Abstract
Purpose
To determine the impact of evaporation on preocular aqueous tear (AT) loss in normal subjects (controls) and patients with keratoconjunctivitis sicca (KCS).
Methods
Eighteen patients (32 eyes) with KCS with or without associated meibomian gland dysfunction (MGD) and 11 sex-matched controls had AT evaporation determined between relative humidity (RH) of 20% and 45% using an evaporometer. AT volume, flow, and turnover were determined with a fluorophotometer.
Results
Evaporative rates increased significantly when the RH was changed from 40%–45% to 20%–25% (P < .001). This change was similar in all groups and on average accounted for a 99.43% increase. There were no statistically significant differences in evaporative rate between controls, the KCS alone group, and the KCS/MGD group. Dry eye patients exhibited a decreased tear turnover when compared to controls. Evaporative contribution to tear loss at 40%–45% RH was 23.47% for controls, 30.99% for “classic” KCS patients, and 25.44% for KCS/MGD patients. At 20%–25% RH, the evaporative contribution was 41.66% for controls, 57.67% for classic KCS patients, and 50.28% for KCS/MGD patients.
Conclusions
RH significantly impacts evaporation regardless of the presence of dry eye disease and probably accounts for the increased dry eye symptoms in people (controls and dry eye patients) in conditions of low RH (eg, deserts, airplane cabins, cold dry seasons). Contribution of evaporation to tear loss tends to be higher than previously described. The percent contribution is dependent on environmental conditions such as RH. There was a trend toward increased contribution to AT loss in dry eye patients vs controls, but statistical significance was not reached.
INTRODUCTION
A normal functioning ocular tear film is essential for avoiding dry eye signs and symptoms.1 The ocular tear film structure is complex. Although its detailed structure is not completely elucidated, some features are well established.2 The tear film is composed of the following: a mucin layer immediately adjacent to the corneal epithelium that is produced by specialized conjunctival cells and ocular surface epithelial cells; an aqueous layer produced by the main lacrimal gland and its accessories; and an outer layer composed of polar and nonpolar lipids (meibum) that are derived mainly from the secretions produced by the meibomian glands.2 The intact outer lipid layer is thought to stabilize the tear film and prevent evaporation of the aqueous layer.1
Between blinks, the aqueous layer of the tear film evaporates to some extent in all subjects, even those without dry eye disease. This results in a change in overall tear film tonicity (osmolarity), which, in turn, may cause the appearance of areas on the ocular surface where the tear film has disappeared. It is generally accepted that the spreading and maintenance of the aqueous layer is dependent upon an intact outer lipid layer. Eyelid blinking replenishes the lipid outer layer covering the aqueous layer, relubricating and redistributing the lipid layer across the ocular surface.3 The redistribution of the lipid layer also facilitates the spread of the underlying aqueous layer as well.
Evaporometry studies have demonstrated that lower RH results in an increased rate of ocular surface tear film evaporation not only in dry eye subjects4 but also in subjects without evidence of dry eyes.5,6 The tear film is exposed to great variations of RH in different environmental conditions, and like any other aqueous solution exposed to air of less than 100% RH, it evaporates, especially in eyes with abnormalities of the overlying lipid layer. The evaporative rate is inversely related to RH, and the change in evaporation is similar in controls and in dry eye patients.5 The results of these studies could be extrapolated to different situations where the humidity conditions are low or extremely low, such as deserts, pressurized cabins of commercial airplanes,7 and other arid environments.
Fluorophotometric analyses have shown some variations in values, depending on the techniques used8; however, its use has allowed us to indirectly measure the dynamics of the tear film (ie, tear volume, tear flow, and tear turnover).
The contribution of evaporation to total tear loss has been reported to be around 10%,9 but other investigators have hypothesized that this contribution might be higher and that there could also be a difference between the contribution in normal subjects when compared with dry eye patients.10
Disruptions of the normal composition, quantity, and physiology of the ocular tear film can cause a vicious cycle of increased tear film evaporation and, therefore, the occurrence of dry eye symptoms. Clinically, it is apparent that dry eye symptoms are exacerbated in patients with any degree of dry eye states, or may be initiated in normal subjects under certain environmental conditions, including low relative humidity (RH), wind, closed working environments, occupational and environmental pollutants, and arid areas.
