient mutants and support the idea that pnp is not an essential gene in P. putida. The lack of a truncated peptide in the mutants was somewhat surprising since the 3′ interrupted gene could be transcribed from its natural promoter (see below). It is possible that the transcript of the truncated gene is functionally inactivated very rapidly and/or that the truncated peptide is particularly labile and is promptly degraded (for example, following tmRNA-mediated tagging) (18).
FIG. 2.
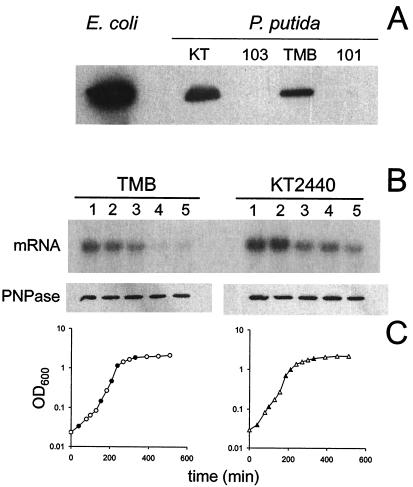
(A) Western blot analysis of PNPase in wild-type and mutant P. putida strains. Cultures of E. coli and P. putida strains KT2440 (KT), PPM103 (103), TMB, and PPM101 (101) were grown in LD broth at 30°C (P. putida strains) or 37°C (E. coli) to an optical density at 600 nm of 0.4 and analyzed by Western blotting by using polyclonal antibodies raised against E. coli PNPase (a generous gift from A. J. Carpousis). Equal portions (1.5 μg) of proteins were loaded in all lanes. Immunoreactive bands were revealed by using the Amersham ECL Western blotting reagent. (B) Expression of pnp in different culture growth phases at 30°C. pnp mRNA and PNPase from samples of P. putida TMB and KT2440 cultures in different growth phases were analyzed by Northern and Western blotting, respectively, as described previously (43). The size of the mRNA is about 2.2 kb. (C) Growth curves. Solid symbols indicate the times when the samples analyzed in panel B were taken. OD600, optical density at 600 nm.
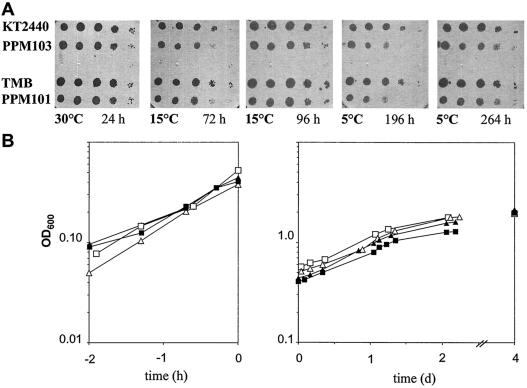
In contrast to what was observed in E. coli (4, 31, 43), P. putida pnp mutants could grow at low temperatures. As shown in Fig. 3A, the efficiency of plating for both mutants was comparable to that for the wild-type control strains at temperatures as low as 5°C, although the colonies appeared later and were smaller than the wild-type colonies. This was confirmed in liquid cultures; at 30°C, the generation times of the mutants were about 60% longer than those of the wild-type strains (about 1 h). After a temperature downshift to 9°C, both the wild-type and pnp mutant P. putida strains continued to grow with comparable generation times (about 22 h) and, unlike E. coli (25, 34), did not exhibit acclimation growth arrest (Fig. 3B).
FIG. 3.
Growth of P. putida pnp mutants at low temperatures. (A) Overnight cultures (grown in LD broth at 30°C with aeration) of the wild-type and pnp mutant strains indicated on the left were serially diluted (100 to 10−4) in Bertani tray wells, replica plated onto five LD agar plates, and incubated at different temperatures and for different times, as indicated below the images, until visible colonies were observed. (B) The same strains were grown in LD broth at 30°C to an optical density at 600 nm (OD600) of about 0.5 and then shifted to 9°C (time zero). The optical density at 600 nm is plotted versus time in hours before the temperature downshift (left graph) or in days after the temperature downshift (right graph). Symbols: □, TMB; ▪, PPM101; ▵, KT2440; ▴, PPM103.
pnp expression during growth at 30°C and upon cold shock.
