Abstract
Purpose
To examine the relationship of retinopathy in persons without diabetes mellitus to the 15-year cumulative incidence of diabetes mellitus and hypertension.
Methods
A total of 3,402 persons 43 to 86 years of age without diabetes mellitus (1,879 without diabetes or hypertension) at the time of a baseline examination in 1988–1990 had follow-up examinations in 1993–1995, 1998–2000, and/or 2003–2005. Diabetes mellitus was defined by a combination of history, serum glucose levels, and glycosylated hemoglobin levels, and hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg and/or use of antihypertensive medications. Retinopathy at baseline was determined by masked gradings of stereoscopic fundus photographs using standardized protocols.
Results
Retinopathy was present in 7.3% of the nondiabetic persons in the cohort and 5.4% of the nondiabetic, nonhypertensive cohort at baseline. The 15-year cumulative incidence of diabetes was 12.5% and of hypertension 54.1%. While controlling for age, persons with retinopathy were more likely to develop diabetes mellitus (odds ratio, 95% confidence interval, P value: 1.70, 1.17–2.48, P = .005) and hypertension (1.62, 1.18–2.23, P = .003) than persons without retinopathy. While controlling for other risk factors (eg, blood pressure, glucose level, cardiovascular disease history), the associations of retinopathy with incident diabetes mellitus (1.35, 0.90–2.03, P = .15) and hypertension (1.48, 1.05–2.07, P = .02) became attenuated but remained statistically significant for hypertension. In stratified analyses, retinopathy was associated with incident diabetes in persons younger than 65 years (1.80, 1.12–2.89, P = .02)
Conclusions
While controlling for other risk factors, retinopathy in nondiabetic individuals is associated with the incidence of hypertension and, in younger persons, with the incidence of diabetes mellitus.
INTRODUCTION
Retinopathy in persons without diabetes or retinal vein occlusion is common, occurring in 1% to 15% of the nondiabetic general population.1–9 It is usually manifest by one or two retinal microaneurysms or blot hemorrhages.4 When present in nondiabetic persons, these lesions are associated with older age and the presence of systemic hypertension.1,2,4,6,8,10 Independent of hypertension and other risk factors, retinopathy has been found to be associated with increased risk of incident carotid artery plaque, subclinical stroke detected on magnetic resonance imaging, stroke mortality, congestive heart failure, and reduced cognitive performance on standardized neuropsychological tests.7–18 These associations suggest that isolated retinopathy signs are markers of systemic vascular disease. There are, however, few data regarding whether retinopathy in nondiabetic persons predicts the subsequent development of diabetes and hypertension, independent of other risk factors.19,20 The purpose of this report is to examine the relationships of retinopathy in nondiabetic persons to the 15-year cumulative incidence of diabetes mellitus and hypertension.
METHODS
POPULATION
Methods used to identify and describe the population have appeared in previous reports.21,22 In brief, a private census of the population of Beaver Dam, Wisconsin (99% white), was performed from fall 1987 to spring 1988 in people 43 to 84 years of age.21 Of the 5,924 eligible individuals, 4,926 participated in the baseline examination in 1988 to 1990.22 Of the 4,542 surviving participants, 3,684 (81.1%) participated in the 5-year follow-up examination in 1993 to 1995.23 Comparisons between participants and nonparticipants at baseline and the 5-year follow-up examination have appeared elsewhere.22,23 Of the 3,334 surviving participants in the baseline and second examination, 2,764 (82.9%) participated in the 10-year follow-up examination between March 1, 1998, and June 9, 2000.24 Comparisons between participants and nonparticipants at baseline and the 10-year examination have appeared elsewhere.23 Of the 2,480 surviving participants who were examined at the baseline, 5-year, and 10-year follow-up examinations, 2,119 (85.4%) participated in the 15-year follow-up examination between March 31, 2003, and April, 30, 2005.25 The mean and median times between the baseline and 15-year follow-up examination were 14.9 years (standard deviation = 0.5 year) and 14.8 years, respectively.
Comparisons between participants and nonparticipants at the 15-year follow-up have been presented elsewhere.25 In general, persons who did not participate in the 15-year follow-up were older at baseline than those who did. After adjusting for age, persons who did not participate were more likely to have fewer years of education completed, to have higher systolic blood pressure, and to have more pack-years smoked than persons who participated. After adjusting for age and gender, nondiabetic participants with retinopathy at baseline were as likely to participate as those in whom retinopathy was absent (data not shown).
PROCEDURES
Similar procedures were used at the baseline and follow-up examinations.26–34 Informed consent was obtained and institutional review board approval was granted at the beginning of each examination.
A standardized questionnaire was administered. It included the following questions pertinent to this report: “Has a doctor ever said you had diabetes, sugar in your urine, or high blood sugar?” and “How old were you when you learned this?” There also were questions regarding use of diet and oral hypoglycemic agents or insulin for the management of hyperglycemia and questions regarding history of cigarette smoking, hypertension, and use of antihypertensive medications for the management of high blood pressure.