The purpose of this study was to experimentally determine the impact of evaporation on aqueous tear (AT) loss in subjects without dry eye and patients with keratoconjunctivitis sicca (KCS) under different RH conditions. This laboratory approach makes it possible to determine and compare the impact of AT evaporation on a variety of parameters in control subjects and dry eye patients.
METHODS
STUDY POPULATIONS
The protocol, consent forms, and data accumulation methods used in these studies were previously approved by the University of Texas Southwestern Medical Center Institutional Review Board. Eligibility requirements for the KCS patients included a prior diagnosis of dry eye with symptoms of foreign body sensation or dryness and interpalpebral fissure conjunctival vital dye staining along with a decreased tear film meniscus on slit-lamp biomicroscopy. Patients were excluded if either eye had clinically evident lid or ocular surface inflammation. Eyes were then assigned to a “classic” KCS group if they showed normal-appearing meibomian secretions (clear and easily expressed). In contrast, eyes with meibomian glands that were either difficult to express or had turbid secretions were assigned to the KCS plus meibomian gland dysfunction (MGD) group. In all cases, both eyes of any study patient had identical type of dry eye. Sex-matched subjects with no evidence of ocular surface or drying disorders were assigned to the control group, and both eyes of each control were studied.
TEAR VOLUME, FLOW, AND TURNOVER
Background fluorescence was determined prior to the instillation of 0.5 μL of 0.5% sodium fluorescein onto the ocular surface. Utilizing a fluorophotometer (Fluorotron Master; Ocumetrics, Mountain View, California), eight measurements were taken for each eye to determine tear fluorescence. The first two measurements were done 1 minute apart, and subsequent measurements were repeated at 3-minute intervals until completion at 19 minutes.11 These data were used to calculate tear volume, tear flow, and tear turnover as previously described.4,12–14
Tear turnover is the percentage change in fluorescein concentration in a given amount of time; there are no units of measure. Tear flow is the amount of tear fluid flowing past the cornea in a given amount of time and is expressed as microliters per minute (μL/min).11 Tear volume refers to the initial tear volume determined by regressing the concentration to time zero, thereby obtaining the decay constant (k) and the concentration at instillation.4
TEAR SURFACE EVAPORATION
An evaporometer (Oxdata, Portland, Oregon) utilized a pump to direct room air through a drying tube into a form-fitting goggle that created a closed environment and contained a humidity/temperature sensor.15 Dry air was pumped into the goggle to reduce RH to 15%, at which time the pump was turned off. The RH within the goggle was allowed to rise. The increase in humidity due to evaporation from skin or evaporating tears was measured. The process was carried out first with the eye closed and then with it open; the difference was the tear evaporation rate.14 Using the original formula published by Rolando and Refojo,16 we calculated the evaporative rates under two different ranges of increasing RH, 20% to 25% and 40% to 45%. The area of the interpalpebral ocular surface was used to calculate evaporation per unit area; the image of the area was captured with the use of a digital camera, and the area was calculated directly with the aid of computer software (Adobe Photoshop, version 6.0.1.2001; Adobe Systems, San Jose, California),14 expressed as μL/cm2/min.
EVAPORATIVE OUTFLOW
Evaporative outflow as a percentage of available tear volume was determined for each individual study eye by multiplying the evaporative rate by the real area divided by the volume.
EVAPORATIVE CONTRIBUTION TO TOTAL AT LOSS
The contribution of tear evaporation to total AT loss was determined for each individual study eye by dividing the evaporative outflow by tear turnover. It is expressed as a percent.
STATISTICAL ANALYSES
Statistical analyses were carried out using SigmaStat 2.03 (Systat Software Inc, Richmond, California). The Student t test and Mann-Whitney rank sum test were applied with statistical significance determined at the P < .05 level. Data from each eye of all study subjects were included in the analyses.
RESULTS
STUDY POPULATIONS
The entire study group consisted of 32 eyes (18 subjects) with at least one eye of each subject meeting the study criteria. The study eyes were analyzed in the following manner: Eight patients (15 eyes) had classic KCS (mean age, 58.2 ± 13.7 years), and ten patients (17 eyes) had KCS plus clinical evidence of MGD (mean age, 59.8 ± 20 years). Eleven subjects with no evidence of ocular surface disease were included as a control group (mean age, 42.4 ± 11.8 years). Only one dry eye subject contributed a study eye to each group. There was no evidence that either age or gender was a significant factor for a predilection to classic KCS or KCS plus MGD.
