Abstract
Purpose
The goal is to analyze the long-term visual outcome of extremely low-birth-weight children.
Methods
This is a retrospective analysis of eyes of extremely low-birth-weight children on whom vision testing was performed. Visual outcomes were studied by analyzing acuity outcomes at ≥36 months of adjusted age, correlating early acuity testing with final visual outcome and evaluating adverse risk factors for vision.
Results
Data from 278 eyes are included. Mean birth weight was 731g, and mean gestational age at birth was 26 weeks. 248 eyes had grating acuity outcomes measured at 73 ± 36 months, and 183 eyes had recognition acuity testing at 76 ± 39 months. 54% had below normal grating acuities, and 66% had below normal recognition acuities. 27% of grating outcomes and 17% of recognition outcomes were ≤20/200. Abnormal early grating acuity testing was predictive of abnormal grating (P < .0001) and recognition (P = .0001) acuity testing at ≥3 years of age. A slower-than-normal rate of early visual development was predictive of abnormal grating acuity (P < .0001) and abnormal recognition acuity (P < .0001) at ≥3 years of age. Eyes diagnosed with maximal retinopathy of prematurity in zone I had lower acuity outcomes (P = .0002) than did those with maximal retinopathy of prematurity in zone II/III. Eyes of children born at ≤28 weeks gestational age had 4.1 times greater risk for abnormal recognition acuity than did those of children born at >28 weeks gestational age. Eyes of children with poorer general health after premature birth had a 5.3 times greater risk of abnormal recognition acuity.
Conclusions
Long-term visual development in extremely low-birth-weight infants is problematic and associated with a high risk of subnormal acuity. Early acuity testing is useful in identifying children at greatest risk for long-term visual abnormalities. Gestational age at birth of ≤ 28 weeks was associated with a higher risk of an abnormal long-term outcome.
INTRODUCTION
Prematurity (defined as gestational age <37 weeks at birth) and low birth weight (defined as infants weighing <2,500 g at birth) are the leading causes of neonatal mortality and morbidity in the United States. More reliable public health statistics are maintained for birth weight than for gestational age. In the United States there were 4,091,063 births in 2003.1 Of these, 12.3% (~503,200) were preterm births, occurring at <37 completed weeks of gestation, and 7.9% (~323 194) were low-birth-weight (LBW) infants. In 2003, 1.4% of births (~ 57,275) were very low-birth-weight (VLBW) infants weighing <1,500 g at birth.1,2 For 2002 (the most recent year of complete data), the National Center for Health Statistics at the Centers for Disease Control and Prevention, reported 29,113 (0.7%) extremely low-birth-weight (ELBW) infants weighing <1,000 g at birth.3 Of these births, 6,268 (21.5%) were infants born weighing <500 g.3
Although survival of infants with birth weights below 500 g and/or less than 24 weeks of gestation occurs very infrequently, the National Institute of Child Health and Human Development reports that more than 70% survival of infants born weighing 501 to 1,000 g.4 Similarly, most large centers report that 50% of infants weighing 500 to 700 g commonly survive.5 These smallest of infants, however, run the largest risk of long-term morbidity.
The major, permanent complications of extreme prematurity include neurological deficits (cerebral palsy or mental retardation) and visual impairment or blindness. About 9% of survivors sustain severe intracranial hemorrhages and another 7% have periventricular leukomalacia, both of which are associated with development of cerebral palsy. 4 Only limited information exists on the long-term visual outcomes of ELBW infants.
RATIONALE
As a sense, vision contributes significantly to the social and economic success of an individual. With increasing numbers of neurologically and visually impaired ELBW infants surviving, it becomes more important to understand the origin, development, and impact of these impairments. Understanding is especially critical for the families of children at risk for a lifetime of blindness or significant visual impairment.
Several studies have addressed the predictive value of infant acuity testing for long-term outcomes in LBW children. Infant preferential-looking acuity is predictive of acuity outcome in cohorts of children with retinopathy of prematurity (ROP)6 following intraventricular hemorrhage7 and cystic leukomalacia.8 None of these studies focused on ELBW infants, and none of them examined the rate of acuity development during infancy as a predictive factor for long-term acuity.
It is the smallest and least mature infants at birth who are at greatest risk of having the most limited, posterior area of retinal vascularization at birth. These eyes are the most likely to develop significant ROP in zone I of the retina, as defined by the International Classification of Retinopathy of Prematurity (ICROP).9 Zone I ROP has consistently been associated with poor long-term visual acuity outcomes in more than 75% of cases.10–12 Most of the children with zone I disease also had developmental delay and neurological disability.
HYPOTHESES
The hypotheses of this thesis are as follows:
Long-term acuity outcomes of eyes in ELBW children will be reduced when compared with those of age-matched, normal children born at term.
Grating acuity results during infancy will be predictive of long-term acuity outcomes.
Children with a history of zone I ROP will have poorer long-term acuity outcomes than children with zone II or III disease.
The smallest and sickest infants will have the poorest long-term acuity outcomes.
AIMS OF THESIS
The overall goal of this thesis is to analyze the long-term visual outcome in eyes of ELBW children. To achieve this goal, the four specific aims of this thesis are to:
Compare the long-term grating acuity and recognition acuity outcomes with age-matched, normal children born at term.
Evaluate the predictive value of grating acuity tests conducted during infancy for long-term acuity outcome.
Determine the impact of zone I disease on acuity outcome
Analyze the impact of neonatal factors on acuity outcome.
HISTORY OF PREMATURE BIRTHS
Despite ongoing research into the causes and methods of preventing or stopping preterm labor, the rate of LBW deliveries remained relatively stable at 6.8% to 8% from 1950 through 1990.13 During the 1990s, however, the rate of preterm births increased in the United States.14, 15 Demissie and coworkers14 report that preterm births increased 15.6% (from 8.8% to 10.2%) between 1989 and 1997 among whites in the United States, whereas the preterm birth rate among blacks decreased by 7.6% (from 19.0% to 17.5%). Branum and Schoendorf15 note that between 1981 and 1998, the preterm birth rate increased 23% among white and 3% among black infants.
Physicians observed as early as the 1930s that prematurely born infants, if given supplemental oxygen to breathe after birth, could be kept alive at a much higher rate than infants left only in room air. Following this observation, numerous subsequent advances in neonatal care led to dramatically increased weight-specific survival rates in preterm, LBW infants during the next 60 years. The most remarkable increases in survival rates have occurred in the ELBW category, from 6% in 1950 to 195416 to 26% in 1973 to 1975,17 with some centers reporting survival rates of 50% in infants weighing 500 to 1,000 g born in 1987-1988.18 During the 1990s, the widespread use of surfactant increased this survival rate even further.19, 20 For decades, however, physicians raised concern that increased survival rates of smaller and smaller infants, unaccompanied by decreased morbidity rates, would lead to increased numbers of impaired survivors. Recent reports tend to substantiate these concerns.
In 1989, Aylward and colleagues21 performed a meta-analysis of 80 papers published during the 1980s. They found that the LBW group had significantly lower intelligence quotients/developmental quotients than control subjects (97.8 vs 103.8). However, they did not find a difference in the mean intelligence quotients/developmental quotients (IQ/DQ) scores among the LBW, VLBW, and ELBW subgroups.
In 1998, Horwood and coworkers22 published their results from long-term follow-up of cognitive, behavioral, and educational outcomes in a cohort of 298 children of VLBW in New Zealand who were born in 1986. They assessed behavior, cognitive ability, school performance, and the need for special education at 7 to 8 years of age (middle childhood) and compared these measures with the same measures in the general population of similarly aged children. They showed significantly higher rates of problems and poorer levels of functioning across all outcome measures in the VLBW group compared to that of the general population. Differences persisted even after controlling for differences in social, family, and other characteristics of the two groups. Also, differences persisted despite controlling for the degree of sensorineural disability. Furthermore, they found evidence of an increasing risk of problems with behavior, cognitive ability, school performance, and the need for special education with decreasing birth weights, noting that ELBW children had higher rates of problems and difficulties than others in this VLBW cohort.
Also, in 1998, Wolke23 reported that 25% of VLBW children had severe or multiple psychological problems and an additional 25% had moderate or mild problems. Most prevalent were attention deficit, lowered IQ, and schooling problems.
In a group of 283 infants that survived extremely premature birth (22 to 25 weeks of gestational development), Wood and associates24 found that at 30 months corrected age, 30% had delayed development that was 2 SD or more below normal as measured by the Bayley Mental and Psychomotor Developmental Indexes. They reported that 10% had severe neuromotor disability, 10% had useful but not fully correctable vision, 3% had significant hearing loss, and 2% were blind with only light perception vision or less.
Cognitive ability leading to educational disadvantage in young adulthood has been documented by Hack and associates.25 They compared a group of 242 VLBW infants born between 1977 and 1979 (mean birth weight, 1,179 g; mean gestational age at birth, 29.7 weeks) with 233 controls from the same population with normal birth weights. The VLBW group had a lower mean IQ (87 vs 92) and lower academic achievement scores (P < .001 for both comparisons). The VLBW group was less likely to have graduated from high school (74% vs 83%, P = .04), and the male VLBW subgroup, but not the female subgroup, was significantly less likely than the normal birth weight controls to be enrolled in post–high school studies (30% vs 53%, P = .002). The VLBW group also had higher rates of neurosensory impairments (10% vs <1%, P = .001).
HISTORY OF RETINOPATHY OF PREMATURITY
In 1942, Terry26,27 observed the development of a new problem in the eyes of prematurely born infants that he termed retrolental fibroplasia (RLF). By 1950, RLF was identified as a major public health problem for the increasing number of surviving prematurely born infants and the leading cause of blindness in children in the United States.28 Research studies found a strong relationship between the development of RLF (now called retinopathy of prematurity, or ROP) and the unmonitored use of high levels of supplemental oxygen.29–34 Toward the end of the 1950s, restricted use of supplemental oxygen dramatically reduced the incidence of blindness from ROP, but mortality and morbidity increased.35 The number of deaths from hyaline membrane disease of the lungs36 also increased significantly during this time, along with a higher incidence of cerebral palsy from hypoxia of the brain.37
By the 1960s, knowledge expanded about the treatment of respiratory distress syndrome in premature infants.38 At the same time, technology advanced enough to allow monitoring of oxygen use in infants. New techniques included arterial-blood gas analysis, transcutaneous oxygen monitoring, and the use of continuous-pulse oximetry monitoring. These advances in monitoring techniques played a key role in lowering the incidence of blindness from ROP in the 1970s, despite an increased survival of VLBW infants.39 Nonetheless, as more and more smaller and less mature infants survive, we see less of an effect of oxygen monitoring on lowering the incidence of ROP,40,41 which is increasing at a rate inversely proportionate to the birth weight and gestational age of surviving infants. Some described this effect as a “second epidemic” of ROP in the industrialized countries of the world39 and as a first epidemic in those middle and lower income countries that experienced an improvement in neonatal care of prematurely born infants during the last decade.42–44
PATHOGENESIS OF RETINOPATHY OF PREMATURITY
The role of oxygen as an etiologic factor in the production of ROP was first suggested by Campbell in 1951.45 Ashton46 proposed the concept of ROP as a sequence of events beginning with vasoconstriction due to increased retinal oxygen exposure followed by vaso-obliteration and nonperfusion. He theorized that subsequent vasoproliferation occurred in response to a vasoformative factor elaborated from hypoxic retina. At the time, little evidence supported this theory. This proposed mechanism relied on the observed vasoconstriction, and the remainder of the theoretic sequence was without direct support. It assumed that nonvascularized peripheral retina was ischemic despite higher-than-normal choroidal oxygen tensions and also that developing endothelial cells behave similarly to mature cells in diabetic retinopathy.