In E. coli, regulation of PNPase appears to be quite complex at both optimum and low growth temperatures. At 37°C PNPase controls its own expression by promoting degradation of its RNase III-processed mRNA (24, 39, 40), whereas upon cold shock this autogenous regulation seems to be alleviated and leads to a substantial transient increase in pnp mRNA. During the first 1 h of cold adaptation the pnp transcript level increases more than 10-fold, while transcription termination is suppressed and polycistronic transcript species appear. Later, the transcript profile returns to the preshift condition. In contrast, the PNPase level slowly increases up to two- or threefold in cold-adapted cells, when transcript abundance returns to an almost preshock level (4, 33, 43). Such an expression pattern implies that pnp mRNA, unlike the mRNA encoding true cold-induced proteins (19, 21), is not efficiently translated during cold acclimation. Control of the pnp mRNA level is also exerted in E. coli at 37°C in a growth phase-dependent manner, and this mRNA is more abundant in early-exponential-phase cultures than in late-exponential-phase cultures. The PNPase level, however, remains constant throughout the culture growth phases, suggesting that there is translational control (43).
As shown in Fig. 2B (upper gel), both P. putida KT2440 and TMB at 30°C expressed a 2.2-kb transcript that hybridized to the pnp-specific probe; the length of this transcript is consistent with the distance from the major 5′ end detected by primer extension (see below) and the predicted transcription terminator downstream of pnp.
The signal was strong in the early exponential phase, and the intensity decreased with increasing cell density. Western blot analysis with anti-PNPase antibodies, however, revealed that the total amount of PNPase protein was constant at all optical densities (Fig. 2B, lower gel). Therefore, the expression pattern of the P. putida pnp gene throughout exponential growth at the optimum growth temperature paralleled the profile observed at 37°C in E. coli (43) at both the RNA and protein levels.
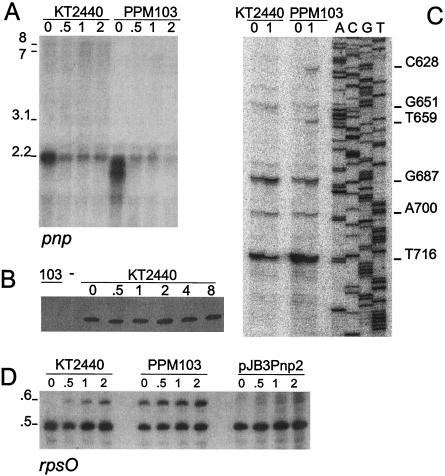
In contrast, upon a temperature downshift to 9°C, the level of the 2.2-kb transcript expressed by the wild-type strains substantially decreased (Fig. 4A). Nevertheless, the level of PNPase remained constant, as determined by Western blotting (Fig. 4B). In the mutant, the truncated pnp gene produced a smeared signal whose intensity at 30°C was greater than the intensity of the wild-type signal, whereas upon cold shock the intensity decreased, as observed in the wild type. Therefore, expression of P. putida pnp in the cold seemed peculiar in that the level of the pnp transcript decreased significantly immediately after the temperature shift, whereas the PNPase level remained constant for several hours, suggesting that pnp mRNA is translatable during cold acclimation. In addition, in contrast to E. coli (43), transcription antitermination did not seem to occur (only very faint 7- to 8-kb smeared signals, which were detected better with longer autoradiographic exposures, appeared 1 h after a temperature downshift). This finding nicely correlates with the lack in P. putida of the cold-inducible deaD gene downstream of pnp.
FIG. 4.