Given these attributes and the study design, which focused on obtaining clinical data and did not evaluate therapy, each eye of each subject that met inclusion criteria was included in the study and analyses. Four subjects contributed only one eye to the analyses because the other eye failed to meet inclusion/exclusion criteria.
STUDY FINDINGS
A summary of the test results for all study eye groups is presented in Tables 1 and 2. Normal eyes provided comparative data.
TABLE 1.
FLUOROPHOTOMETRIC TEAR PARAMETERS*
| GROUP | TEAR VOLUME (μL) | TEAR FLOW (μL/MIN) | TEAR TURNOVER (%/MIN) |
|---|---|---|---|
| Controls | 1.99 ± 1.54 | 0.30 ± 0.24 | 16.33 ± 7.12 |
| Dry eye group | |||
| Classic KCS | 2.05 ± 1.87 | 0.28 ± 0.29 | 14.38 ± 7.87 |
| KCS + MGD | 3.40 ± 3.20 | 0.27 ± 0.20 | 13.72 ± 10.74 |
KCS = keratoconjunctivitis sicca; MGD = meibomian gland dysfunction.
Expressed as mean ± SD.
TABLE 2.
INCREASE IN RATE OF EVAPORATION AFTER RELATIVE HUMIDITY (RH) REDUCTION FROM 40%–45% TO 20%–25%
| GROUP | EVAPORATION AT 40%–45% RH*(μL/CM2/MIN) | EVAPORATION AT 20%–25% RH*(μL/CM2/MIN) | RELATIVE INCREASE IN EVAPORATION†(%) | PVALUE‡ |
|---|---|---|---|---|
| Controls | 0.037 ± 0.016 | 0.065 ± 0.022 | 85.59 | < .001 |
| All KCS | 0.034 ± 0.020 | 0.067 ± 0.043 | 108.09 | < .001 |
| Classic KCS | 0.037 ± 0.022 | 0.067 ± 0.031 | 118.32 | < .001 |
| KCS + MGD | 0.032 ± 0.018 | 0.068 ± 0.053 | 97.85 | < .001 |
| All subjects | 0.035 ± 0.018 | 0.066 ± 0.037 | 99.43 | < .001 |
KCS = keratoconjunctivitis sicca; MGD = meibomian gland dysfunction.
Expressed as mean ± SD.
Mean percentage change after the simulated decrease of 20% in RH within each group.
Comparison of the evaporative rates under the two RH ranges within each group.
TEAR VOLUME, FLOW, TURNOVER, AND THICKNESS
The eyes of patients enrolled in this study were typically noted to have a decreased tear film meniscus on clinical examination at the time of screening. The eyes with KCS plus MGD showed an increased tear volume when compared with the normal eye and the classic KCS eyes. The classic KCS group had approximately the same tear volume as the normal eyes. Tear flow results were similar in the three groups (Table 1). All dry eye groupings exhibited decreased tear turnover when compared with the normal group, although statistical significance was not reached.
TEAR EVAPORATION STUDIES
Evaporation rates (Table 2) were determined using the actual ocular surface area for each eye utilizing a digital image and software measurements and applying a standard formula.16 The evaporation rates for the dry eye groups and the controls were very similar and did not show statistically significant difference.
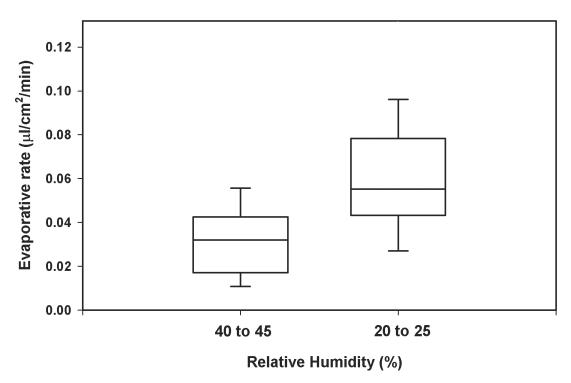
When the RH conditions were changed from 40%–45% to 20%–25%, the evaporative rates increased significantly in all three groups. The impact of this change in RH on evaporation was similar in all groups, and on average there was a 99.43% increase in evaporation (P < .001) (Figure 1).