In the 1980s, Kretzer and Hittner47–50 proposed modifications to the Ashton hypothesis of ROP pathophysiology. Their theory held that mesenchymal spindle cells, derived from the hyaloid artery, invade the nerve fiber layer and migrate centrifugally in the avascular retina. As these primitive cells migrate, they differentiate into endothelial cells that form the developing, expanding retinal vasculature. It is this interface of differentiating mesenchymal spindle cells and newly forming vascular endothelium that is the site of insult in ROP. Increased oxygenation, with its attendant free-radical generation, overwhelms the immature antioxidant systems of the preterm retina. Loss of control over differentiation occurs, and the damaged mesenchymal cells form abnormal neovascular changes at the leading zone of retinal vascular development.
Kretzer and Hittner used electron microscopic examination of postmortem preterm retinas to assess cell damage to the mesenchymal spindle cells. They demonstrated an increase in gap junctioning between adjacent spindle cells in eyes that developed ROP. Spindle cell gap junctions serve primarily as indicators of cell membrane dysfunction. Thus an increase in the number of these gap junctions was interpreted as reflecting an abnormal state of stress placed upon the developing retinal vascular system.47,48
Kretzer and Hittner also demonstrated an increase in rough endoplasmic reticulum in the spindle cells associated with increased numbers of gap junctions. They theorized that the increase in rough endoplasmic reticulum (RER) indicated an increased cellular secretion of angiogenic factors.49,51 Finally, they proposed that the increased gap junctioning of spindle cells and increased RER would decrease (down-regulate) as the cells matured. This down-regulation would also be accompanied by myofibroblasts invading the vitreous, leading to vitreous contraction and tractional retinal elevation.51
Although some investigators still subscribed to Ashton’s theory,52 the new ideas proposed by Kretzer and Hittner had the elegance of being grounded in hard evidence and demonstrated physiology. Also, the new ideas coincided with the authors’ strident but controversial advocacy of vitamin E therapy for ROP.53–57
In both theories, Ashton’s or Kretzer and Hittner’s, the common denominator is the cytotoxicity of oxygen via free radical production and the lack of mature antioxidant mechanisms to deal with it in the youngest preterm infants. Penn and associates58 validated this concept of an oxygen-induced injury and subsequent nonperfusion leading to neovascularization in the retina.
The process of neovascularization of the retina was further elucidated with Miller’s description of vascular endothelial growth factor as the stimulant for ocular neovascularization.59 In the process of normal retinal vascularization, a delicate balance exists between hypoxia that develops in the differentiating, thickening fetal retina and the release of vascular endothelial growth factor (VEGF) that stimulates vascular growth. Provis and coworkers60 have shown histologically that VEGF expression in the normal fetal retina occurs just anterior to the leading edge of the developing retinal vessels.
In the retina of a prematurely born infant, although retinal differentiation continues, retinal vascularization may be stopped or delayed by oxidative stresses and other injuries to the developing vascular precursor cells. This delay, in turn, leads to hypoxia in which excessive amounts of VEGF production occur in the area of demarcation between the vascularized and nonvascularized retina stimulating the neovascular process of ROP.61–64
FACTORS AFFECTING VISUAL DEVELOPMENT OF PRETERM INFANTS
It is known that premature birth can have a range of effects on the visual system ranging from ROP-related complications of the eye to visual sequelae ascribed to cortical complications of preterm delivery. These effects may lead to a lifetime of visual impairment. Crofts and associates65 have quantified the risk of visual impairment as being 26 times greater for VLBW infants than for those with birth weights of 2,500 to 3,000 g.
Structures in the visual system mature according to a genetically preprogrammed timeline beginning at conception. Preterm birth presents the incompletely developed visual system with a variety of situations not encountered in full-term visual development. Preterm infants are subjected to two basic alterations from normal that can affect the processes of normal visual development.66,67 First, the visual system is exteriorized prematurely, and second, it is subjected to diseases and complications that are known to affect prematurely born infants.
The preterm birth exposes the developing visual system to numerous environmental exposures from which it would otherwise be protected in utero. These differences include higher-than-normal oxygen tension, light exposure, and nutritional changes. The effect of elevated oxygen exposure on the developing retina and its relationship to ROP have been detailed already in the section “History of Retinopathy of Prematurity.”
The developing, in utero fetus essentially has no light exposure or stimulation of the visual system. When born prematurely, the child is thrust into a lighted environment that is very different. Despite recent trends to reduce and cycle lighting in the nursery,68–72 this new environment creates light exposure of the ocular structures and early stimulation of the visual system.
Precocious light exposure has been theorized to play a role in affecting both structure and function of the developing visual system. In 1998, it was proven that light exposure does not have an effect on the development of ROP.73 Furthermore, information from this study and others has shown that an early visual experience for the eye and brain neither accelerates nor delays the normal timing of visual development as measured by psychophysical or electrophysiological means.6,74–77
Prior to birth, the fetus receives all its nourishment by the placental transfer of nutrients from the mother. The visual system has very stringent nutritional requirements, especially as they pertain to the development of photoreceptor outer segments and cell membranes in the visual cortex. In both of these tissues, over 60% of their structural materials are lipids that have a high concentration of docosahexaenoic acid.78,79 Once separated from the maternal supply of docosahexaenoic acid, the preterm infant is at significant risk for docosahexaenoic acid deficiency.80,81 This is because the preterm infant has limited capacity to synthesize docosahexaenoic acid from its precursor fatty acids. Thus, it cannot meet the demands of the growing tissues for docosahexaenoic acid if there is no direct dietary supply. Although present in breast milk, until 2002, docosahexaenoic acid was not present in commercially available infant formulas in the United States. Without a dietary source of docosahexaenoic acid, preterm infants demonstrate poorer visual function than those having a dietary source of docosahexaenoic acid.80,81
The second set of factors that can affect the process of normal visual development in a preterm infant consists of disease, complications, and untoward effects related to the preterm birth. ELBW infants are particularly at risk for these factors. Complications of prematurity, including sepsis,82–84 intraventricular hemorrhage,7,85 periventricular leukomalacia, and necrotizing enterocolitis, are recognized risk factors for ROP and cortical visual impairment.
LONG-TERM OUTCOME
Three major epidemiological studies of long-term outcomes in LBW infants have been reported.86–89 All three studies included only very small numbers of children with birth weight <1,001 g, and their cohorts were biased toward larger LBW infants with only mild ROP. Nonetheless, these studies report poor visual outcomes in about 3% of the preterm population and severe visual impairment in about 1%. In addition, these studies found higher rates of refractive error, strabismus, and cortical visual impairment among children with a history of preterm birth than among age-matched children who were born at term.
There is a higher prevalence of myopia among preterm infants than among full-term infants.90,91 Within the preterm population, myopia is associated with poorer general health status as well as severity of ROP and treatment with cryotherapy. Other refractive errors, including astigmatism and anisometropia, are also more prevalent among children with a history of preterm birth than among children born at term.92,93 Uncorrected refractive errors may lead to chronic blurring that, in turn, may affect long-term visual acuity development. Myopia of prematurity is a special case in which myopia may progress so rapidly that it may cause visual deprivation amblyopia. Anisometropic refractive errors place the child at risk for secondary visual disorders, including amblyopia and strabismus.
The prevalence of strabismus among preterm infants and children ranges from about 3% in 6-month-olds without ROP to 57% in 5-year-olds who were born at less than 28 weeks postconception.94,95 The high prevalence of strabismus appears to be related to the high prevalence of refractive errors, severity of ROP, and general health status. Not only is strabismus more prevalent among preterm than full-term children, but the types of strabismus are different; exotropia is more common among LBW children. Strabismus presents a risk for amblyopia and abnormal binocular vision, including reduced stereoacuity.
Cortical visual impairment in preterm children is often associated with congenital infections or malformations, perinatal asphyxia, intraventricular hemorrhage, periventricular leukomalacia, or other neurological disorders. Severe intraventricular hemorrhage is strongly associated with poor acuity outcome, strabismus, and nystagmus.96,97 Cortical visual impairment is often clustered with other visual deficits, including ocular motility disturbances, optic nerve hypoplasia, and reading disorders.
NEONATAL FACTORS
It is difficult to sort out the effects of complications of preterm birth from the direct effects of ROP on long-term visual outcome. Complications including sepsis, intraventricular hemorrhage, periventricular leukomalacia, and necrotizing enterocolitis are recognized risk factors for both ROP and cortical visual impairment. Within the ELBW group, these conditions are highly prevalent and, along with general health status indices (such as length of hospital stay and number of days ventilated), must be considered in the interpretation of risk for poor long-term acuity.
STUDY SUBJECTS AND METHODS
STUDY SUBJECT RECRUITMENT AND INFORMATION
Subjects were recruited by searching the database of patients evaluated at the Pediatric Laboratory at the Retina Foundation of the Southwest in Dallas, Texas, between 1983 and 2002. The following inclusion criteria were used:
Patients, who as infants, underwent ophthalmic assessment at the Retina Foundation of the Southwest
Birth weight of ≤ 1,000 g
Patients currently more than 3 years old according to adjusted age (Adjusted age is equal to the chronologic age of the prematurely born infant minus the amount of prematurity.)
Children identified as meeting the above criteria were then contacted and asked to return for follow-up testing. Recruitment of the potential study subjects consisted of a minimum of three telephone calls to the parents or guardian asking them to return the child for follow-up. If telephone contact was unsuccessful, then a letter was sent to the last known address for the patient. If the address was no longer valid, then the patient’s ophthalmologist was contacted for updated address information, and local telephone directories and the Internet were searched for a new address and phone number. Information on patients was gathered from a variety of sources, including hospital records, office charts, parent interviews, and directly from queries of referring physicians (usually ophthalmologists).
Information for each eye of each patient was entered into a computerized database using the Microsoft Excel application. Demographic information recorded for each patient included last name, first name, gender, gestational age at birth in weeks, birth weight, name of referring physician, and adjusted age at visual evaluation. Additional information regarding length of stay in hospital after birth, total number of days of ventilation, number of readmissions to hospital in first year of life, necrotizing enterocolitis (yes or no), intraventricular hemorrhage (no, grade mild or grade moderate/severe), periventricular leukomalacia (yes or no), and sepsis (yes or no) was gathered from an interview with the parents or guardian, usually at the time of the initial visual acuity testing. Necrotizing enterocolitis, periventricular leukomalacia, intraventricular hemorrhage, sepsis, and total number of days of ventilation were used as proxies for severity of illness after birth. In addition, the results of orthoptic and sensory testing as described below were recorded in the database record. The refractive error for each eye was recorded in the database.
Information regarding ROP in each eye was gathered either directly from the patients’ records and/or from direct communication of the author with the examining pediatric ophthalmologist. All ROP assessments were performed either by the author or by a pediatric ophthalmologist trained by the author in ROP examination techniques. Both the author and the examining pediatric ophthalmologist were certified Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) Study examiners who screen infants at local neonatal intensive care units on a weekly basis. The stage and zone of ROP was graded according to ICROP.98,99 Information recorded in the database included cicatricial outcome grading of ROP; the use of interdictive therapy, such as laser photocoagulation or cryotherapy; and corrective surgical interventions for ROP, such as scleral buckling, vitrectomy, and vitrectomy with lensectomy. In addition, when available, the zone of ROP at the time of maximal stage of ROP was recorded. Also entered into the database was information regarding other eye conditions, such as cataract, glaucoma, aphakia, nystagmus, amblyopia, and corneal abnormality.
The author obtained all sensory outcome data as part of routine clinical, ophthalmological follow-up or during attendance at a research laboratory visit at the Retina Foundation of the Southwest. Examinations of all study subjects included visual acuity testing by grating acuity techniques and, in some cases, by recognition acuity measurement. A subset of the study subjects underwent additional follow-up testing, including orthoptic assessment and completion of a Children’s Visual Function Questionnaire and Developmental Skills Survey by the parent(s) or guardian, contrast sensitivity testing, and stereopsis.