Blood pressure was measured according to the Hypertension Detection and Follow-up Program protocol.29 Nonfasting blood specimens also were obtained from participants. Serum glucose level was determined using the hexokinase method,30 and plasma glycosylated hemoglobin was determined using affinity chromatography (Isolab Inc, Akron, Ohio).31
Stereoscopic 30° color fundus photographs centered on the disc (Diabetic Retinopathy Study standard field 1) and macula (Diabetic Retinopathy Study standard field 2) and a nonstereoscopic color fundus photograph temporal to but including the fovea (modified Diabetic Retinopathy Study standard field 3) were taken in each eye.32 Additional fundus photographs were taken if any lesions were found outside these fields.
Retinopathy was defined using a classification derived from studies of diabetic retinopathy but herein used to describe the presence of such lesions in the absence of diabetes. The presence of retinal hemorrhages, microaneurysms, cotton-wool spots, hard exudates, intraretinal microvascular abnormalities, venous beading, new vessels on the disc and elsewhere, and preretinal and vitreous hemorrhages was graded in a masked fashion using an abbreviation of the modified Airlie House classification scheme.33,34 The presence of other retinal disease, such as central and branch retinal arterial or venous occlusion, retinal cholesterol emboli, and surface wrinkling retinopathy was graded using a detailed protocol.
When two eyes of a participant were discrepant in the presence of a lesion, the grade assigned for the participant was that of the more severely involved eye. For example, in assigning the presence of retinal microaneurysms, if they were present in one eye but not the other, the participant would be considered to have retinal microaneurysms. When lesions could not be graded in one eye, the participant was assigned a score equivalent to that in the other eye.
DEFINITIONS
Current age was defined as the age at the time of the baseline examination. Retinopathy was defined to include presence of microaneurysms and/or blot hemorrhages, and/or more severe retinopathy lesions (eg, hard exudates, cotton-wool spots, intraretinal microvascular abnormalities, retinal new vessels). Microaneurysms and blot hemorrhages were also analyzed separately. The mean systolic blood pressure was the average of the two systolic blood pressure determinations, and the mean diastolic blood pressure was the average of the two diastolic blood pressure determinations at baseline. A person was defined as having a positive history if he or she responded positively to the questions regarding cardiovascular disease and stroke at baseline. Hypertension was defined as a mean systolic blood pressure of 140 mm Hg or greater, and/or a mean diastolic blood pressure of 90 mm Hg or greater, and/or a history of hypertension with use of antihypertensive medication. Diabetes was defined as a history of diabetes mellitus, treated with insulin, oral hypoglycemic agents, and/or diet. Newly diagnosed diabetes mellitus was defined by a glycosylated hemoglobin value that was greater than 2 standard deviations above the mean for a given age-sex group (ie, 43 to 54 years of age, men > 9.5% and women > 9.6%; 55 to 64 years of age, men > 9.4% and women > 10.0%; 65 to 74 years of age, men > 9.6% and women > 9.6%; and 75 years of age or older, men > 9.5% and women > 9.6%) and a random blood glucose value greater than 200 mg/dL. Primary care physicians were consulted whenever there was doubt about past diagnosis.
Incident diabetes mellitus was defined in the cohort without diabetes at baseline, whereas incidence of hypertension was defined among those who also did not have hypertension at baseline (ie, nondiabetic and nonhypertensive).
Cigarette smoking status was defined as follows: subjects were classified as having never smoked if they reported having smoked fewer than 100 cigarettes in their lifetime; as ex-smokers if they had smoked more than this number of cigarettes in their lifetime but had stopped smoking before the baseline examination; and as current smokers if they had not stopped.
STATISTICS
SAS was used for statistical analysis.35 Cumulative incidence was estimated by the product-limit method,36 and age-adjusted rates were computed by the direct method. Tests for differences between rates were conducted by the log-rank test.37 Multivariable models were constructed by discrete logistic hazard regression.38 Time-varying covariates were employed as follows. For each separate 5-year follow-up interval, the value of each covariate at the beginning of the interval, or previous value if that value was missing, was included in the model. For example, the baseline value of retinopathy was included for the interval between baseline and the 5-year examination. The value at the 5-year examination was included for the interval between the 5- and 10-year examinations, and the value at the 10-year examination was included for the interval between the 10- and 15-year examinations. Time-varying covariates for other parameters that are subject to change were defined similarly.
RESULTS
Of the 3,904 persons examined at both the baseline and at least one follow-up examination, we excluded 39 because photographs could not be graded for retinal lesions and 36 because of central or branch retinal venous or arterial occlusions or macular edema at baseline. For incidence of diabetes, we also excluded 350 persons with diabetes, suspected diabetes, or no diabetes information at baseline, and 77 persons missing diabetes information at follow-up, leaving 3,402 persons for analysis. For incidence of hypertension, we further excluded 288 with diabetes and 1,623 with hypertension at baseline and 40 persons missing hypertension information at follow-up, leaving 1,878 persons for analysis. Characteristics of both groups are presented in Table 1. Retinopathy was present in 7.3% of the nondiabetic group and 5.4% of the nondiabetic nonhypertensive group. The 15-year cumulative rate of disappearance of retinopathy was 92% in the nondiabetic group and 95% in the nondiabetic nonhypertensive group.