Expression profile of pnp and rpsO upon cold shock. Cultures of KT2440 (pnp+), PPM103 (pnp), and PPM103 containing pJB3Pnp2 (a plasmid expressing P. putida pnp from the plac promoter) were grown in LD broth at 30°C with aeration to an optical density at 600 nm of 0.4, quickly cooled to 9°C in an ice-water bath, and transferred to 9°C in a refrigerated water bath with aeration. Aliquots were obtained immediately before the temperature downshift (zero time) and at different times after the temperature downshift (indicated in hours above the lanes); RNA was extracted as described previously (12) and processed as follows. (A) Northern blot analysis of pnp transcripts. Fifteen micrograms of RNA was resolved by 1.5% agarose gel electrophoresis and analyzed by Northern blot hybridization with the radiolabeled riboprobe pnp transcribed with T7 polymerase from pGERF1, as previously described (7, 12). The approximate sizes of the RNAs (in kilobases) are indicated on the left. (B) Expression of PNPase upon cold shock. Western blotting with PNPase polyclonal antibodies was performed by using 1-μg portions of total P. putida protein extracts. Lane 103 contained a sample of PPM103 (pnp) extract. (C) Primer extension of pnp transcripts. Oligonucleotide FG243 labeled at the 5′ end with 32P was hybridized to 10 μg of total RNA, extended with reverse transcriptase, and electrophoresed in a 6% polyacrylamide sequencing gel. The sequencing reactions (lanes A, C, G, and T) were performed by using the FG243 primer and plasmid pRF17 as a template. The coordinates of the 5′ end signals are indicated on the right. (D) Northern blot analysis of rpsO transcripts. RNA was resolved by 5% acrylamide denaturing gel electrophoresis and analyzed by Northern blot hybridization with radiolabeled oligonucleotide FG432.
We also determined the 5′ end of the pnp transcripts by primer extension analysis (Fig. 4C). With RNA from cells grown at 30°C up to the mid-exponential phase, several signals were observed, and the major signals mapped 35 and 63 nt upstream of the putative start codon. One hour after a temperature downshift to 9°C the intensity of the pnp-proximal signal decreased, while the distal signal became stronger. In the pnp mutant the pnp-proximal signal was more intense than that in the wild type, whereas the distal signal was fainter; as in the wild type, 1 h after a temperature downshift to 9°C the intensity of the pnp-proximal signal decreased, while the distal signal became stronger and additional signals appeared. Although the primer extension analysis was not quantitative, the data are in agreement with Northern blotting data.
In E. coli pnp may be transcribed from the following two promoters: P1, located upstream of rpsO, by reading through a Rho-independent terminator; and P2, located in the rpsO-pnp intergenic region. Both transcripts are efficiently processed by RNase III at a site downstream of P2 (36). The upstream rpsO and downstream pnp monocistronic transcripts are then processed further. In the equivalent P. putida region a single promoter upstream of rpsO was predicted. Whether a second promoter is present in the intergenic region remains to be ascertained; however, the multiple 5′ ends found by primer extension analysis suggest that there is extensive endonucleolytic mRNA processing in the rpsO-pnp intergenic region, which appears to be modulated both by PNPase and by cold shock. As in E. coli, such endonucleolytic processing appears to be very efficient, since we could not detect dicistronic transcripts (expected to be 2.7 kb long) by Northern blotting.
Overall, our data suggest that PNPase may negatively control the abundance of pnp mRNA at the optimum temperature but does not seem to be responsible for its downregulation in the cold. It may be concluded that the pnp expression pattern in P. putida during cold acclimation differs from that observed in E. coli at the level of mRNA and at the level of protein abundance.
rpsO transcription analysis.
To test whether the transcript profile observed in response to cold shock was specific for pnp, we analyzed the transcription pattern of the adjacent upstream gene, rpsO. At 30°C a single signal at ca. 0.5 kb was detected for the wild-type strains, and this signal remained constant in the cold acclimation phase. However, a ca. 0.6-kb RNA, possibly a precursor of the 0.5-kb transcript, appeared, and its intensity progressively increased during cold shock. In contrast, in the mutant strains the 0.6-kb signal was present also at 30°C, suggesting that PNPase is involved in maturation of the rpsO transcript. Accordingly, the longer signal disappeared when the mutant was complemented by the P. putida pnp gene expressed from the pJB3Pnp2 plasmid (Fig. 4D). This preliminary transcription analysis of the rpsO transcripts indicated that PNPase plays a significant, albeit not indispensable, role in the processing of the main rpsO mRNA from a longer precursor.