SENSORY TESTING
Visual Acuity Measurements
Visual acuity testing of all study subjects was performed by postdoctoral fellows who were trained in techniques of grating and recognition acuity testing of preverbal infants and children. All testing was performed in the Pediatric Eye Research Laboratory at the Retina Foundation of the Southwest. Grating acuity testing was chosen as a technique because it could be performed on all study subjects regardless of their verbal skills or cognitive abilities. Recognition acuity testing was used whenever possible in addition to grating acuity testing because it not only evaluates resolutional ability of the visual system but reflects some cognitive processes in conjunction with the visual system.
Grating Acuity Testing
Grating acuity testing was performed on all infants and children using a forced-choice preferential looking (FPL) protocol.100 For the FPL protocol testing, Teller acuity cards were used as stimuli (Figure 1). The mean luminance of the acuity cards was 1.60 log candelas per square meter. For infants, an observer made a forced choice as to the preferred direction of an infant’s gaze for each card (Figure 2). Older children were asked to point to the location of the grating on a demonstration card. All test sessions began with low spatial frequency stimulus (.23 cycles/degree) and progressed to higher frequency stimuli. A two down–one up staircase procedure was used to converge on the acuity threshold in eight reversals. Step size was approximately one octave (two cards) until the first reversal was obtained. Then, step size was reduced to approximately .5 octave (one card) for the remainder of the test. Acuity was estimated as the mean of the last six reversals. Normative acuity data are available for this protocol, including mean acuity and 95% tolerance limits.101
FIGURE 1.
Six Teller acuity cards showing varying grating widths from fine (on left) to coarse (on right). Note “peephole” in center of each card for observation of eye movement.
FIGURE 2.
Monocular forced-choice preferential looking grating acuity testing. Toddler (held by mother) points at gratings on Teller cards, which are presented by tester/observer.
Recognition Acuity Testing
Recognition visual acuity was tested using crowded HOTV optotypes displayed on an electronic visual acuity system.102 Figure 3 shows a typical presentation of the letter “O” using this system. The Amblyopia Treatment Study (ATS) acuity testing protocol was used.103 This recognition acuity testing was selected in lieu of the Early Treatment Diabetic Retinopathy Study protocol because the young age of the study subject population made it more likely to achieve a reliable and reproducible testing session.
FIGURE 3.
Crowded Letter “O”: Letter “O” with crowding bars around it, as used for HOTV recognition acuity testing.
Children were asked either to name the letters or to match the letters by pointing to the appropriate letter on a matching card provided to them. The ATS protocol employs four stages for testing a child’s acuity in each eye. First, screening of acuity is performed by having the subject read one letter per line starting with the 20/100 optotype down to the point of failure. Then, the second stage starts by moving back up two lines from the missed letter and then going down to smaller letter lines until at least two letters are missed on a line. The third stage is the reinforcement stage that requires the tester to go back up three lines from the last missed line and present only one letter on each line, again moving toward smaller letters. This is done with some verbal encouragement. Last, stage 4 requires the tester to repeat the last missed line of letters in stage 3 and continue until two of the four letters on the line are missed. Using the ATS protocol, acuity is scored as the smallest letter size for which at least three presentations (three or three or three of four) are correctly identified. This test protocol is currently in wide use for randomized clinical trials in pediatric ophthalmology; normative data are available, including mean acuity and 95% tolerance limits.104–107
Visual acuity is reported as the logarithm of the minimal angle of resolution (logMAR). Minimal angle of resolution is calculated by taking the inverse of the visual acuity fraction. For instance, 20/40 becomes 2 and 20/100 becomes 5. Their logarithms of the minimal angle of resolution (logMARs) are .3 and .7, respectively. LogMAR and common Snellen vision equivalents are shown in Table 1. In order to include low levels of vision in the analysis, light perception and no light perception were coded as 2.0 and 2.5 logMAR, respectively. Owing to the asymmetry of ROP and other eye conditions between the two eyes of a given patient, all visual acuity results are presented as eyes rather than as patients.
TABLE 1.
LOGMAR VISUAL ACUITIES AND THEIR SNELLEN VISUAL ACUITY EQUIVALENTS
| logMAR | .1 | 0 | .1 | .2 | .3 | .4 | .5 |
| Snellen | 20/15 | 20/20 | 20/25 | 20/32 | 20/40 | 20/50 | 20/60 |
| logMAR | .6 | .7 | .8 | .9 | 1.0 | 1.1 | 1.2 |
| Snellen | 20/80 | 20/100 | 20/125 | 20/160 | 20/200 | 20/250 | 20/320 |
| logMAR | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 2.0 | 2.5 |
| Snellen | 20/400 | 20/500 | 20/630 | 20/800 | 20/1000 | LP | NLP |
logMAR = log of minimal angle of resolution; LP = light perception; NLP = no light perception.
Since the range of normal acuity varies considerably with age during the first 3 years of life, acuities in preterm children were compared to norms for adjusted-age at the time of testing. The acuities measured were compared with the mean of normal for age and also with the lower limit of the normal range for age. The lower limit of the normal range is 1.96 SD below the mean of normal and includes 95% of the normal population.
ORTHOPTIC ASSESSMENT
Orthoptic assessment was performed by a trained orthoptist. It included evaluation of ductions and versions, cover/uncover testing, and alternate cover testing to detect the presence of manifest and latent strabismus. If a child was found to have a manifest strabismus, then a prism cover test was used to measure the angle of deviation at distance and near fixation. The presence or absence of strabismus was evaluated because of its importance as an indicator for the potential of amblyopia as a cause of a visual deficit.
The refractive error of each eye was evaluated in one of three ways. All study subjects were under the ongoing care of a pediatric ophthalmologist. If the child wore glasses, then the ophthalmologist was asked about the refractive status. If that was not possible, then the child’s glasses were read with a lensometer and the reading recorded. In children who did not wear glasses, a Nikon Retinomax K-plus Auto Refract Keratometer (Nikon USA, Melville, New York) was used to screen for significant, previously undetected refractive errors.
RESULTS
STUDY SUBJECTS
A total of 149 patients (298 eyes) who underwent ophthalmic assessment at the Retina Foundation of the Southwest were identified as having a birth weight of ≤ 1,000 g and being more than 3 years of adjusted age. Of these 149 patients, 10 were excluded because of lost patient records (9) and shaken baby syndrome (1).108–110 (The child with shaken baby syndrome was excluded because of the possibility that the cause of any visual abnormality might be due to injury rather than to factors related to prematurity.) The remaining 139 children (278 eyes) comprise the study subject cohort. Of these, 58 children were reevaluated at the Retina Foundation of the Southwest specifically for the additional long-term follow-up testing portion of this study. The remaining 81 children’s records were identified as containing sufficient data to be included in the study even though the patients could not be located or were unwilling to return for the additional long-term follow-up testing. All children in this study had their last visual acuity testing at 36 months or more of adjusted age.
Table 2 shows the general demographics of the entire study cohort. The cohort is subdivided into 58 children who were seen for the additional long-term follow-up testing and into those 81 who were not. The mean birth weight of the entire group was 731 g, and the mean gestational age at birth was 26 weeks. The average age at the time of final acuity testing was 70.8 months (adjusted age) for the whole cohort. The mean birth weight of the patients who were evaluated with additional follow-up testing is lower that those in the group without additional follow-up testing—680 g vs 767 g, respectively (t = 3.63; P = .0004). Likewise, the gestational age at birth is on average 2 weeks less in the group of patients seen for additional follow-up testing (25 weeks) than the group who were not (27 weeks; t = 3.96; P = .0001). As might be expected, the mean adjusted age at the time of the last vision testing was higher in the group of patients seen for additional follow-up testing than in the group that were not: − 88 months vs 57 months (t = 5.40; P < .0001). Despite these differences between the two subgroups of patients, there does not appear to be an obvious reason of this self-selection. These differences are not considered to be of substantive importance to the study results.
TABLE 2.
DEMOGRAPHICS OF THE 139 EXTREMELY LOW-BIRTH-WEIGHT STUDY SUBJECTS INCLUDED IN THIS REPORT
| VARIABLE | ALL PATIENTS (n = 139) | PATIENTS SEEN FOR ADDITIONAL FOLLOW-UP TESTING (n = 58) | PATIENTS NOT SEEN FOR ADDITIONAL FOLLOW-UP TESTING (n = 81) | DIFFERENCE BETWEEN GROUPS |
|---|---|---|---|---|
| Birth weight (g) | ||||
| Mean | 731 ± 146 | 680 ± 137 | 767 ± 142 | t = 3.36 |
| Median | 725 | 680 | 765 | |
| Range | 396 – 992 | 396 – 964 | 420 – 992 | |
| Gestational age at birth (weeks) | ||||
| Mean | 26 ± 2.2 | 25 ± 2.0 | 27 ± 2.1 | t = 3.96 |
| Median | 26 | 25 | 26 | |
| Range | 22 – 33 | 22 – 29 | 22 – 33 | |
| Adjusted age at last vision test (months) | 70.8 | 87.7 | 57.4 | t = 5.40 |
t = t test.
VISUAL ACUITY DEVELOPMENT IN ELBW INFANTS
Early Grating Acuity
For the purposes of this study, early grating acuity testing is defined as being performed at 6 months of adjusted age or younger. Because the mean normal acuity for age and the lower tolerance limit of normal vary considerably between birth at term (40 weeks gestational age) and at 6 months, visual acuity was evaluated as the difference from the established norms for mean acuity and the lower limit of normal for adjusted age.101
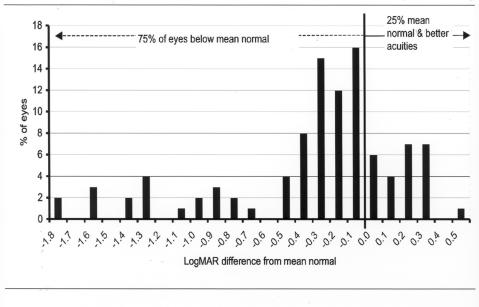
A total of 100 eyes (36%) of the entire cohort of ELBW infants were tested by grating acuity techniques at 6 months of adjusted age or younger. Figure 4 shows the results of the early grating acuity differences from mean normal. Early grating acuities of these 100 eyes ranged from 1.8 log units below to .5 log units above the mean of the normal acuity range for adjusted age. Seventy-five eyes (75%) had acuity levels below the normal mean, and only 25 eyes (25%) were at or above the mean of normal for age.
FIGURE 4.
Early grating acuity differences from mean normal: Chart shows grating acuity difference from mean normal for adjusted age in 100 eyes of infants tested at 6 months or less adjusted age. Visual acuity difference is expressed as logMAR.
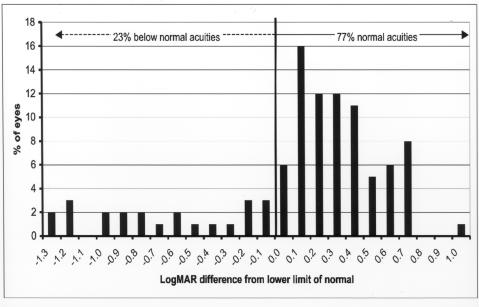
When early grating acuity results were compared with the lower tolerance limit of the normal range for adjusted age, 23 eyes (23%) were below the lower limit of the normal range. Seventy-seven eyes (77%) were within the normal visual range for adjusted age (Figure 5).
FIGURE 5.
Early grating acuity differences from lower limit of normal: Chart shows grating acuity difference from the lower limit of the normal range for adjusted age in 100 eyes of infants tested at 6 months or less of adjusted age. Visual acuity difference is expressed as logMAR.