TABLE 1.
BASELINE CHARACTERISTICS OF NONDIABETIC AND NONDIABETIC NONHYPERTENSIVE GROUPS IN THE BEAVER DAM EYE STUDY, 1988–1990
| NONDIABETIC GROUP | NONDIABETIC, NONHYPERTENSIVE GROUP | |||
|---|---|---|---|---|
| CHARACTERISTIC | N* | MEAN ± SD OR % | N | MEAN ± SD OR % |
| Age, yr | 3402 | 60.0 ± 10.5 | 1878 | 57.6 ± 9.8 |
| Body mass index, kg/m2 | 3391 | 28.6 ± 5.3 | 1874 | 27.6 ± 4.8 |
| Glycosylated hemoglobin, % | 3402 | 5.7 ± 0.7 | 1869 | 5.7 ± 0.7 |
| Serum glucose, mg/dL | 3402 | 98 ± 14 | 1873 | 97 ± 14 |
| Serum total cholesterol, mg/dL | 3402 | 233 ± 43 | 1873 | 229 ± 42 |
| Serum HDL cholesterol, mg/dL | 3397 | 53 ± 18 | 1870 | 54 ± 18 |
| Systolic blood pressure, mm Hg | 3401 | 130 ± 19 | 1878 | 119 ± 11 |
| Diastolic blood pressure, mm Hg | 3401 | 78 ± 10 | 1878 | 74 ± 8 |
| Pack-years smoked | 3392 | 17 ± 25 | 1871 | 16 ± 23 |
| Sex, % male | 3402 | 43.3 | 1878 | 43.2 |
| Cardiovascular disease history, % | 3367 | 10.8 | 1861 | 8.0 |
| Hypertension, % | 3401 | 45.8 | NA | NA |
| Any retinopathy, % | 3402 | 7.3 | 1878 | 5.4 |
| Microaneurysms only, % | 3320 | 5.0 | 1851 | 4.1 |
| Blot hemorrhages only, % | 3199 | 1.4 | 1790 | 0.8 |
NA = not available.
Numbers vary due to missing data.
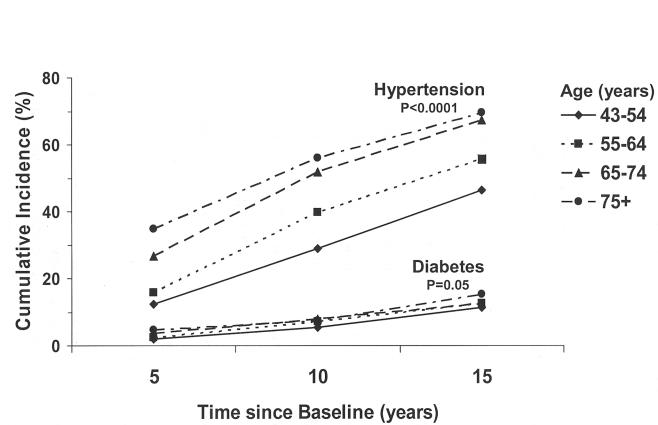
The 15-year cumulative incidence of diabetes was 12.5% (95% confidence interval [CI], 11.2 to 13.8). The cumulative incidence of diabetes was higher in nondiabetic hypertensive persons at baseline compared to nondiabetic normotensive persons (17.4% vs 8.7%, P < .001). The 15-year cumulative incidence of hypertension was 54.1% (95% CI, 51.6 to 56.6). Figures 1 and 2 show the relationship of 15-year cumulative incidence of diabetes and hypertension by age and sex. The incidence of hypertension increased with age and was similar in men and women. The incidence of diabetes increased with age and was higher in men than women.
FIGURE 1.
The 15-year cumulative incidence of hypertension and diabetes by age in persons without diabetes at baseline in the Beaver Dam Eye Study; P value is test of trend
FIGURE 2.
The 15-year cumulative incidence of hypertension and diabetes by sex in persons without diabetes at baseline in the Beaver Dam Eye Study; P value is test of trend.
The presence of microaneurysms only, blot hemorrhages only, or any retinopathy at baseline was associated with a higher 15-year cumulative incidence of diabetes or hypertension (Table 2). While controlling for age, these relationships remained except for the relations of blot hemorrhages to the incidence of hypertension and diabetes, which were no longer statistically significant.
TABLE 2.