FIGURE 1.
Box-and-whisker plot representation of evaporative rates in 18 dry eye patients collected at two different relative humidity conditions similar to those found at sea level and during flights (P < .001, evaporative rate at 40%–45% relative humidity vs 20%–25%). Whiskers represent the upper and lower limits of the data set.
EVAPORATIVE CONTRIBUTION TO TEAR LOSS
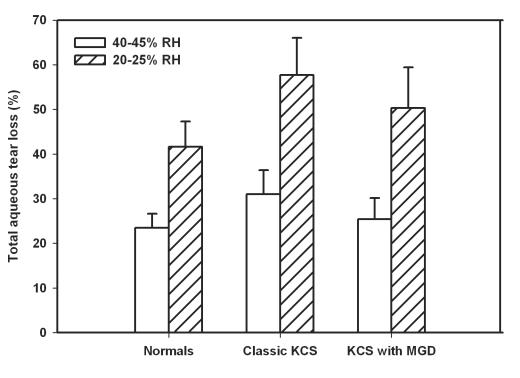
When percent evaporative contribution to tear loss was calculated (Table 3), the relative contribution of evaporation becomes more evident and was RH-dependent. At 40% to 45% RH, for normal eyes, evaporation accounts for 23.47% of turnover, whereas in eyes with classic KCS it is 30.99% and for KCS plus MGD it is 25.44%. At the lower RH condition, 20% to 25%, the evaporation accounts for 41.66% in normal eyes, 57.67% in classic KCS eyes, and 50.28% in eyes with KCS plus MGD (Figure 2). Thus there was a trend toward increased evaporative contribution to tear loss in the dry eye patients when compared with the normals, although the difference was not statistically significant (Table 3).
TABLE 3.
EVAPORATIVE CONTRIBUTION TO TEAR LOSS
| EVAPORATIVE CONTRIBUTION TO TEAR LOSS AT 20%–25% RH | EVAPORATIVE CONTRIBUTION TO TEAR LOSS AT 40%–45% RH | |||
|---|---|---|---|---|
| GROUP | %* | PVALUE | %* | PVALUE |
| Controls | 41.66 ± 23.20 | (.187)† | 23.47 ± 13.08 | (.376)† |
| Dry eye | ||||
| Classic KCS | 57.67 ± 32.25 | (.114)‡ | 30.99 ± 20.99 | (.227)‡ |
| KCS + MGD | 50.28 ± 35.41 | (.417)‡ | 25.44 ± 18.17 | (.725)‡ |
KCS = keratoconjunctivitis sicca; MGD = meibomian gland dysfunction; RH = relative humidity.
Expressed as mean ± SD.
Controls vs all dry eye.
Compared to controls.
FIGURE 2.
Evaporative contribution to tear loss at different relative humidity (RH) conditions (40%–45% and 20%–25%). The contribution of evaporation to total aqueous tear loss in dry eye patients, either with (10 patients) or without (8 patients) meibomiam gland dysfunction (MGD), when compared to 11 normal subjects did not reach statistical significance. Error bars represent the standard error of the mean. KCS = keratoconjunctivitis sicca.
DISCUSSION
In this clinical laboratory study, new information has been gained in two areas: the impact of evaporation to total AT loss and the contribution of evaporation in this process in normal subjects and dry eye patients. We have shown that the contribution of evaporation to tear loss tends to be higher than the 10% that was described in the past,9 with values up to 40% to 60% when eyes are exposed to 20% to 25% RH. It is also important to mention that this percent contribution to tear loss can increase in magnitude depending on the existent environmental conditions. This change relies mainly on the fact that the evaporative rates increase significantly after minimum reduction of RH.5
The use of the evaporometry approach in combination with fluorophotometry analyses in the assessment of patients with dry eye syndrome has provided additional understanding of the behavior of normal and diseased tear films under different RH conditions.