Late Grating Acuity
Final grating acuity outcomes were measured at 3 years of adjusted age or older for 248 eyes (89%) of the study group. At 36 months of age, the mean of normal grating acuity is 20/40 (.3 logMAR) and the range of normal is from 20/80 (.6 logMAR) to 20/15 (−.1 logMAR). Figure 6 shows that late grating acuities of the 248 study eyes ranged from 20/15 to no light perception (NLP; 2.5 logMAR). The mean adjusted age at the time of final grating acuity testing was 73 ± 36 months with a range of 36 to 236 months; 101 eyes (41%) had final grating acuity outcomes of 20/40 (.3 logMAR) or better, 80 eyes (32%) were between 20/50 (.4 logMAR) and 20/160 (.9 logMAR), 67 eyes (27%) were 20/200 (1.0 logMAR) or worse, and 44 eyes (18%) were worse than 20/800 (1.6 logMAR).
FIGURE 6.
Late grating acuity outcomes: Chart shows distribution of grating acuity vision in 248 eyes tested at 36 months or more of adjusted age. Visual acuity is expressed as logMAR.
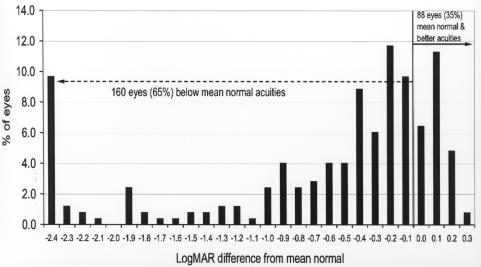
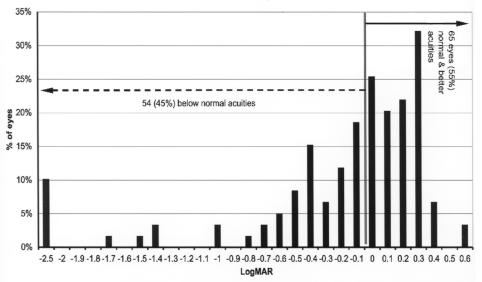
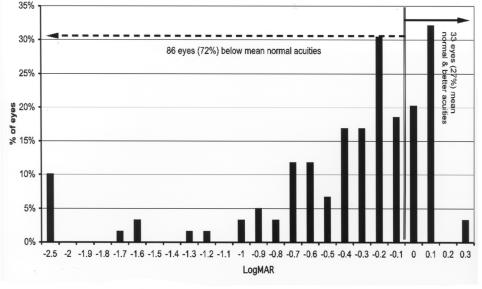
Figure 7 shows the final grating acuity differences from the mean of normal for adjusted age for these 248 eyes. The mean normal acuity at 48 months or more adjusted age is .1 logMAR (20/25). Acuity levels ranged from 2.4 log units below the mean of normal to 0.3 log units above mean normal. At 36 months or more of adjusted age, 160 eyes (65%) were below mean normal and 88 eyes (35%) were at or above the mean of normal.
FIGURE 7.
Late grating acuity differences from mean normal: Chart shows grating acuity difference from mean normal for adjusted age of 248 eyes of children tested at 36 months or more of adjusted age. Visual acuity difference expressed as logMAR.
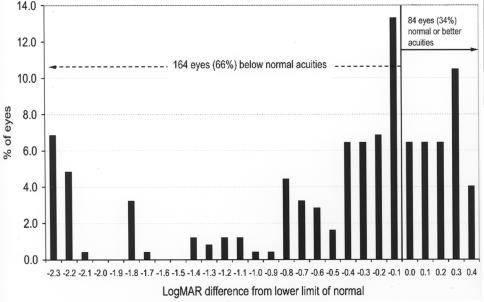
At 36 and 48 months of age, the lower limit of the normal range is 20/80 (.6 logMAR) and 20/40 (.3 logMAR), respectively. When grating acuity outcomes at 36-month adjusted age or older were compared with the lower tolerance limit of the normal range for age, 164 eyes (66%) were below the lower limit of the normal range. Only 84 eyes (34%) were within the normal limits (Figure 8).
FIGURE 8.
Late grating acuity differences from lower limit of normal: Chart shows grating acuity difference from the lower limit of the normal range for adjusted age in 248 eyes of children tested at 36 months or more of adjusted age. Visual acuity difference expressed as logMAR.
Recognition Acuity
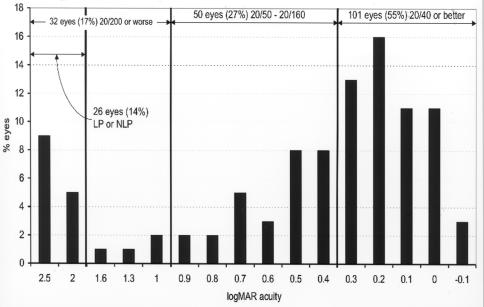
Recognition acuity outcomes were also measured at 3 years of adjusted age or older on 183 eyes (66%) of the study group. Figure 9 shows the HOTV recognition acuity results for these eyes. The mean adjusted age at the time of testing was 76 ± 39 months with a range of 36 to 236 months of age. Acuity levels ranged from 20/15 (−.1 logMAR) to no light perception (NLP) (2.5 logMAR); 101 eyes (55%) had acuities of 20/40 (.3 logMAR) or better. This is somewhat higher than the 41% who had grating acuity of 20/40 or better. This discrepancy is likely due to the fact that a large number of the children in this study had multiple disabilities and were unable to undergo recognition acuity testing for cognitive reasons. Thus the children with higher cognitive abilities were more likely to be capable of recognition acuity testing. An additional 50 eyes (27%) had vision ranging from 20/50 (.4 logMAR) to 20/160 (.9 logMAR). The remaining 32 eyes (17%) were at or below the level of legal blindness; six eyes (3%) were between 20/200 (1.0 logMAR) and 20/800 (1.6 logMAR), and 26 eyes (14%) had light perception only or NLP.
FIGURE 9.
Recognition acuity outcomes: Chart shows distribution of recognition acuity vision in 183 eyes tested at 36 months or more of adjusted age. Visual acuity difference expressed as logMAR. LP = light perception; NLP = no light perception.
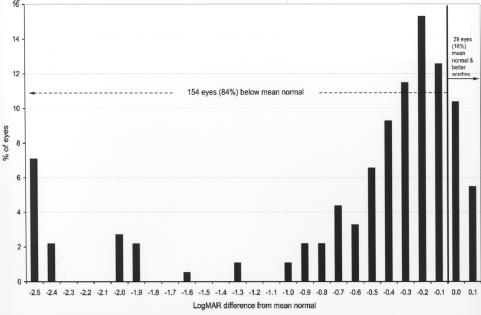
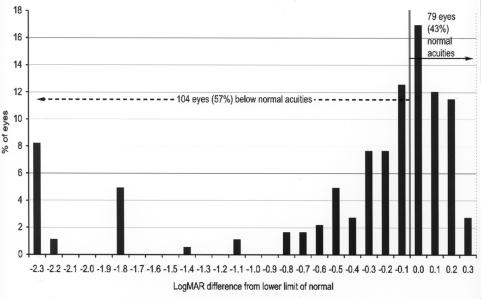
Figures 10 and 11 compare the recognition acuity outcome at 36 months or more of adjusted age with the mean normal value and the lower limit of the normal range of age. The mean normal values used for recognition acuity are as follows: for age 48 months or greater, 20/20 (0 logMAR), and for 36 to 47 months, 20/25 (.1 logMAR). A total of 154 eyes (84%) (Figure 10) were below the mean of the normal range, and 104 eyes (57%) (Figure 11) were below the lower limit of the normal range.
FIGURE 10.
Recognition acuity differences from mean normal: Chart shows recognition acuity difference from mean normal for adjusted age in 183 eyes of children tested at 36 months or more adjusted age. Visual acuity difference expressed as logMAR.
FIGURE 11.
Recognition acuity differences from lower limit of normal: Chart shows recognition acuity difference from the lower limit of normal for adjusted age in 183 eyes of children tested at 36 months or more of adjusted age. Visual acuity difference expressed as logMAR.
Rate of Grating Acuity Development
The rate of grating acuity development in this study cohort was evaluated for those eyes of children who were tested at least twice during the first 18 months of life. A total of 152 eyes were tested at least twice. The number of testing encounters for a given eye ranged from two to seven.
The rate of visual maturation during infancy is calculated as the slope of the best-fit line relating logMAR grating acuity to adjusted age in log months. This makes the rate of visual development into a linear function over time. The range of slopes varied from −4.95 logMAR/log month to 2.93 logMAR/log month. The mean slope of these 152 study eyes was −.72 ± 1.09 logMAR/log month. This average rate of maturation does not differ significantly from the mean slope of acuity development for healthy full-term infants (−.64 ± .21 logMAR/log month).101,111 If the slope of acuity development is > −.2, then the rate of visual development is considered to be abnormally slow.
PREDICTIVE VALUE OF EARLY ACUITY TESTING
The value of early acuity testing of infants’ eyes during the first 18 months of life in predicting late visual outcome is analyzed in four ways:
The results of the rate of early grating acuity development (slope) information (whether normal or subnormal) are compared with late grating acuity outcomes (normal vs abnormal).
The rate of early grating acuity development (slope) is compared with late recognition acuity results (normal vs abnormal).
The results of early grating testing are compared with late grating outcomes.
The results of early grating testing are compared with late recognition acuity outcomes.
All eyes that underwent two or more grating acuity tests within the first 18 months of life and that had late grating acuity testing (≥36 months of adjusted age) were evaluated; 152 eyes met these criteria. A chi-square analysis was then performed comparing the predictive value of the rate (slope) of early visual development with the long-term grating acuity outcomes. An abnormal early slope was predictive of an abnormal late grating acuity outcome (χ2 = 39.8, P < .0001) with a sensitivity of 63%, a specificity of 86%, and an accuracy of 76% (Table 3).
TABLE 3.
COMPARISON OF THE RATE OF EARLY VISUAL DEVELOPMENT (SLOPE) WITH LATE GRATING ACUITY OUTCOMES IN 152 EYES WITH TWO OR MORE GRATING ACUITY TESTS WITHIN THE FIRST 18 MONTHS OF LIFE
| SLOPE | ABNORMAL GRATING ACUITY ≥36 MONTHS ADJUSTED AGE | NORMAL GRATING ACUITY ≥36 MONTHS ADJUSTED AGE |
|---|---|---|
| Abnormal | 41 eyes | 12 eyes |
| Normal | 24 eyes | 75 eyes |
| Sensitivity = 63% | χ2 = 39.8 | |
| Specificity = 86% | P < .0001 | |
| Accuracy = 76% |
χ2 = chi-square.
The rate of early visual development (slope) was also compared with recognition acuity outcome at 3 years or more adjusted age. There were 152 eyes that underwent these testing criteria; however, recognition acuity testing data on one eye could not be retrieved. This analysis on 151 eyes also showed that a below normal rate of visual development early in life was correlated with abnormal recognition acuity outcomes (χ2 = 41.9, P < .0001) with a sensitivity of 64%, a specificity of 87%, and an accuracy of 77% (Table 4).
TABLE 4.
COMPARISON OF THE RATE OF EARLY VISUAL DEVELOPMENT (SLOPE) WITH LATE RECOGNITION ACUITY OUTCOMES IN 151 EYES WITH TWO OR MORE GRATING ACUITY TESTS WITHIN THE FIRST 18 MONTHS OF LIFE
| SLOPE | ABNORMAL RECOGNITION ACUITY ≥36 MONTHS ADJUSTED AGE | NORMAL RECOGNITION ACUITY ≥36 MONTHS ADJUSTED AGE |
|---|---|---|
| Abnormal | 42 eyes | 11 eyes |
| Normal | 24 eyes | 74 eyes |
| Sensitivity = 64% | χ2 = 41.9 | |
| Specificity = 87% | P < .0001 | |
| Accuracy = 77% |
χ2 = chi-square.