15-YEAR CUMULATIVE INCIDENCE OF DIABETES AND HYPERTENSION BY BLOT HEMORRHAGES, MICROANEURYSMS, AND ANY RETINOPATHY AT BASELINE IN THE BEAVER DAM EYE STUDY
| OUTCOME | LESION | PRESENCE | N | % | OR | 95% CI | P | AGE-ADJUSTED % | OR | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | Blot hemorrhage | Absent | 3154 | 11.8 | 1.00 | * | .04 | 12.2 | 1.00 | * | .06 |
| Present | 45 | 21.6 | 2.27 | 1.04–4.96 | 30.9 | 2.11 | 0.96–4.64 | ||||
| Microaneurysms | Absent | 3154 | 11.8 | 1.00 | * | .006 | 12.2 | 1.00 | * | .008 | |
| Present | 166 | 21.5 | 1.81 | 1.17–2.79 | 24.9 | 1.80 | 1.17–2.78 | ||||
| Any retinopathy | Absent | 3154 | 11.8 | 1.00 | * | .003 | 12.2 | 1.00 | * | .005 | |
| Present | 248 | 20.3 | 1.74 | 1.20–2.53 | 19.9 | 1.70 | 1.17–2.48 | ||||
| Hypertension | Blot hemorrhage | Absent | 1776 | 53.2 | 1.00 | * | .03 | 57.4 | 1.00 | * | .15 |
| Present | 14 | 84.7 | 2.40 | 1.04–5.53 | 83.1 | 1.85 | 0.80–4.33 | ||||
| Microaneurysms | Absent | 1776 | 53.2 | 1.00 | * | .01 | 57.4 | 1.00 | * | .03 | |
| Present | 75 | 66.3 | 1.58 | 1.10–2.28 | 66.7 | 1.52 | 1.05–2.21 | ||||
| Any retinopathy | Absent | 1776 | 53.2 | 1.00 | * | .0005 | 57.4 | 1.00 | * | .003 | |
| Present | 102 | 71.1 | 1.75 | 1.27–2.40 | 69.3 | 1.62 | 1.18–2.23 |
CI = confidence interval; OR = odds ratio.
There was an age effect for the relationship between retinopathy and incident diabetes. In younger persons less than 65 years of age, the incidence of diabetes was higher in those with retinopathy as compared with those without retinopathy (24.3% vs 11.1%), whereas in persons 65 years of age or older, the incidence of diabetes was similar between those with and without retinopathy (11.5% vs 13.1%, P test of interaction = .02). In multivariable models in those less than 65 years of age, while controlling for other covariates, blot hemorrhages only and any retinopathy at baseline were both associated a with higher cumulative incidence of diabetes (Table 3). While controlling for other factors (ie, sex, serum glucose, glycosylated hemoglobin, body mass index, serum high-density lipoprotein cholesterol, smoking status, and cardiovascular disease history at baseline), the presence of any retinopathy (odds ratio [OR] 2.40, 95% CI 1.07 to 5.38, P = .03) but not retinal microaneurysms only (OR 1.56, 95% CI 0.58 to 4.20, P = .37) was associated with incident diabetes in those who were less than 65 years of age and normotensive at baseline. In those less than 65 years of age who were hypertensive at baseline, higher odds of diabetes incidence were found for any retinopathy (OR 1.59, 95% CI 0.89 to 2.84, P = .12), or retinal microaneurysms only (OR 1.68, 95% CI 0.87 to 3.26, P = .13), but neither relation was statistically significant. Further analyses using multivariable models with time-dependent covariates showed only a statistically significant relationship of blot hemorrhages only and incident diabetes (Table 4). There were no relationships found for any retinopathy, microaneurysms only, or blot hemorrhages only and incident diabetes in those older than 65 years of age (Tables 3 and 4).
TABLE 3.
ASSOCIATION OF BLOT HEMORRHAGES, MICROANEURYSMS, AND ANY RETINOPATHY WITH INCIDENCE OF DIABETES AND HYPERTENSION IN MULTIVARIABLE MODELS
| OUTCOME | LESION | OR | 95% CI | P |
|---|---|---|---|---|
| Diabetes* | ||||
| Age < 65 yr | Blot hemorrhage | 4.16 | 1.52–11.38 | .006 |
| Microaneurysms | 1.60 | 0.92–2.77 | .10 | |
| Any retinopathy | 1.80 | 1.12–2.89 | .02 | |
| Age ≥ 65 yr | Blot hemorrhage | 0.33 | 0.04–2.52 | .29 |
| Microaneurysms | 1.07 | 0.40–2.88 | .90 | |
| Any retinopathy | 0.74 | 0.32–1.70 | .48 | |
| Hypertension† | Blot hemorrhage | 1.95 | 0.81–4.70 | .14 |
| Microaneurysms | 1.40 | 0.95–2.06 | .09 | |
| Any retinopathy | 1.48 | 1.05–2.07 | .02 | |
CI = confidence interval; OR = odds ratio.
Controlling for sex, glucose, glycosylated hemoglobin, body mass, hypertension, serum high-density lipoprotein (HDL) cholesterol, smoking, and cardiovascular disease history at baseline.
Controlling for age, sex, systolic blood pressure, diastolic blood pressure, serum HDL cholesterol, and cardiovascular disease history at baseline.
TABLE 4.