We found a trend toward increased contribution of evaporation to total AT loss in dry eye patients, either with or without MGD, when compared with controls, but this difference did not reach statistical significance. Importantly, our study clearly demonstrates that evaporation occurs in the presence of a normal tear film, especially in low RH, and provides supporting evidence for the onset of dry eye symptoms in such environmental conditions as low RH that pilots encounter in commercial airplanes.7
Despite the fact that the difference between the two RH experimental conditions was only 20%, this reduction in RH had a statistically significant impact on the evaporative rate for each of our three study groups of approximately a 99% increase in AT evaporation. The inclusion of young normal subjects allowed an analysis of the impact of low RH on normal tear films and also the assessment of tear loss patterns. The tear evaporative behavior of their normal tear films under the humidity conditions tested was not different from that seen in dry eye patients.
There are likely other contributory factors present in nonlaboratory low RH conditions that contribute to increased evaporation rates, including air movement and noxious contaminants in the atmosphere. However, it is now clear that AT evaporation in low RH is probably sufficient to trigger the appearance of symptoms due to increased tear loss from evaporation. The presence of relatively normal tear volume in clinical dry eye patients suggests that qualitative alterations in preocular surface tears may play a greater role in the expression of disease than quantitative changes.
PEER DISCUSSION
DR J. DANIEL NELSON
The impact of relative humidity on evaporation from the tear film has been widely investigated in both normal and dry eye subjects. The results are often difficult to interpret and compare because of the use of different measurement units, different measurement techniques, and different experimental conditions. In this present work McCulley and colleagues obtained a mean evaporation rate in normal controls of 0.037+ 0.016 μL/cm2/min at 40–45% RH, which is comparable to previously published evaporation rates in normal subjects in range of the 0.025–0.094 μL/cm2/min (4.1–15.6 × 10−7 g/cm−2/s) 1. The variability in evaporation rates can be attributed to measurement technique and experimental conditions. On the other hand, the evaporation rates obtained in this study at 40–45% RH for both the “classic KCS” (0.037 + 0.022 μL/cm2/min) and “KCS+MGD” groups (0.032 + 0.018 μL/cm2/min) are much lower then those previously reported (0.088–0.143 μL/cm2/min).2 What accounts for this difference? There could be technical reasons relating to the particular methods used to measure evaporation rates, or, perhaps it was how dry eye and MGD were defined. Agreement as to what subjective and objective findings constitute a diagnosis of dry eye, and to a lesser extent meibomian gland dysfunction (MGD), is not consistent throughout the literature. In examining Table 1, it appears that individuals with “KCS” have similar fluorophotometric tear parameters as normal controls. This begs the question- What is the difference between “KCS” patients and normal controls? In this study, keratoconjunctivitis sicca (KCS) was defined by symptoms and conjunctival vital dye staining, along with a decreased tear film meniscus. In other reports in the literature different definitions for dry eye are used. Some investigators believe that it is ocular surface inflammation that is responsible for many of the symptoms and findings in KCS. It is not unusual for conjunctival vital dye staining and a decreased tear meniscus to exist in the absence of ocular surface inflammation. As patients with ocular surface inflammation were excluded from this study, it appears that the KCS subjects in this study exhibited clinical characteristics that were more similar to the normal controls than individuals with ocular surface inflammation.
In this study evaporation rates increased significantly with a lowering of the relative humidity. The evaporation rates at 20–25% RH are approximately twice those at 40–45% RH for both normal and KCS subjects. In addition, the evaporative contribution to total tear loss was similar for both the “Classic KCS” and “KCS+MGD” patients. I am puzzled that both “Classic KCS” and “KCS+MGD” eyes show similar evaporation rates as normal controls and more puzzled that the presence of “MGD” did not affect evaporation rates or total tear loss (Table 2). This suggests that evaporation has a significant effect on both normal and dry eye patients and, contrary to “dry eye dogma”, that the presence of MGD has no additional effect on evaporation rates.
The authors also show that the contribution of evaporation to tear turnover is higher than previously reported and dependent on the environmental relative humidity. Although the authors could not demonstrate statistical significance, there was a trend toward increased evaporative contribution to tear loss in the dry eye patients compared to normal subjects. It is very interesting that even with similar fluorophotometric tear parameters and evaporation rates; evaporation seems to have more of an effect on tear turnover in the dry eye patients compared to normal controls.