The 100 eyes that underwent early grating acuity testing (≤6 months adjusted age) and that underwent late grating acuity testing at ≥ 36 months adjusted age were compared for the predictive value of early grating acuity testing. This analysis showed that early grating testing was predictive of late grating outcome acuity in this group (χ2 = 24.38, P < .0001) with a sensitivity of 46%, a specificity of 95%, and an accuracy of 76% (Table 5).
TABLE 5.
COMPARISON OF THE LATE GRATING ACUITY OUTCOMES WITH EARLY GRATING ACUITY TESTING RESULTS FOR 100 EYES THAT UNDERWENT BOTH EVALUATIONS
| ABNORMAL GRATING ACUITY ≥36 MONTHS ADJUSTED AGE | NORMAL GRATING ACUITY ≥36 MONTHS ADJUSTED AGE | |
|---|---|---|
| Abnormal grating at 6 months | 18 eyes | 3 eyes |
| Normal grating at 6 months | 21 eyes | 58 eyes |
| Sensitivity = 46% | χ2 = 24.38 | |
| Specificity = 95% | P <.0001 | |
| Accuracy = 76% |
χ2 = chi-square.
Finally, results of early grating acuity were compared with results of recognition acuity testing. A total of 99 eyes underwent both of these testing sessions. When the normal vs abnormal early grating eyes were compared with the normal vs abnormal recognition acuity results, early grating testing was predictive of recognition acuity (χ2 = 14.39, P = .0001) with a sensitivity of 39%, a specificity of 93%, and an accuracy of 69% (Table 6).
TABLE 6.
COMPARISON OF THE RECOGNITION ACUITY OUTCOMES WITH EARLY GRATING ACUITY TESTING RESULTS FOR 99 EYES THAT UNDERWENT BOTH EVALUATIONS
| ABNORMAL RECOGNITION ACUITY ≥36 MONTHS ADJUSTED AGE | NORMAL RECOGNITION ACUITY ≥36 MONTHS ADJUSTED AGE | |
|---|---|---|
| Abnormal grating 6 months | 17 eyes | 4 eyes |
| Normal grating 6 months | 27 eyes | 51 eyes |
| Sensitivity = 39% | χ2 = 14.39 | |
| Specificity = 93% | P = .0001 | |
| Accuracy = 69% |
χ2 = chi-square.
ANALYSIS OF RISK FACTORS AFFECTING VISUAL ACUITY OUTCOMES IN ELBW CHILDREN
Zone of Disease at Time of Maximal ROP
Accurate data regarding the zone of ROP at the time of maximal disease were available for 208 eyes of 106 infants. Sixty-seven eyes of 34 patients were graded as having zone I disease, 132 eyes of 67 patients had zone II disease, and 9 eyes of 5 patients had zone III disease. This determination of zone was made by the author reviewing the records of the ROP screening exams made at the time of the examinations. Eyes with ROP were classified as being “zone I” eyes if at the time of reaching their maximal stage of ROP they were graded as having at least one clock hour sector of any stage of ROP in zone I. Otherwise, eyes were placed in the zone II/III category. Maximal disease recorded in either zone II or zone III was combined into a single group (zone II/III) because of the small number of eyes (nine) with maximal disease in zone III.
Table 7 compares the characteristics of the two groups. The zone I group eyes were of infants who were significantly smaller and less mature, as judged by birth weight and gestational weight at birth, than were the infants in the Zone II/III group of eyes. A total of 50 of the 67 eyes (75%) in the zone I group received ablative treatment of the peripheral avascular retina with laser photocoagulation or cryotherapy for severe ROP, whereas only 47 of 141 eyes (33%) in the zone II/III group received ablative treatment. This higher proportion of zone I group receiving ablative treatment than the zone II/III group is statistically significant (χ2 = 7.92; P = .0049).
TABLE 7.
CHARACTERISTICS OF THE PATIENTS (AND EYES) IN WHICH MAXIMAL ROP WAS IN ZONE I VS THOSE IN WHICH MAXIMAL ROP WAS IN ZONE II OR III
| CHARACTERISTIC | ZONE I EYES | ZONE II/III EYES | DIFFERENCE |
|---|---|---|---|
| No. of eyes (patients) | 67 (34) | 141 (72) | |
| Range of BW (g) | 396 – 964 | 420 – 992 | |
| Mean BW (g) ± SD | 670 ± 123 | 745 ± 150 | χ2 = 9.3
P = .0023 |
| Median BW (g) | 695 | 750 | χ2 = 10.2
P = .0014 |
| Range of GA (weeks) | 22 – 29 | 22 – 33 | |
| Mean GA (weeks) ± SD | 24.9 ± 1.6 | 26.4 ± 2.2 | χ2 = 4.0
P = .0455 |
| Median GA (weeks) | 25.0 | 26.0 | χ2 = 4.2
P = .0404 |
| No. eyes with laser/cryotherapy | 50 (75%) | 47 (33%) | χ2 = 7.92
P = .0049 |
BW = birth weight; GA = gestational age at birth; ROP = retinopathy of prematurity; SD = standard deviation; χ2 = chi-square.
Sixty-seven eyes of 34 patients were graded as having maximal ROP in zone I and underwent acuity testing at 36 months of adjusted age or older. A total of 59 eyes (88%) were tested with grating acuity techniques, and 50 eyes (75%) were tested with recognition acuity testing techniques. A total of 141 eyes of 72 patients who had their maximal disease in zone II/III underwent acuity testing at 36 months or older adjusted age. Of these zone II/III group eyes, 119 eyes (84%) were evaluated using grating acuity techniques and 102 (72%) were evaluated by recognition acuity methods (Table 8).
TABLE 8.
COMPARISON OF VISUAL ACUITY OUTCOMES AT ≥36 MONTHS OF ADJUSTED AGE FOR EYES IN WHICH MAXIMAL ROP WAS IN ZONE I VS EYES IN WHICH MAXIMAL ROP WAS IN ZONE II OR III
| TOTAL EYES (PTS) | ZONE I EYES (PTS) | ZONE II/III EYES (PTS) | DIFFERENCE | |
|---|---|---|---|---|
| 208 (106) | 67 (34) | 141 (72) | ||
| Grating acuity | 178 (86%) | 59 (88%) | 119 (84%) | |
| < mean normal acuity for adj age | 135 (76%) | 49 (83%) | 86 (72%) | z = 5.97 |
| < normal acuity for adj age | 97 (54%) | 43 (73%) | 54 (45%) | z = 3.73 |
| Recognition acuity | 152 (73%) | 50 (75%) | 102 (72%) | |
| Below normal acuity for adj age | 74 (49%) | 37 (74%) | 37 (36%) | z = 3.72 |
adj = adjusted; pts = patients.
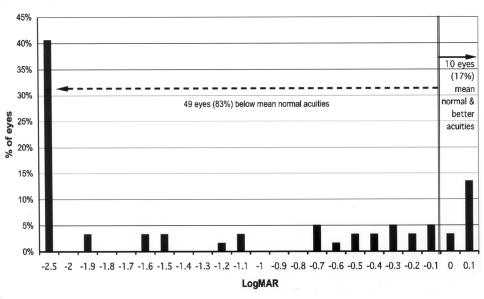
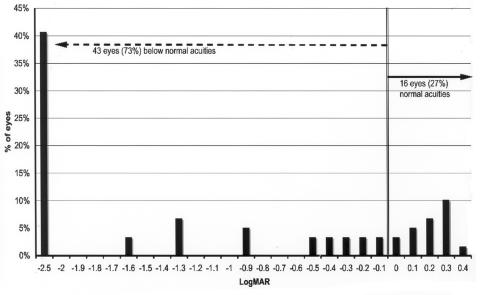
Grating acuity at 36 months or more adjusted age was compared between the zone I and the zone II/III groups (Figures 12 through 15). Overall, 49 of 59 eyes (83%) of the zone I group had grating acuity below mean normal vs 86 eyes (72%) of the zone II/III group (z = 5.97; P < .0001). When compared with the lower tolerance limit for normal, 43 of 59 eyes (73%) in the zone I group were below this limit compared with 54 eyes (45%) of zone II/III eyes. This difference was significant (z = 3.73; P = .0002).
FIGURE 12.
Visual outcomes for Zone I eyes compared with the mean of normal for age: Grating acuity difference from mean normal for adjusted age in 59 eyes with maximal ROP occurring in zone I. Visual acuity difference expressed as logMAR.
FIGURE 15.
Visual outcomes for Zone II/III eyes Compared with the lower limit of normal for age: Grating acuity difference from lower limit of normal range for adjusted age in 119 eyes with maximal ROP occurring in zone II/III. Visual acuity difference expressed as logMAR.
For recognition acuity, 37 of 50 eyes (74%) of the zone I group had acuity below normal vs 37 of 102 eyes (36%) of the zone II/III eyes (z = 3.72; P = .0002). Moreover, 28% of children with zone I disease had bilateral recognition acuity outcomes of 1.0 logMAR (20/200) or worse vs only 2% of the children with zone II/III disease (z = 2.83; P = .0047).
Other Risk Factors
Risk factors shown in Table 9 were evaluated for poor long-term visual acuity outcome (≥3-line deficit from mean normal). In addition, gestational age at birth and birth weight were evaluated as risk factors for poor refractive outcome (hypermetropia ≥ +3.5 diopters [D], any myopia, myopia ≥ −6D) and for poor alignment outcome (strabismus). For risk factor analysis, each of the variables was stratified as shown in Table 9. Mantel-Haenszel odds ratios were computed for all risk factors. Because the incidence of the adverse outcome (poor visual acuity) was more than 10%, odds ratios were corrected to relative risks to appropriately assess the magnitude of the associations.
TABLE 9.
STRATIFICATION OF RISK FACTORS THAT WERE EVALUATED FOR POOR LONG-TERM VISUAL ACUITY OUTCOME
| RISK FACTOR | GROUPS |
|---|---|
| Zone of maximal ROP | Zone I |
| Gestational age at birth | ≤28 wk vs >28 wk |
| Birth weight | ≤750 g vs >750 g |
| General health | ≤60 days ventilation vs >60 days |
| Gender | Male vs female |
| IVH | Grade 3–4 vs Grade 0–2 |
| NEC | Yes/no |
| PVL | Yes/no |
| Sepsis | Yes/no |
IVH = intraventricular hemorrhage; NEC = necrotizing enterocolitis; PVL =
Children born at ≤28 weeks of gestation had 4.1 times (95% CI = 2.2 to 6.2) greater risk of abnormal recognition acuity outcome than children born at >28 weeks of gestation. Children with poorer general health, who were hospitalized initially for more than 3 months postpartum, had 5.3 times greater risk of abnormal recognition acuity outcome than children whose initial duration of hospitalization was 3 months or less. The other risk factors were not significantly associated with risk for poor recognition acuity outcomes.
Children born at ≤25 weeks of gestational age had 1.7 times (95% CI = 1.2–2.0) greater risk of developing myopia than children born at >25 weeks of gestation. Birth weight was not significantly associated with risk for poor refractive outcome. Neither gestational age at birth nor birth weight posed a significant risk for strabismus.