In conclusion, the patterns of pnp regulation upon cold shock in E. coli and P. putida are markedly different, and P. putida pnp mutants did not show cold sensitivity, unlike other previously studied gram-positive and gram-negative organisms (19). It thus appears that involvement of PNPase in cold adaptation is not a universal feature of (mesophilic) bacteria and that control of PNPase expression in the cold evolved independently in different lines. Whether this reflects more general differences in cold adaptation mechanisms in Pseudomonas remains open to further investigation.
TABLE 2.
Oligonucleotides used
| Oligonu- cleotide | Sequencea | Coordinatesb |
|---|---|---|
| FG143 | CCXRRXMGXMGXGARATHGGXCA YGG | 395-404c |
| FG144 | CCCATXGCDATXCCXGCXACXGSXG CYTT | 458-467c |
| FG172 | ACCGGCTACCTTGAAGTCCA | 2243-2224 |
| FG231 | GTCTGCACCGTGGTTTCC | 1089-1106 |
| FG390 | TGATCCGTATGGTCAACCACCGTCGT AAGCTGC | 396-430 |
| FG393 | TTTGAAGGGTTTTCTCTATTGCGTTAC GGTCG | 2888-2857 |
| FG432 | GAAAGCTTATTAGCGACGCAGGCCCA | 501-483 (HindIII 5′ tail) |
| FG243 | AGAAACCCTTGCCTGGATCAGCCTG | 918-894 |
Oligonucleotide sequences are 5′ to 3′, and the IUB/GCG code is used. Bases missing in the P. putida sequence are underlined.
Coordinates in GenBank accession number Y18132.
Degenerate primer FG143 and FG144 correspond to amino acids 395 to 404 and 458 to 467, respectively, of E. coli PNPase (SwissProt accession no. P05055) and bracket the region from position 1978 to position 2123 of P. putida, as assessed a posteriori.
Acknowledgments
We are grateful to S. Zangrossi for technical assistance, M. Allaria, who participated in cloning of pnp, A. J. Carpousis for the generous gift of antibodies against PNPase, K. Kaniga for providing the plasmid vector for mutagenesis, and F. Briani for reading the manuscript.
R.F. was supported by a fellowship from Università degli Studi di Milano. This research was supported in part by grants from the Ministero dell'Università e della Ricerca Scientifica (Programmi di Rilevante Interesse Nazionale 2001 and FIRB 2001).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagdasarian, M., R. Lurz, B. Ruckert, F. C. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 3.Baggi, G., D. Catelani, C. Sorlini, and V. Treccani. 1987. Microbial degradation of methylbenzenes: metabolism of 1,2,4-trimethylbenzene by a Pseudomonas putida. Ann. Microbiol. 32:45. [Google Scholar]
- 4.Beran, R. K., and R. W. Simons. 2001. Cold-temperature induction of Escherichia coli polynucleotide phosphorylase occurs by reversal of its autoregulation. Mol. Microbiol. 39:112-125. [DOI] [PubMed] [Google Scholar]
- 5.Bestetti, G., P. Di Gennaro, E. Galli, B. Leoni, Pellizzoni, Sello, and Bianchi. 1994. Bioconversion of substityute naphtalenes to the corresponding salicylic acid. Appl. Microbiol. Biotechnol. 40:791-793. [Google Scholar]
- 6.Blatny, J. M., T. Brautaset, L. Winther, K. Haugan, and S. Valla. 1997. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl. Environ. Microbiol. 63:370-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briani, F., S. Zangrossi, D. Ghisotti, and G. Dehò. 1996. A Rho-dependent transcription termination site regulated by bacteriophage P4 RNA immunity factor. Virology 223:57-67. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, Z. F., and M. P. Deutscher. 2003. Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc. Natl. Acad. Sci. 100:6388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, Z. F., Y. Zuo, Z. Li, K. E. Rudd, and M. P. Deutscher. 1998. The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J. Biol. Chem. 273:14077-14080. [DOI] [PubMed] [Google Scholar]