Dr. McCulley and colleagues are to be congratulated on this interesting paper that shows 1) that going from a high RH to low RH increases evaporation in both normal and dry eye subjects and 2) that evaporation contributes significantly to tear turnover and this may be more so in dry eye patients. I would be interested in the author’s comments as to 1) Why baseline tear volume, tear flow and tear turnover measurements are relatively similar between normal controls and the “Classical KCS” and “KCS + MGD” patients; 2) Why are tear evaporation rates similar between normal subjects, “Classical KCS” and “KCS + MGD” patients; and 3) Have we been misguided in our classifying dry eyes as “evaporative or non-evaporative”.
REFERENCES
- 1.Tomlinson A, Khanal S. Assessment of tear film dynamics: Quantification approach. Ocular Surface. 2005;3:81–95. doi: 10.1016/s1542-0124(12)70157-x. [DOI] [PubMed] [Google Scholar]
DR JAY H. KRACHMER
All of us have seen patients with the same relative aqueous tear volume, tear meniscus, and Schirmer test. Yet only some have symptoms of dry eye. From your studies, how big a role does the evaporation play in causing symptoms, assuming they do not have meibomian gland disease or other factors like that?
DR RONALD KLEIN
I was interested in the comparisons of difference between your controls and cases. You did not address other factors besides age differences and medication usage that might influence the tear flow.
DR ALLAN J. FLACH
You mentioned medications. What do you tell these patients about diet and water ingestion during your studies?
DR MICHAEL A. LEMP
In your patients who had meibomian gland dysfunction (MGD), did any of them fall into the category of what Dr William Mathers calls obstructive meibomian gland dysfunction? He certainly reported increased evaporative tear rates in those patients. As to your comments on the possible effects of hyperosmolarity in the tear film, I agree with you and the theory of Tiffany and Bron, i.e. that is, the tear film is compartmentalized. If you don’t have free exchange between the marginal tear strip and the exposed area over the surface of the eye, therefore the delta, the changes between normal and dry eye patients, may be on the order of 20 milliosmols in the marginal tear strip. But if you look where most of the evaporation is occurring, and no free exchange between the surface of the eye, that delta may be instead of 20 or 30 millosmols, may be 300 milliosmols; that may be responsible for some of the damage that we see on the ocular surface
DR THOMAS O. WOOD
When dry eye patients come in to see me, the first thing that I look at their hands. Virtually all of them have tissue in their hands. And if I sit there and talk to them long enough, they’ll be wiping at their eyes. It seems to me like most of these are post-menopausal women and they all wipe their eyes. This may be responsible for 75 percent of their symptoms and can explain the Rose Bengal staining, the change in osmolarity, and the change in the Schirmer test. If I can convince these ladies to stop rubbing their eyes, they do not need much treatment.
DR ALAN H. FRIEDMAN
Did you try to segregate baseline from reflex tearing, as to the rate of evaporation? Were topical anesthetics used at any time during the studies?
DR JAMES P. MCCULLEY
No topical anesthetics were used in the study. Dry eye patients are a heterogeneous group. I selected patients who had typical symptoms of dry eyes, had interpalpebral fissure vital staining consistent with dry eyes, did not have any other disease process, and that did not have clinically evident inflammation at the slit lamp. However, most probably had cytokines and other markers of inflammation since inflammation certainly does occur. Whenever you disturb the normal physiology, as a dry eye does, a universal response is inflammation. So, we were selecting patients at a certain stage of disease.
Evaporation clearly is a very potent force and much more important than I realized. We have all recognized clinically over the years that environmental circumstances can adversely affect our patient population. A patient from Dallas who goes skiing in Colorado may have a decompensation of their dry eye state because of the lower humidity in the mountains, the increased evaporation, and hence greater expression of disease with increased symptoms and increased ocular surface staining.