ASSOCIATION OF BLOT HEMORRHAGES, MICROANEURYSMS, AND ANY RETINOPATHY WITH INCIDENCE OF DIABETES AND HYPERTENSION IN MULTIVARIABLE MODELS WITH TIME-DEPENDENT COVARIATES
| OUTCOME | LESION | OR | 95% CI | P |
|---|---|---|---|---|
| Diabetes* | ||||
| Age < 65 yr | Blot hemorrhage | 3.68 | 1.23–10.96 | .02 |
| Microaneurysms | 0.97 | 0.46–2.05 | .94 | |
| Any retinopathy | 1.15 | 0.64–2.09 | .64 | |
| Age ≥ 65 yr | Blot hemorrhage | 0.37 | 0.05–2.84 | .34 |
| Microaneurysms | 1.12 | 0.41–3.07 | .83 | |
| Any retinopathy | 0.74 | 0.32–1.76 | .50 | |
| Hypertension† | Blot hemorrhage | 2.50 | 1.30–4.81 | .006 |
| Microaneurysms | 1.30 | 0.87–1.95 | .20 | |
| Any retinopathy | 1.45 | 1.04–2.03 | .03 | |
CI = confidence interval; OR = odds ratio.
Controlling for sex, glucose, glycosylated hemoglobin, body mass, hypertension, serum high-density lipoprotein (HDL) cholesterol, smoking, and cardiovascular disease history.
Controlling for age, sex, systolic blood pressure, diastolic blood pressure, serum HDL cholesterol, and cardiovascular disease history.
There was no similar effect of age for the incidence of hypertension by retinopathy status at baseline (P test of interaction = .19; data not shown). In multivariable models controlling for other covariates, the only significant association found was between any retinopathy at baseline and the 15-year cumulative incidence of hypertension (Table 3). This association remained in multivariable models with time-dependent covariates (Table 4). In the latter models, a statistically significant association between retinal blot hemorrhages only and incident hypertension was found.
The 5-year mean change in systolic or diastolic blood pressure was not statistically different by the presence or absence of retinopathy lesions at baseline in the nonhypertensive group (data not shown). The 5-year mean change in casual blood glucose or glycosylated hemoglobin measurements was not statistically different by the presence or absence of retinopathy lesions at baseline in the nondiabetic group (data not shown).
DISCUSSION
Our data show a prospective relation of retinopathy at baseline in nondiabetic persons to an increased cumulative incidence of hypertension but not diabetes over the 15-year period of the study. After adjusting for other risk factors at baseline, nondiabetic persons without hypertension with any retinopathy at baseline were approximately 50% more likely to develop hypertension compared with persons without retinopathy. Retinopathy at baseline, although not related to incident diabetes after accounting for age and common risk factors in the whole cohort, was associated with incident diabetes in persons younger than 65 years.
The higher risk of hypertension in nondiabetic people with retinopathy is consistent with the cross-sectional associations of retinopathy with hypertension in nondiabetic persons in Beaver Dam and in other studies,1,2,4,6,8,10 In Evans County, Georgia, retinopathy was present in 2.3% of white males and 4.9% of white females whose diastolic blood pressure was greater than 100 mm Hg.1 In a population-based study of 855 men 50 years of age in Göthenburg, Sweden, mean systolic and diastolic blood pressures were significantly (P < .05) higher in those subjects with retinopathy than in those without these signs.2 However, not all data from other studies are consistent with these findings.7,10 Most of these analyses were cross-sectional, and few examined the relation of retinopathy to incident hypertension, independent of other risk factors. Only one study has examined the relation of retinopathy to incident hypertension. In a 3-year follow-up of the Atherosclerosis Risk in Communities (ARIC) Study cohort, after controlling for average systolic and diastolic blood pressures over the preceding 6 years, body mass index, waist-to-hip ratio, and other risk factors, the odds of developing hypertension were approximately 30% higher in nondiabetic normotensive persons with retinopathy present compared to those without retinopathy at baseline. However, the association was not statistically significant (P = .14).20 In a case-control study, Cugini and colleagues39 showed that normotensive nondiabetic persons with retinopathy have higher mean ambulatory systolic blood pressure compared to those without retinopathy. The higher risk of developing hypertension in our study may be due, in part, to higher mean ambulatory blood pressure that is not detected by casual blood pressure measurements.
Few other studies have investigated whether retinopathy in nondiabetic persons is a preclinical marker of future diabetes. Although a number of cross-sectional studies in nondiabetic individuals have shown that retinopathy signs may be associated with impaired glucose metabolism, not all studies have consistently linked retinopathy signs with hyperglycemia.6,40–42 In the Blue Mountains and Hoorn studies, retinopathy in nondiabetic participants was not related to higher fasting glucose levels.
There are few prospective data linking retinopathy in nondiabetic persons to subsequent risk of clinical diabetes. In the ARIC study, Wong and colleagues,19 while controlling for age, sex, race, blood pressure, fasting blood glucose levels, body mass index, and fasting insulin, found retinopathy not to be associated with the 3-year incidence of diabetes mellitus (OR, 1.1; 95% CI, 0.7 to 1.9). They attributed this to retinopathy in nondiabetic persons being a sign of the effect of higher blood pressure rather than hyperglycemia on the retinal vasculature. In that study, however, they reported that in persons with a family history of diabetes, retinopathy was associated with a higher odds (OR, 2.3) of developing diabetes. We did not have a family history of diabetes mellitus in our study.