DISCUSSION
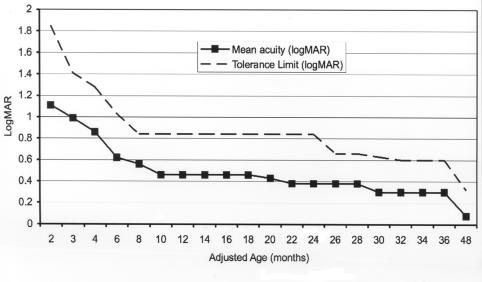
Visual development is a learned ability that is a coordinated process between the eyes and the brain. In a full-term infant this process of visual development starts at birth and is not complete until 3 to 4 years of age. At birth, a full-term infant has a grating visual acuity level of about 1.5 logMAR (20/600). Acuity improves rapidly during the first few months, and by age 1 year vision is normally about .48 logMAR (20/60). Visual acuity finally reaches adult levels of resolution (20/20) at about 36 to 48 months of age101 (Figure 16). In the preterm infant, even when the eye is free of disease, the process of visual acuity maturation is delayed by the amount of prematurity. Therefore, when age is corrected for prematurity, the rate and timing of visual acuity development in healthy preterm children are similar to that of full-term children.6
FIGURE 16.
Normal grating acuity development based on data from normal full-term infants. (Courtesy of Eileen E. Birch, PhD, Dallas, Texas)
The group of subjects in this study does not represent a birth cohort. Therefore, as such, the results are not representative of the risks and occurrence of events in the general population. On the other hand, it is very difficult to assess those risks in population cohorts that have very few adverse events, such as visual impairment in a general population of prematurely born babies. Therefore, although this study’s cohort has the disadvantage that it may not be used to accurately estimate the absolute value of the risk associated with each factor in the general population, it has the advantage of a high prevalence of adverse events that can be used to estimate associations with risk more easily. In this way, this study does illuminate factors on which it may be important to focus.
LONG-TERM GRATING ACUITY AND RECOGNITION ACUITY OUTCOMES
In this population of 278 eyes of 139 ELBW children on whom long-term visual outcome data was acquired, 248 eyes had 3-year grating acuity outcome measurements, whereas only 183 eyes had HOTV recognition acuity measurement. Although the mean age for the time of outcome testing was similar for each technique (73 ± 36 months for grating; 76 ± 39 months for recognition), the smaller number of eyes successfully tested with recognition acuity techniques reflects the fact that a relatively large number of neurodevelopmentally impaired children are included in this cohort. This difference is also reflected in the larger percentage of those study subjects able to perform recognition acuity testing who achieved 20/40 or better acuity than in the group of subjects who were able to perform grating acuity testing (55% vs 41%; P =.003). Recognition acuity testing requires more cognitive ability than the reflexive responses elicited by grating acuity testing. The most extensive long-term visual acuity outcome data set for LBW children was published in a series of papers by the CRYO-ROP study group. A similar difficulty in testing neurologically impaired patients was also noted by the CRYO-ROP Cooperative Group in their 5½-year outcome study. They were unable to obtain information on 100 eyes during recognition acuity testing and attributed this failure primarily to neurodevelopmental impairment.112
Visual outcome, as measured at 36 months or more of adjusted age, was outside of the normal tolerance limits in over half the eyes (Figures 8 and 11) and was below the mean of normal in over 65% (Figures 7 and 10). One hundred and thirty-four eyes (54%) tested with grating acuity and 104 eyes (57%) tested by recognition acuity were outside the normal range; 160 of the 248 eyes (65%) tested with grating testing were below the mean of normal, and 154 of 183 eyes (84%) were below the mean with HOTV testing.
Others have reported reduced long-term acuity outcomes in LBW infants, regardless of ROP outcome.86,96,113–116 Even among LBW infants with no apparent retinal or cortical sequelae of premature birth, subnormal long-term binocular visual acuity outcomes have been reported.116,117 Longitudinal studies of visual acuity maturation in ELBW infants are scarce in the ROP literature. Courage and Adams118 reported grating acuity data from 26 ELBW infants and found that most grating acuities fell within the normal tolerance limits, although they tended to be below the average acuity of postconceptional age-matched full-term infants after 9 months of age.
The data from the CRYO-ROP study and this present study are summarized and compared in Tables 10 through 12. Because most of the patients included in the present study’s cohort received cryotherapy or laser treatment for threshold ROP, the most appropriate comparisons are with the treated eyes from the CRYO-ROP Study.
TABLE 10.
A COMPARISON OF 3½ -YEAR CRYO-ROP STUDY GRATING ACUITY OUTCOME DATA WITH GRATING ACUITY OUTCOMES OF THIS EXTREMELY LOW-BIRTH-WEIGHT (ELBW) COHORT
| TREATED EYES (n = 193) 187 EYES TESTED | CONTROL EYES (n = 192) 189 EYES TESTED | COMBINED EYES (n = 385) 376 EYES TESTED | ELBW COHORT (n = 175) | |
|---|---|---|---|---|
| Favorable | 89 (47.6%) | 65 (34.4%) | 154 (41.0%) | 115 (65.7%) |
| Normal | 38 (20.3%) | 35 (18.5%) | 73 (19.4%) | 58 (33.1%) |
| Below normal | 51 (27.3%) | 30 (15.9%) | 81 (21.5%) | 57 (32.6%) |
| Unfavorable | 98 (52.4%) | 124 (65.6%) | 222 (59.0%) | 60 (34.3%) |
| Poor vision | 41 (21.9%) | 27 (14.3%) | 68 (18.1%) | 44 (25.1%) |
| Blind/low vision | 57 (30.5%) | 97 (51.3%) | 154 (41.0%) | 16 (9.1%) |
CRYO-ROP = Cryotherapy for Retinopathy of Prematurity.
TABLE 12.
A COMPARISON OF 5½ -YEAR CRYO-ROP STUDY SNELLEN ACUITY OUTCOME DATA WITH 5½ -YEAR SNELLEN ACUITY OUTCOMES OF THIS EXTREMELY LOW-BIRTH-WEIGHT (ELBW) COHORT
| TREATED EYES (n=233) (222 EYES TESTED) | CONTROL EYES (n=230) (222 EYES TESTED) | COMBINED EYES (n=463) (444 EYES TESTED) | ELBW COHORT (n=48) | |
|---|---|---|---|---|
| Favorable | 104 (46.8%) | 77 (34.7%) | 181 (40.8%) | 43 (89.6%) |
| 20/40 or better | 28 (12.6%) | 37 (16.7%) | 65 (14.6%) | 25 (52.1%) |
| <20/40, but 20/60 or better | 14 (6.3%) | 11 ( 5.0%) | 25 ( 5.6%) | 8 (16.7%) |
| <20/60, but >20/200 | 62 (27.9%) | 29 (13.1%) | 91 (20.5%) | 10 (20.8%) |
| Unfavorable | 118 (53.1%) | 145 (65.3%) | 263 (59.2%) | 5 (10.4%) |
| ≤20/200 | 48 (21.6%) | 39 (17.6%) | 87 (19.6%) | 1 ( 2.1%) |
| Blind | 70 (31.5%) | 106 (47.7%) | 176 (39.6%) | 4 ( 8.3%) |
CRYO-ROP = Cryotherapy for Retinopathy of Prematurity
Grating acuity outcome at 3.5 years of age from the CRYO-ROP Study and this ELBW cohort is summarized in Table 10. It should be noted that only ELBW children who were tested at exactly the same age as those in the CRYO-ROP group (32 to 53 months) are included in this table. Even though the cohort in the present study is composed of only ELBW children, the author observed a higher rate of favorable grating acuity outcome than found in the CRYO-ROP cohort (66% vs 48%) and a much lower rate of blindness (9% vs 31%). These differences may be attributed to improved neonatal care and/or changes in the treatment timing and techniques for severe ROP (ie, the use of laser photocoagulation instead of cryotherapy).
In the present cohort, recognition acuity outcome at 3.5 years of age (Table 11) has an even higher rate of favorable outcome compared to the grating acuity data in Table 10. Using CRYO-ROP Study criteria, over 80% of this ELBW cohort’s eyes had a favorable long-term recognition acuity outcome. Note, however, that HOTV acuity testing requires a certain level of cognitive ability to perform, so those patients with more severe neurodevelopmental limitations, who are most likely to have the worst visual acuity outcome, did not contribute data to this table. The observed rate of favorable recognition acuity outcome was higher than found in the CRYO-ROP cohort (81% vs 53%), and the rate of blindness was lower (17% vs 37%).
TABLE 11.
A COMPARISON OF 3½ -YEAR CRYO-ROP STUDY HOTV RECOGNITION ACUITY OUTCOME DATA WITH 3½ -YEAR HOTV RECOGNITION ACUITY OUTCOMES OF THIS EXTREMELY LOW-BIRTH-WEIGHT (ELBW) COHORT
| TREATED EYES (n=203) 148 EYES TESTED | CONTROL EYES (n=203) 146 EYES TESTED | COMBINED EYES (n=406) 294 EYES TESTED | ELBW COHORT (n=64) | |
|---|---|---|---|---|
| Favorable | 79 (53.4%) | 62 (42.5%) | 141 (48.0%) | 52 (81.3%) |
| Normal | 43 (29.1%) | 42 (28.8%) | 85 (28.9%) | 38 (59.4%) |
| Below normal | 36 (24.3%) | 20 (13.7%) | 56 (19.1%) | 14 (21.9%) |
| Unfavorable | 69 (46.6%) | 84 (57.5%) | 153 (52.0%) | 12 (18.8%) |
| Poor vision | 14 (9.5%) | 6 (4.1%) | 20 (6.8%) | 1 (1.5%) |
| Blind/low vision | 55 (37.2%) | 78 (53.4%) | 133 (45.2%) | 11 (17.2%) |
CRYO-ROP = Cryotherapy for Retinopathy of Prematurity.
At 5.5 years of age (Table 12), the present cohort of eyes of ELBW children has a much higher rate of favorable recognition acuity outcome than that found in the CRYO-ROP cohort (90% vs 47%). Over 50% of the ELBW subjects’ eyes in the present study achieved 20/40 or better recognition acuity, whereas only 13% of the CRYO-ROP cohort achieved 20/40 or better. The observed rate of blindness was lower than found in the CRYO-ROP cohort (8% vs 32%).
An additional factor that could have had an impact on the relatively low rate of blindness may be the follow-up criteria. The CRYO-ROP study continued to follow and assess each child in the study cohort at each age regardless of whether they had a severe visual impairment. In the current study of ELBW eyes, if no visual function was detected on repeated testing, then follow-up may have ceased before 3 years of age, a factor which could account for the lower rate of blindness reported here.
PREDICTIVE VALUE OF GRATING ACUITY TESTS
In this ELBW cohort, grating acuity at ≤6 months of age was predictive of long-term grating acuity outcome with an accuracy of 69% (Table 6); early grating acuity was predictive of long-term recognition acuity outcome with an accuracy of 76% (Table 5). Two earlier studies have found similar accuracy rates in LBW cohorts.6,119 The predictive value of grating acuity tests is improved when testing is conducted at later ages or at multiple ages during infancy.6,120 This finding led to the examination of the rate (slope) of grating acuity development during the first 18 months of life as a predictor of long-term visual acuity outcome. This approach improved the accuracy to 76% and 77% (Tables 3 and 4) for long-term grating and recognition acuity outcomes.
Infant grating acuity tests, then, can provide valuable predictive information, but they should be interpreted cautiously, particularly for infants who score within the normal range. Infants with initially normal grating acuity scores still warrant observation, because one third will have long-term visual impairment of some degree, and even in the absence of visual acuity deficits, they are at risk for myopia, anisometropia, and strabismus.