As for the tear volume, I am not certain exactly what is happening. We did not separate out reflex tearing from basal tearing. However we did not stimulate the patients, and we did not use topical anesthetics, as that would have complicated the study. There is a phase in the development of dry eyes, where there is a feedback from the ocular surface to the lacrimal gland and the lacrimal gland starts to secrete more tears. There is probably also an inflammatory component to it, and may result from some lacrimal gland burnout, and then a later stage of the disease where tear film, tear production, tear flow, and tear volume are decreased. I theorize that we are simply catching this patient population in that stage of the disease. I do think the evaporation does explain many of the patients’ symptoms when they’re exposed to low relative humidity environments. Our normal patients did not have vital staining or any symptoms. None of the patients had evidence of systemic disease. On entry into the study, none of the controls had any topical eye medications and the dry eye patients had only artificial tears. None of the patients were taking systemic medication or had a systemic disease that might contribute to the diagnosis of dry eyes. The patients did not eat during the course of the test, which took less than an hour, and I did not alter their fluid intake. This was not a longitudinal study.
There were patients that had an obstructive component to their MGD and their secretions were turbid. I am pleased that Dr. Lemp agrees with Tiffany and Bron, among others, but it was David Meadows, Ph.D. that suggested to me the compartmentalization impact and how a 20 milliosmolar increase in our tear meniscus measurement of tear osmolarity could indeed be clinically significant.
Our patient population did not have a tissue in their hand and did not have the mucous fishing syndrome which I described a number of years ago. They had no evidence of vital staining outside the interpalpebral fissure and had no evidence of mechanical trauma.
REFERENCES
- 1.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 2.McCulley JP, Shine WE. Tear film structure and dry eye. Contactologia. 1998;20:145–149. [Google Scholar]
- 3.Mishima S. Some physiological aspects of the precorneal tear film. Arch Ophthalmol. 1965;73:233–241. doi: 10.1001/archopht.1965.00970030235017. [DOI] [PubMed] [Google Scholar]
- 4.Mathers WD, Daley TE. Tear flow and evaporation in patients with and without dry eye. Ophthalmology. 1996;103:664–669. doi: 10.1016/s0161-6420(96)30637-4. [DOI] [PubMed] [Google Scholar]
- 5.McCulley JP, Aronowicz JD, Uchiyama E, et al. Correlations in a change in aqueous tear evaporation with a change in relative humidity and the impact. Am J Ophthalmol. 2006;141:758–760. doi: 10.1016/j.ajo.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 6.Ousler GW, 3rd, Abelson MB, Nally LA, et al. Evaluation of the time to “natural compensation” in normal and dry eye subject populations during exposure to a controlled adverse environment. Adv Exp Med Biol. 2002;506:1057–1063. doi: 10.1007/978-1-4615-0717-8_150. [DOI] [PubMed] [Google Scholar]
- 7.McCarty DJ, McCarty CA. Survey of dry eye symptoms in Australian pilots. Clin Experiment Ophthalmol. 2000;28:169–171. doi: 10.1046/j.1442-9071.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. The Ocular Surface. 2005;3:81–95. doi: 10.1016/s1542-0124(12)70157-x. [DOI] [PubMed] [Google Scholar]
- 9.Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998;17:565–596. doi: 10.1016/s1350-9462(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 10.Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004;78:389–394. doi: 10.1016/s0014-4835(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 11.van Best JA, Benitez del Castillo JM, Coulangeon LM. Measurement of basal tear turnover using a standardized protocol. European concerted action on ocular fluorometry. Graefes Arch Clin Exp Ophthalmol. 1995;233:1–7. doi: 10.1007/BF00177778. [DOI] [PubMed] [Google Scholar]
- 12.Mathers WD, Lane JA, Sutphin JE, et al. Model for ocular tear film function. Cornea. 1996;15:110–119. doi: 10.1097/00003226-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Eter N, Gobbels M. A new technique for tear film fluorophotometry. Br J Ophthalmol. 2002;86:616–619. doi: 10.1136/bjo.86.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCulley JP, Shine WE, Aronowicz J, et al. Presumed hyposecretory/hyperevaporative KCS: tear characteristics. Trans Am Ophthalmol Soc. 2003;101:141–152. [PMC free article] [PubMed] [Google Scholar]
- 15.Mathers WD, Binarao G, Petroll M. Ocular water evaporation and the dry eye. A new measuring device. Cornea. 1993;12:335–340. doi: 10.1097/00003226-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Rolando M, Refojo MF. Tear evaporimeter for measuring water evaporation rate from tear film under controlled conditions in humans. Exp Eye Res. 1983;36:25–33. doi: 10.1016/0014-4835(83)90086-6. [DOI] [PubMed] [Google Scholar]