We found an age effect in the relationship between retinopathy and incident diabetes. In persons less than 65 years, the presence of any retinopathy and blot hemorrhages only at baseline was associated with 15-year cumulative incident diabetes, after controlling for other covariates. This is consistent with previous studies, which show that the association of retinopathy signs with cardiovascular mortality is stronger in younger compared with older people.14 Only the association with blot hemorrhages remained after multivariable modelling with time-dependent covariates. This suggests that changes in glycemia, body mass index, hypertension status, and other processes over the period of the study may explain, in part, the association of any retinopathy with incident diabetes. It is not clear why blot hemorrhages, but not microaneurysms, in the absence of more severe retinopathy is a preclinical marker of diabetes in younger persons. In older people, retinopathy was not related to incident diabetes, as these signs may reflect more heterogeneous etiologies (eg, age, atherosclerosis), unrelated to diabetes.
The strengths of our study include it being a large population-based cohort with long-term follow-up using standard protocols for measuring blood pressure, diabetes status, and retinopathy. Its limitations include the possibility of misclassification of hypertension and diabetes status. Another relative limitation is the possibility of uncontrolled confounding. Selective survival and participation might have resulted if nondiabetic persons with retinopathy at baseline who developed diabetes or hypertension were more likely to die or not participate, reducing the ability to find a relation if it existed.
In summary, our data show that while controlling for other risk factors, retinopathy in nondiabetic individuals is associated with the incidence of hypertension. In younger nondiabetic persons less than 65 years of age, retinopathy, in particular blot hemorrhages, may additionally be related to incidence of diabetes. Our data suggest that blood pressure in normotensive nondiabetic persons with retinopathy should be more closely monitored for detection of hypertension.
PEER DISCUSSION
FREDERICK L. FERRIS III
Dr. Klein and his co-authors have utilized data they have collected during the fifteen year follow-up of patients enrolled in the Beaver Dam Eye Study to address a question that has concerned ophthalmologists for decades. One of the first prevalence studies of eye disease was the Framingham Eye Study.1 Participants in this study had careful eye examinations with fundus photographs in the mid 1970’s. It was noted that some study participants had microaneurysms without evidence of either diabetes or hypertension. The clinical significance of this observation was unclear, because there was no follow-up information on these individuals. Since that time, there have been numerous studies that have reported prevalent retinopathy in persons without diabetes or hypertension.2–8 The reported prevalence of retinopathy has ranged from 2% to 12%. Several of these reports have suggested an association with a possible increased risk of hypertension developing in persons with retinopathy compared with persons who did not have retinopathy.2,5,6 None of the studies has shown an increased risk of developing diabetes in participants who have retinopathy but do not have diabetes.
The fifteen year follow-up of persons with retinopathy in the Beaver Dam Eye Study provides the best information to date to address the question of whether the presence of some retinopathy in persons without diabetes or hypertension is a risk factor for the future development of these two systemic disorders. The cumulative fifteen-year incidence of diabetes and hypertension in the Beaver Dam Eye Study was 12.5% and 54.1% respectively, giving good power to find an association with either disorder if one was present. Overall, it would appear that having some retinopathy does increase one’s risk of developing either diabetes or hypertension. Those with retinopathy apparently have between a 50% increased risk and a doubling of risk for both disorders. When the population was divided into younger (<65 years old) and older groups of participants, there seemed to be a consistent risk for the development of hypertension in both groups, but the increased risk for developing diabetes may be limited to the younger group. Whether the risk of developing diabetes is only increased in persons younger than age 65 who have retinopathy, is not clear. This may be a chance observation in a subgroup analysis or it may be an important observation. It appears that the predictive association of blot hemorrhages with eventual diabetes may be more important than the association with microaneurysms. Microaneurysms (present in 7.3% of the population) seem weakly predictive across all age groups, while blot hemorrhages (present in only 1.4% of the population) may be associated with a two to four times increased risk of developing diabetic retinopathy, especially in persons 65 years old or younger.
Further studies will be necessary to assess the importance of blot hemorrhages as well as possible combinations of retinopathy lesions as predictors. It is particularly interesting that the point estimates for blot hemorrhages as risk factors for the development diabetes range from a three to fourfold increased risk for younger participants to a threefold apparent protection for older patients. The number of persons with these blot hemorrhages is relatively small and the confidence intervals around these risks are large, but this qualitative interaction deserves further investigation. In diabetic retinopathy blot hemorrhages are often associated with non-perfusion and they could be a sign of more severe vascular problems. As the authors suggest, it will be important to assess whether these blot hemorrhages or other retinopathy lesions are associated with competing systemic risks. For example, if blot hemorrhages are associated with an increased mortality risk, this competing risk could explain the apparent conflicting finding of an increased risk of incident diabetes in younger participants and a decreased risk in older participants. It would be important to know if persons with blot hemorrhages were more likely to die in the five year intervals between assessments of diabetes or hypertension. We look forward to these future investigations. In the meantime, the findings from the Beaver Dam Eye Study confirm the clinical suspicions that the presence of retinopathy may be important even if their presence only indicates a moderate increased risk of developing diabetes or hypertension.