ZONE I DISEASE
As expected, eyes with zone I ROP had significantly poorer recognition acuity outcomes than did the group of zone II/III eyes. More zone I eyes than zone II/III eyes had grating acuities outside of the normal tolerance limits (73% vs 45%) (Figures 13 and 15 and Table 8). The CRYO-ROP group reported that over 90% of zone I eyes had unfavorable recognition acuity and grating acuity outcomes at 3.5 years of age, whereas only about 50% of zone II eyes had unfavorable outcomes.121 They concluded that “the more posterior the zone of ROP and the greater the extent of stage 3+ disease, the greater the unfavorable outcome rate.”122 In the present study, 27% of zone I eyes achieved grating acuity within the normal tolerance limits. This increase in favorable grating acuity outcomes in the present cohort may be related to advances in neonatal care and/or ROP treatment that have occurred since the time of the CRYO-ROP study (eg, the introduction of surfactant therapy and the adoption of laser photocoagulation treatment in the 1990s).
FIGURE 13.
Visual outcomes for Zone I eyes compared with the lower limit of normal for age: Grating acuity difference from lower limit of normal range for adjusted age in 59 eyes with maximal ROP occurring in zone I. Visual acuity difference expressed as logMAR.
NEONATAL RISK FACTORS
ROP is known to be a multifactorial condition, that is, it is difficult to identify individual factors that predispose an infant to develop ROP or its sequelae, including long-term visual impairment. In the present study, a gestational age ≤28 weeks was associated with over four times increased risk for below normal recognition acuity outcome and 1.5 times increased risk for subnormal grating acuity outcome. Birth weight was not associated with long-term acuity outcomes. These results may not be surprising, because all LBW children have high risk for poor acuity outcomes89 and it may be difficult to detect further increases in risk over this baseline level. Poor general health, as indicated by 3 or more months duration of postnatal hospitalization (a proxy for severity of illness), also significantly elevated the risk for poor recognition acuity outcome by a factor of 5.
In an earlier study, Hardy and coworkers123 found that using birth weight, gestational age, gender, multiple birth, and ophthalmologic examination findings as factors in a multiple logistic risk model for ROP allowed for identification of a subgroup of high-risk infants in which 36% had unfavorable outcomes at 3 months post-term age. Infants without these risk factors had only a 5% rate of unfavorable outcomes. Although both studies evaluated similar risk factors, the outcome measure in the present study was a long-term functional outcome (visual acuity at ≥3 years of age) rather than the short-term anatomical outcome used by Hardy and colleagues. Tommiska and coworkers124 reported that 23% of ELBW infants had ophthalmic abnormalities and that these abnormalities were associated with lower birth weight and lower gestational age. Taken together with the present study, low gestational age is the common factor that emerges as presenting a strong risk for visual impairment and blindness. This finding suggests that the level of maturation of the infant at birth (as indicated by gestational age) is the most important prognostic factor in long-term visual development.
CONCLUSIONS
In high-income countries, including the United States, United Kingdom, and Scandinavia, advanced healthcare technology provides neonatal intensive care support for LBW infants. ROP is confined mainly to ELBW infants,125 and ROP-related visual impairment is likely to be associated with multiple disabilities.24,65,126 In these countries, ROP-related visual disability accounts for 5% to 8% of childhood visual impairment.65,127,128 This study demonstrates that infant grating acuity testing is useful in identifying eyes at high risk for moderate to severe visual impairment. Gestational age at birth of 28 weeks or less is also associated with significantly elevated risk for abnormal long-term visual acuity outcome.
Currently, there are clearly defined protocols for the screening of children at risk of developing ROP.129,130 However, there are no such guidelines for the long-term visual assessment of LBW children. The outcomes from the present study have implications for the care of ELBW children, and this information can lead to evidence-based practices, thereby improving our knowledge of the potential visual outcome for these children. With continued improvements in neonatal care, it is likely that this population of ELBW survivors will continue to increase over time. Whereas research has focused primarily on enabling these infants to survive, their long-term outcomes, such as vision, may have more lasting relevance to the children and their families and as such must not be neglected.
FIGURE 14.
Visual outcomes for Zone II/III eyes compared with the mean of normal for age: Grating acuity difference from mean normal for adjusted age in 119 eyes with maximal retinopathy of prematurity occurring in zone II/III. Visual acuity difference expressed as logMAR.
ACKNOWLEDGMENTS
The author wishes to acknowledge Eileen E. Birch, PhD (Retina Foundation of the Southwest, Dallas, Texas), and Anna O’Connor, PhD (University of Liverpool, Liverpool, United Kingdom), for their help, review, patience, and encouragement with the preparation of the scientific and statistical information presented in this thesis.
The author is also grateful to Joel N. Leffler, MD (pediatric ophthalmologist, private practice, Dallas, Texas), David R. Stager, Sr, MD (pediatric ophthalmologist, private practice, Dallas, Texas), and David Weakley, MD (University of Texas Southwestern Medical School, Dallas, Texas), for their aid with gathering follow-up data on mutual patients who were included in the study cohort.
REFERENCES
- 1.Martin JA, Kochanek KD, Strobino DM, et al. Annual summary of vital statistics—2003. Pediatrics. 2005;115:619–634. doi: 10.1542/peds.2004-2695. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton BE, Martin JA, Sutton PD. Births: preliminary data for 2003. Natl Vital Stat Rep. 2004;53:1–17. [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2002. Natl Vital Stat Rep. 2003;52:1–113. [PubMed] [Google Scholar]
- 4.Greene MF. Outcomes of very low birth weight in young adults [editorial] N Engl J Med. 2002;346:146–148. doi: 10.1056/NEJM200201173460302. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez JA, Hall DM, Goldson EJ, et al. Impact of infants born at the threshold of viability on the neonatal mortality rate in Colorado. J Perinatol. 2000;20:21–26. doi: 10.1038/sj.jp.7200300. [DOI] [PubMed] [Google Scholar]
- 6.Birch EE, Spencer R. Visual outcome in infants with cicatricial retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1991;32:410–415. [PubMed] [Google Scholar]
- 7.Harvey EM, Dobson V, Luna B, Scher MS. Grating acuity and visual-field development in children with intraventricular hemorrhage. Dev Med Child Neurol. 1997;39:305–312. doi: 10.1111/j.1469-8749.1997.tb07436.x. [DOI] [PubMed] [Google Scholar]
- 8.Eken P, de Vries LS, van Nieuwenhuizen O, et al. Early predictors of cerebral visual impairment in infants with cystic leukomalacia. Neuropediatrics. 1996;27:16–25. doi: 10.1055/s-2007-973742. [DOI] [PubMed] [Google Scholar]
- 9.An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 10.CRYO-ROP Group. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome—stucture and function. Arch Ophthalmol. 1990;108:1408–1416. [PubMed] [Google Scholar]
- 11.McManaway JW, DeMaio R, Blankenship GW. Complications of zone one retinopathy of prematurity treatment. Proc Int Strabismological Assoc. 1995:533–536. [Google Scholar]
- 12.O’Keefe M, Lanigan B, Long VW. Outcome of zone 1 retinopathy of prematurity. Acta Ophthalmol Scand. 2003;81:614–616. doi: 10.1111/j.1395-3907.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 13.Arnold LS, Grad RK. Low birth weight and infant mortality: a health policy perspective. NAACOGS Clin Issu Perinat Womens Health Nurs. 1992;3:1–12. [PubMed] [Google Scholar]
- 14.Demissie K, Rhoads GG, Ananth CV, et al. Trends in preterm birth and neonatal mortality among blacks and whites in the United States from 1989 to 1997. Am J Epidemiol. 2001;154:307–315. doi: 10.1093/aje/154.4.307. [DOI] [PubMed] [Google Scholar]
- 15.Branum AM, Schoendorf KC. Changing patterns of low birthweight and preterm birth in the United States, 1981–98. Paediatr Perinat Epidemiol. 2002;16:8–15. doi: 10.1046/j.1365-3016.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- 16.Silverman WA. The statistics of deaths of premature infants. In: Dunham’s Premature Infants, Third Edition New York: Harper & Brothers, 1961.
- 17.Hack M, Fanaroff AA, Mekatz IR. The low-birth-weight infant—evolution of a changing outlook. N Engl J Med. 1979;301:1162–1165. doi: 10.1056/NEJM197911223012105. [DOI] [PubMed] [Google Scholar]
- 18.Hack M, Horbar JD, Malloy MH, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics. 1991;87:587–597. [PubMed] [Google Scholar]
- 19.El-Metwally D, Vohr B, Tucker R. Survival and neonatal morbidity at the limits of viability in the mid 1990s: 22 to 25 weeks. J Pediatr. 2000;137:616–622. doi: 10.1067/mpd.2000.109143. [DOI] [PubMed] [Google Scholar]
- 20.Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002;110:143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 21.Aylward GP, Pfeiffer SI, Wright A, et al. Outcome studies of low birth weight infants published in the last decade: a metaanalysis. J Pediatr. 1989;115:515–520. doi: 10.1016/s0022-3476(89)80273-2. [DOI] [PubMed] [Google Scholar]
- 22.Horwood LJ, Mogridge N, Darlow BA. Cognitive, educational, and behavioural outcomes at 7 to 8 years in a national very low birthweight cohort. Arch Dis Child Fetal Neonatal Ed. 1998;79:F12–F20. doi: 10.1136/fn.79.1.f12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolke D. Psychological development of prematurely born children. Arch Dis Child. 1998;78:567–570. doi: 10.1136/adc.78.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood N, Marlow N, Costeloe K, et al. Neurologic and developmental disability after extremely preterm birth. N Engl J Med. 2000;343:378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 25.Hack M, Flannery D, Schluchter M, et al. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 26.Terry TL. Extreme prematurity and fibroplastic overgrowth of persistent vascular sheath behind each crystalline lens. I. Preliminary report. Am J Ophthalmol. 1942;25:203–204. doi: 10.1016/j.ajo.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Terry T. Fibroblastic overgrowth of persistent tunica vasculosa lentis in premature infants. II. Report of cases. Clinical aspects. Arch Ophthalmol. 1943;29:36–53. [PMC free article] [PubMed] [Google Scholar]
- 28.Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc. 1968;66:940–985. [PMC free article] [PubMed] [Google Scholar]
- 29.Patz A, Eastham A, Higginbotham DH, Kleh T. Oxygen studies in retrolental fibroplasia. II. The production of microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953;36:1511–1522. [PubMed] [Google Scholar]
- 30.Patz A, Hoeck LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol. 1952;35:1248–1253. doi: 10.1016/0002-9394(52)91140-9. [DOI] [PubMed] [Google Scholar]
- 31.Kinsey VE. Retrolental fibroplasia: cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol. 1956;56:481–543. [PubMed] [Google Scholar]
- 32.Lanman JT, Guy LP, Dancis J. Retrolental fibroplasia and oxygen therapy. JAMA. 1954;155:223–226. doi: 10.1001/jama.1954.03690210001001. [DOI] [PubMed] [Google Scholar]
- 33.Ashton N, Ward B, Serpell G. Communications. Role of oxygen in the genesis of RLF. Br J Ophthalmol. 1953;37:513–520. doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of RLF. Br J Ophthalmol. 1954;38:397–430. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolton DPG, Cross KW. Further observations on the cost of preventing retrolental fibroplasia. Lancet. 1974;1:445–448. doi: 10.1016/s0140-6736(74)92395-2. [DOI] [PubMed] [Google Scholar]
- 36.Avery ME, Oppenheimer EH. Recent increase in mortality from hyaline membrane disease. J Pediatr. 1960;57:553–559. doi: 10.1016/s0022-3476(60)80083-2. [DOI] [PubMed] [Google Scholar]
- 37.McDonald AD. Cerebral palsy in children of very low birth weight. Arch Dis Child. 1963;38:579–588. doi: 10.1136/adc.38.202.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warley MA, Gairdner D. Respiratory distress syndrome of the newborn—principles in treatment. Arch Dis Child. 1962;37:455–465. doi: 10.1136/adc.37.195.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert C. Retinopathy of prematurity: epidemiology. Community Eye Health. 1997;10:22–24. [Google Scholar]
- 40.Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326:1050–1054. doi: 10.1056/NEJM199204163261603. [DOI] [PubMed] [Google Scholar]
- 41.Flynn JT, Bancalari E, Bawol R, et al. Retinopathy of prematurity: a randomized, prospective trial of transcutaneous oxygen monitoring. Ophthalmology. 1987;94:630–638. doi: 10.1016/s0161-6420(87)33400-1. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert C, Canovas R, Kocksch R, Foster A. Causes of blindness and severe visual impairment in children in Chile. Dev Med Child Neurol. 1994;36:334–343. doi: 10.1111/j.1469-8749.1994.tb11853.x. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert C, Foster A. Causes of blindness in children attending four schools for the blind in Thailand and the Philippines—a comparison between urban and rural blind populations. Int Ophthalmol. 1993;17:229–234. doi: 10.1007/BF01007745. [DOI] [PubMed] [Google Scholar]
- 44.O’Sullivan J, Gilbert C, Foster A. The causes of childhood blindness in South Africa. S Afr Med J. 1997;87:1691–1695. [PubMed] [Google Scholar]
- 45.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia. A clinical approach. Med J Aust. 1951;2:48. [PubMed] [Google Scholar]
- 46.Ashton N. The pathogenesis of retrolental fibroplasia. Ophthalmology. 1979;86:1695–1699. doi: 10.1016/s0161-6420(79)35330-1. [DOI] [PubMed] [Google Scholar]
- 47.Kretzner FL, Hittner HM. Vitamin E questioned [reply to letter] Pediatrics. 1984;5:734–735. [Google Scholar]
- 48.Kretzner FL, Hittner HM. Human retinal development: relationship to the pathogenesis of retinopathy of prematurity. In: McPherson AR, Hittner HM, Kretzner FL, eds. Retinopathy of Prematurity: Current Concepts and Controversies. Toronto: BC Decker; 1986.