- 10.Clements, M. O., S. Eriksson, A. Thompson, S. Lucchini, J. C. Hinton, S. Normark, and M. Rhen. 2002. Polynucleotide phosphorylase is a global regulator of virulence and persistency in Salmonella enterica. Proc. Natl. Acad. Sci. USA 99:8784-8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coburn, G. A., and G. A. Mackie. 1999. Degradation of mRNA in Escherichia coli: an old problem with some new twists. Prog. Nucleic Acids Res. Mol. Biol. 62:55-108. [DOI] [PubMed] [Google Scholar]
- 12.Dehò, G., S. Zangrossi, P. Sabbattini, G. Sironi, and D. Ghisotti. 1992. Bacteriophage P4 immunity controlled by small RNAs via transcription termination. Mol. Microbiol. 6:3415-3425. [DOI] [PubMed] [Google Scholar]
- 13.Deutscher, M. P., and N. B. Reuven. 1991. Enzymatic basis for hydrolytic versus phosphorolytic mRNA degradation in Escherichia coli and Bacillus subtilis. Proc. Natl. Acad. Sci. USA 88:3277-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donovan, W. P., and S. R. Kushner. 1986. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 83:120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 16.Fontanella, L., S. Pozzuolo, A. Costanzo, R. Favaro, G. Dehò, and P. Tortora. 1999. Photometric assay for polynucleotide phosphorylase. Anal. Biochem. 269:353-358. [DOI] [PubMed] [Google Scholar]
- 17.Ghisotti, D., R. Chiaramonte, F. Forti, S. Zangrossi, G. Sironi, and G. Dehò. 1992. Genetic analysis of the immunity region of phage-plasmid P4. Mol. Microbiol. 6:3405-3413. [DOI] [PubMed] [Google Scholar]
- 18.Gillet, R., and B. Felden. 2001. Emerging views on tmRNA-mediated protein tagging and ribosome rescue. Mol. Microbiol. 42:879-885. [DOI] [PubMed] [Google Scholar]
- 19.Golovlev, E. 2003. Bacterial cold shock response at the level of DNA transcription, translation, and chromosome dynamics. Microbiology 72:1-7. [PubMed] [Google Scholar]
- 20.Goverde, R. L., J. H. J. Huis in't Veld, J. G. Kusters, and F. R. Mooi. 1998. The psychrotrophic bacterium Yersinia enterocolitica requires expression of pnp, the gene for polynucleotide phosphorylase, for growth at low temperature (5 degrees C). Mol. Microbiol. 28:555-569. [DOI] [PubMed] [Google Scholar]
- 21.Graumann, P. L., and M. A. Marahiel. 1998. A superfamily of proteins that contain the cold-shock domain. Trends Biochem. Sci. 23:286-290. [DOI] [PubMed] [Google Scholar]
- 22.Hayes, R., J. Kudla, G. Schuster, L. Gabay, P. Maliga, and W. Gruissem. 1996. Chloroplast mRNA 3′-end processing by a high molecular weight protein complex is regulated by nuclear encoded RNA binding proteins. EMBO J. 15:1132-1141. [PMC free article] [PubMed] [Google Scholar]
- 23.Higashitani, A., N. Higashitani, S. Yasuda, and K. Horiuchi. 1994. A general and fast method for mapping mutations on the Escherichia coli chromosome. Nucleic Acids Res. 22:2426-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarrige, A. C., N. Mathy, and C. Portier. 2001. PNPase autocontrols its expression by degrading a double-stranded structure in the pnp mRNA leader. EMBO J. 20:6845-6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, P. G., M. Mitta, Y. Kim, W. Jiang, and M. Inouye. 1996. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, P. G., R. A. VanBogelen, and F. C. Neidhardt. 1987. Induction of proteins in response to low temperature in Escherichia coli. J. Bacteriol. 169:2092-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 28.Kessler, B., V. de Lorenzo, and K. N. Timmis. 1992. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol. Gen. Genet. 233:293-301. [DOI] [PubMed] [Google Scholar]