REFERENCES
- 1.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study Monograph. Surv Ophthalmol. 1980;24(suppl):335–610. [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Wand Q. Blood pressure, hypertension and retinopathy in a population. Trans Am Ophthalmol Soc. 1993;91:207–226. [PMC free article] [PubMed] [Google Scholar]
- 3.Nagi DK, Pettitt DJ, Klein R, Knowler WC. Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Diabet Med. 1997;14:449–456. doi: 10.1002/(SICI)1096-9136(199706)14:6<449::AID-DIA367>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Yu T, Mitchell P, Berry G, Li W, Wang JJ. Retinopathy in older persons without diabetes and its relationship to hypertension. Arch Ophthalmol. 1998;116:83–89. doi: 10.1001/archopht.116.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard MAT, Brothers BS, King MS, et al. ARIC Study Group. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the atherosclerosis risk in communities study. Ophthalmolology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons. Ophthalmology. 2003;110:658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 7.van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003;121:245–251. doi: 10.1001/archopht.121.2.245. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Barr EL, Tapp RJ, et al. Diabetes Obesity and Lifestyle (AusDiab) study. Am J Ophthalmol. 2005;140:1157–1159. doi: 10.1016/j.ajo.2005.07.030. [DOI] [PubMed] [Google Scholar]
DR DENNIS M. ROBERTSON
Can you determine from your database whether some of these patients may have endocrine ophthalmopathy or Graves Disease? We were surprised in a recent study from Mayo Clinic in which the patients with endocrine ophthalmopathy were randomized for treatment with external radiation to determine if the ophthalmopathy would be reduced. Pre-treatment, a number of patients with Graves Disease had microaneursyms and we surmised that these patients might have a higher sensitivity to developing complications induced by these minimal doses of radiation. When we reviewed the literature, we did not find any studies other than ours that identified micro aneurysms associated with Graves Disease.
DR LEE M. JAMPOL
“Retinopathy” is a noun in search of an adjective. You are looking for an etiologic adjective to describe this entity and that is certainly worthwhile. Meanwhile, I have always thought that you need a descriptive adjective to give this entity a name. Many people are confused when they read your publications about “retinopathy,” they are not sure what you are describing.
DR RONALD KLEIN
The relationship of blot hemorrhages to death is very important and we looked at this retinal lesion as a risk factor for mortality. In persons with diabetes, we have found a relationship of the presence and severity of retinopathy with higher risk of mortality.1 While controlling for other risk factors we have found no relationship of either retinal microanuerysms or blot hemorrhages with overall mortality in the nondiabetic Beaver Dam population (Klein R et al. unpublished data).
We have never looked at the relationship of Graves disease with retinopathy suggested by Dr Robertson. We have obtained a history of thyroid disease as well as a history of radiation, and plan to examine this relationship in the Beaver Dam population.
I agree with Dr Jampol’s comments. When we know essential hypertension is present in the absence of diabetes we often label the presence of microaneurysms and blot hemorrhages as hypertensive retinopathy. When diabetes is present, we call it diabetic retinopathy.2 Many of you may be aware of the New England Journal of Medicine paper that came out about the COL4A1, which is a a gene encoding type IV collagen α1 (COL4A1), a basement-membrane protein, mutationsof which may affect small blood vessels.3 Genetic epidemiological studies may better enable us to label retinopathy with an adjective for the noun. This information may be of help in what to tell an individual without diabetes or hypertension in terms of their prognosis who is found to have retinal microaneursyms and blot hemorrhages. What I did not mention in the paper is that nearly all of the lesions disappear over the five-year intervals and that is very different from the natural history of lesions found in diabetic retinopathy.
REFERENCES
- 1.Klein R, Klein BEK, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117:1487–1495. doi: 10.1001/archopht.117.11.1487. [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Mitchell P. Hypertensive retinopathy. NEJM. 2004;35:2310–2317. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 3.Gould DB, Phalan FC, van Mil SE, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. NEJM. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.McDonough JJ, Garrison GE, Hames CG. Blood pressure and hypertensive disease among negroes and whites; a study in Evans, County, Georgia. Ann Intern Med. 1964;61:208–228. doi: 10.7326/0003-4819-61-2-208. [DOI] [PubMed] [Google Scholar]
- 2.Svardsudd K, Wedel H, Aurell E, et al. Hypertensive eye ground changes. Prevalence, relation to blood pressure and prognostic importance. The study of men born in 1913. Acta Med Scand. 1978;204:159–167. [PubMed] [Google Scholar]
- 3.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24:335–610. [PubMed] [Google Scholar]
- 4.Klein R. Retinopathy in a population-based study. Trans Am Ophthalmol Soc. 1992;90:561–594. [PMC free article] [PubMed] [Google Scholar]
- 5.Stolk RP, Vingerling JR, de Jong PT, et al. Retinopathy, glucose, and insulin in an elderly population. The Rotterdam Study. Diabetes. 1995;44:11–15. doi: 10.2337/diab.44.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Yu T, Mitchell P, Berry G, et al. Retinopathy in older persons without diabetes and its relationship to hypertension. Arch Ophthalmol. 1998;116:83–89. doi: 10.1001/archopht.116.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis? The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20:1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Sharp PS, Chaturvedi N, Wormald R, et al. Hypertensive retinopathy in Afro-Caribbeans and Europeans. Prevalence and risk factor relationships. Hypertension. 1995;25:1322–1325. doi: 10.1161/01.hyp.25.6.1322. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Wu SY, Hyman L, et al. Diabetic retinopathy in a black population: the Barbados Eye Study. Ophthalmology. 1999;106:1893–1899. doi: 10.1016/s0161-6420(99)90398-6. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, et al. Hypertension and retinopathy, arteriolar narrowing, and arteriovenous nicking in a population. Arch Ophthalmol. 1994;112:92–98. doi: 10.1001/archopht.1994.01090130102026. [DOI] [PubMed] [Google Scholar]
- 11.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: The Atherosclerosis Risk in Communities Study. Stroke. 2002;33:1487–1492. doi: 10.1161/01.str.0000016789.56668.43. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: The Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekin O, Cukur S, Uraldi C, et al. Relationship between retinopathy and cognitive impairment among hypertensive subjects. A case-control study in the Ankara-Pursaklar region. Eur Neurol. 2004;52:156–161. doi: 10.1159/000081855. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA. 2005;293:63–69. doi: 10.1001/jama.293.1.63. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama T, Date C, Yokoyama T, et al. A 15.5-year follow-up study of stroke in a Japanese provincial city. The Shibata Study. Stroke. 1997;28:45–52. doi: 10.1161/01.str.28.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Mohamed Q, Klein R, et al. Do retinopathy signs in non-diabetic individuals predict the subsequent risk of diabetes? Br J Ophthalmol. 2006;90:301–303. doi: 10.1136/bjo.2005.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 21.Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134:1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Klein BE, Linton KL, et al. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period: the Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BEK, Lee KE, et al. Changes in visual acuity in a population over a 15-year period. The Beaver Dam Eye Study. Am J Ophthalmol. 2006 doi: 10.1016/j.ajo.2006.06.015. In Press. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE. The Beaver Dam Eye Study. Manual of Operations. US Department of Commerce. Springfield, Va: NTIS Accession No. PB91-149823, 1991.
- 27.Klein R, Klein BE. The Beaver Dam Eye Study II. Manual of Operations. US Department of Commerce. Springfield, Va: NTIS Accession No. PB95-273827, 1995.
- 28.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. US Department of Commerce. Springfield,Va: NTIS Accession No. PB91-184267, 1991. [DOI] [PubMed]
- 29.Hypertension Detection and Follow-up Program Cooperative Group. The hypertension detection and follow-up program. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 30.Stein MW. D-glucose determination with hexokinase and glucose-6-phosphate dehydrogenases. In: Bergmeyer HC, ed. Methods of Enzymatic Analysis New York: Academic Press; 1963:177.
- 31.Klenk DC, Hermanson GT, Krohn RI, et al. Determination of glycosylated hemoglobin by affinity chromatography: comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clin Chem. 1982;28:2088–2094. [PubMed] [Google Scholar]
- 32.Diabetic Retinopathy Study Research Group. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21:210–226. [PubMed] [Google Scholar]
- 33.Klein BE, Davis MD, Segal P, et al. Diabetic retinopathy. Assessment of severity and progression. Ophthalmology. 1984;91:10–17. doi: 10.1016/s0161-6420(84)34374-3. [DOI] [PubMed] [Google Scholar]
- 34.Klein R, Klein BE, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93:1183–1187. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 35.SAS Institute Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute Inc, 1999.
- 36.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 37.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 38.Hosmer DW Jr, Lemeshow S. Applied Logistic Regression New York: John Wiley & Sons; 1989:238–245.
- 39.Cugini P, Cruciani F, Turri M, et al. ‘Minimal-change hypertensive retinopathy’ and ‘arterial pre-hypertension,’ illustrated via ambulatory blood-pressure monitoring in putatively normotensive subjects. Int Ophthalmol. 1998;22:145–149. doi: 10.1023/a:1006256106959. [DOI] [PubMed] [Google Scholar]
- 40.Wong TY, Barr EL, Tapp RJ, et al. Retinopathy in persons with impaired glucose metabolism: the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Am J Ophthalmol. 2005;140:1157–1159. doi: 10.1016/j.ajo.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 41.van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003;121:245–251. doi: 10.1001/archopht.121.2.245. [DOI] [PubMed] [Google Scholar]
- 42.Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk in Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]