- 29.Lessl, M., D. Balzer, R. Lurz, V. L. Waters, D. G. Guiney, and E. Lanka. 1992. Dissection of IncP conjugative plasmid transfer: definition of the transfer region Tra2 by mobilization of the Tra1 region in trans. J. Bacteriol. 174:2493-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leszczyniecka, M., D. C. Kang, D. Sarkar, Z. Z. Su, M. Holmes, K. Valerie, and P. B. Fisher. 2002. Identification and cloning of human polynucleotide phosphorylase, hPNPase old-35, in the context of terminal differentiation and cellular senescence. Proc. Natl. Acad. Sci. USA 99:16636-16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luttinger, A., J. Hahn, and D. Dubnau. 1996. Polynucleotide phosphorylase is necessary for competence development in Bacillus subtilis. Mol. Microbiol. 19:343-356. [DOI] [PubMed] [Google Scholar]
- 32.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 33.Mathy, N., A. C. Jarrige, M. Robert-Le Meur, and C. Portier. 2001. Increased expression of Escherichia coli polynucleotide phosphorylase at low temperatures is linked to a decrease in the efficiency of autocontrol. J. Bacteriol. 183:3848-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng, H., J. L. Ingrahm, and M. Mitta. 1962. Damage and derepression in Escherichia coli resulting from growth at low temperatures. J. Bacteriol. 84:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piazza, F., M. Zappone, M. Sana, F. Briani, and G. Dehò. 1996. Polynucleotide phosphorylase of Escherichia coli is required for the establishment of bacteriophage P4 immunity. J. Bacteriol. 178:5513-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Piwowarski, J., P. Grzechnik, A. Dziembowski, A. Dmochowska, M. Minczuk, and P. Stepien. 2003. Human polynucleotide phosphorylase, hPNPase, is localized in mitochondria. J. Mol. Biol. 329:853-857. [DOI] [PubMed] [Google Scholar]
- 36.Regnier, P., M. Grunberg Manago, and C. Portier. 1987. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. Homology of the primary structure of the protein with the RNA-binding domain of ribosomal protein S1. J. Biol. Chem. 262:63-68. [PubMed] [Google Scholar]
- 37.Reiner, A. M. 1969. Characterization of polynucleotide phosphorylase mutants of Escherichia coli. J. Bacteriol. 97:1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiner, A. M. 1969. Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J. Bacteriol. 97:1431-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert-Le Meur, M., and C. Portier. 1992. E. coli polynucleotide phosphorylase expression is autoregulated through an RNase III-dependent mechanism. EMBO J. 11:2633-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert-Le Meur, M., and C. Portier. 1994. Polynucleotide phosphorylase of Escherichia coli induces the degradation of its RNase III processed messenger by preventing its translation. Nucleic Acids Res. 22:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Symmons, M. F., M. G. Williams, B. F. Luisi, G. H. Jones, and A. J. Carpousis. 2002. Running rings around RNA: a superfamily of phosphate-dependent RNases. Trends Biochem. Sci. 27:11-18. [DOI] [PubMed] [Google Scholar]
- 42.Wang, W., and D. H. Bechhofer. 1996. Properties of a Bacillus subtilis polynucleotide phosphorylase deletion strain. J. Bacteriol. 178:2375-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zangrossi, S., F. Briani, D. Ghisotti, M. Regonesi, P. Tortora, and G. Dehò. 2000. Transcriptional and post-transcriptional control of polynucleotide phosphorylase during cold acclimation in Escherichia coli. Mol. Microbiol. 36:1470-1480. [DOI] [PubMed] [Google Scholar]
- 44.Zuker, M., D. Mathews, and D. Turner. 1999. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide, p. 11-43. In J. Barciszewski and B. Clark (ed.), RNA biochemistry and biotechnology. Kluwer Academic Publishers, Dordrecht, The Netherlands.