- 49.Kretzner FL, Hittner HM, Johnson AT, et al. Vitamin E and retrolental fibroplasia: ultrastructural support of clinical efficacy. Ann NY Acad Sci. 1982;393:145–166. doi: 10.1111/j.1749-6632.1982.tb31240.x. [DOI] [PubMed] [Google Scholar]
- 50.Kretzner FL, Hunter DG, Mehta RS, et al. Spindle cells as vasoformative elements in the developing human retina: vitamin E modulation. In: Coates PW, Markwald RR, Kenny AD, eds. Developing and Regenerating Vertebrate Nervous Systems. New York: Alan R Liss; 1983.
- 51.Kretzner FL, McPherson AR, Hittner HM. An intrepretation of retinopathy of prematurity in terms of spindle cells: relationship to vitamin E prophylaxis and cryotherapy. Graefes Arch Clin Exp Ophthalmol. 1986;224:205–214. doi: 10.1007/BF02143055. [DOI] [PubMed] [Google Scholar]
- 52.Phelps DL. Retinopathy of prematurity [reply to letter] N Engl J Med. 1992;327:648–649. doi: 10.1056/NEJM199208273270917. [DOI] [PubMed] [Google Scholar]
- 53.Johnson LH, Schaffer DB, Goldstein DE, Boggs TR. Influence of vitamin E treatment and adult blood transfusions on mean severity of retrolental fibroplasia (MS-RLF) Pediatr Res. 1977;11:535. [Google Scholar]
- 54.Johnson LH, Schaffer DB, Boggs TR. The premature infant, vitamin E deficiency, and retrolental fibroplasia. Am J Clin Nutr. 1974;27:1158–1171. doi: 10.1093/ajcn/27.8.1158. [DOI] [PubMed] [Google Scholar]
- 55.Hittner HM, Godio LB, Rudolph AJ, et al. Retrolental fibroplasia: efficacy of vitamin E in a double-blind clinical study of preterm infants. N Engl J Med. 1981;305:1365–1371. doi: 10.1056/NEJM198112033052301. [DOI] [PubMed] [Google Scholar]
- 56.Vitamin E and Retinopathy of Prematurity. Report of a study by a committee of the Institute of Medicine, Division of Health Sciences Policy. June 1986; v and 24 pp. NTIS Accession No. PB 88 223–656.
- 57.Phelps DL, Rosenbaum AL, Isenberg SJ, et al. Tocopherol efficacy and safety for preventing retinopathy of prematurity: a randomized, controlled, double-masked trial. Pediatrics. 1987;79:489–500. [PubMed] [Google Scholar]
- 58.Penn JS, Tolman BL, Henry MM. Oxygen-induced retinopathy in the rat: relationship of retinal nonperfusion to subsequent neovascularization. IOVS. 1994;35:3429–3435. [PubMed] [Google Scholar]
- 59.Miller JW. Vascular endothelial growth factor and ocular neovascularization. Am J Pathol. 1997;151:13–23. [PMC free article] [PubMed] [Google Scholar]
- 60.Provis JM, Leech J, Diaz CM, et al. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res. 1997;65:555–568. doi: 10.1006/exer.1997.0365. [DOI] [PubMed] [Google Scholar]
- 61.Flynn JT, O’Grady GE, Herrera J, et al. Retrolental fibroplasia: I. Clinical observations. Arch Ophthalmol. 1977;95:217–223. doi: 10.1001/archopht.1977.04450020019002. [DOI] [PubMed] [Google Scholar]
- 62.Flynn JT, Cassady J, Essner D, et al. Fluorescein angiography in retrolental fibroplasia: experience from 1969–1977. Ophthalmology. 1979;86:1700–1723. doi: 10.1016/s0161-6420(79)35329-5. [DOI] [PubMed] [Google Scholar]
- 63.Flynn JT. Acute proliferative retrolental fibroplasia: evolution of the lesion. Graefes Arch Klin Exp Ophthalmol. 1975;195:101–111. doi: 10.1007/BF00417113. [DOI] [PubMed] [Google Scholar]
- 64.Flynn JT. Retinopathy of prematurity. Pediatr Clin North Am. 1987;34:1487–1515. doi: 10.1016/s0031-3955(16)36370-2. [DOI] [PubMed] [Google Scholar]
- 65.Crofts BJ, King R, Johnson A. The contribution of low birth weight to severe vision loss in a geographically defined population. Br J Ophthalmol. 1998;82:9–13. doi: 10.1136/bjo.82.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birch EE, O’Connor AR. Preterm birth and visual development. Semin Neonatol. 2001;6:487–497. doi: 10.1053/siny.2001.0077. [DOI] [PubMed] [Google Scholar]
- 67.O’Connor AR, Fielder AR, Birch EE. Long term ophthalmic outcome of low birth weight children who did not have retinopathy of prematurity. Ital J Pediatr. 2002;28:359–365. doi: 10.1542/peds.109.1.12. [DOI] [PubMed] [Google Scholar]
- 68.Oehler JM. Developmental care of low birth weight infants. Nurs Clin North Am. 1993;28:289–301. [PubMed] [Google Scholar]
- 69.Glotzbach SF, Rowlett EA, Edgar DM, et al. Light variability in the modern neonatal nursery: chronobiologic issues. Med Hypotheses. 1993;41:217–224. doi: 10.1016/0306-9877(93)90234-h. [DOI] [PubMed] [Google Scholar]
- 70.Graven SN. Clinical research data illuminating the relationship between the physical environment & patient medical outcomes. J Healthc Des;1997;9:15–9. 21–4. [PubMed] [Google Scholar]
- 71.Walsh-Sukys M, Reitenbach A, Hudson-Barr D, DePompei P. Reducing light and sound in the neonatal intensive care unit: an evaluation of patient safety, staff satisfaction and costs. J Perinatol. 2001;21:230–235. doi: 10.1038/sj.jp.7200534. [DOI] [PubMed] [Google Scholar]
- 72.Bowie BH, Hall RB, Faulkner J, Anderson B. Single-room infant care: future trends in special care nursery planning and design. Neonatal Netw. 2003;22:27–34. doi: 10.1891/0730-0832.22.4.27. [DOI] [PubMed] [Google Scholar]
- 73.Reynolds JD, Hardy RJ, Kennedy KA, et al. Lack of efficacy of light reduction in preventing retinopathy of prematurity. N Engl J Med. 1998;338:1572–1576. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 74.Birch DG, Birch EE, Hoffman DR, Uauy RD. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. IOVS. 1992;33:2365–2376. [PubMed] [Google Scholar]
- 75.Birch EE, Birch DG, Petrig B, Uauy RD. Retinal and cortical function of very low birthweight infants at 36 and 57 weeks postconception. Clin Vis Sci. 1990;5:363–373. [Google Scholar]
- 76.Kennedy KA, Ipson MA, Birch DG, et al. Light reduction and the electroretinogram of preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;76:F168–173. doi: 10.1136/fn.76.3.f168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fielder AR, Moseley MJ. Environmental light and the preterm infant. Semin Perinatol. 2000;24:291–298. doi: 10.1053/sper.2000.8597. [DOI] [PubMed] [Google Scholar]
- 78.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129–138. doi: 10.1016/s0022-3476(05)81247-8. [DOI] [PubMed] [Google Scholar]
- 79.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 80.SanGiovanni JP, Parra-Cabrera S, Colditz GA, et al. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000;105:1292–1298. doi: 10.1542/peds.105.6.1292. [DOI] [PubMed] [Google Scholar]
- 81.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 82.Tadesse M, Dhanireddy R, Mittal M, Higgins RD. Race, Candida sepsis, and retinopathy of prematurity. Biol Neonate. 2002;81:86–90. doi: 10.1159/000047189. [DOI] [PubMed] [Google Scholar]
- 83.Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retinopathy of prematurity. Pediatrics. 1998;101:654–657. doi: 10.1542/peds.101.4.654. [DOI] [PubMed] [Google Scholar]
- 84.Gunn TR, Easdown J, Outerbridge EW, Aranda JV. Risk factors in retrolental fibroplasia. Pediatrics. 1980;65:1096–1100. [PubMed] [Google Scholar]
- 85.Scher MS, Dobson V, Carpenter NA, Guthrie RD. Visual and neurological outcome of infants with periventricular leukomalacia. Dev Med Child Neurol. 1989;31:353–365. doi: 10.1111/j.1469-8749.1989.tb04004.x. [DOI] [PubMed] [Google Scholar]
- 86.Darlow BA, Clemett RS, Horwood J, Mogridge N. Prospective study of New Zealand infants with birth weight less than 1500g and screened for ROP: visual outcome at age 7–8 years. Br J Ophthalmol. 1997;81:935–940. doi: 10.1136/bjo.81.11.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holmstrom G, el Azazi M, Kugelberg U. Ophthalmological long term follow up of preterm infants: a population based, prospective study of the refraction and its development. Br J Ophthalmol. 1998;82:1265–1271. doi: 10.1136/bjo.82.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larsson EK, Rydberg AC, Holmstrom GE. A population-based study of the refractive outcome in 10-year-old preterm and full-term children. Arch Ophthalmol. 2003;121:1430–1436. doi: 10.1001/archopht.121.10.1430. [DOI] [PubMed] [Google Scholar]
- 89.O’Connor AR, Stephenson TJ, Johnson A, et al. Long term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–18. doi: 10.1542/peds.109.1.12. [DOI] [PubMed] [Google Scholar]
- 90.Choi MY, Park IK, Yu YS. Long term refractive outcome in eyes of preterm infants with and without retinopathy of prematurity: comparison of keratometric value, axial length, anterior chamber depth and lens thickness. Br J Ophthalmol. 2000;84:138–143. doi: 10.1136/bjo.84.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pennefather PM, Tin W, Strong NP, et al. Refractive errors in children born before 32 weeks gestation. Eye. 1997;11:736–743. doi: 10.1038/eye.1997.188. [DOI] [PubMed] [Google Scholar]
- 92.Tabbara KF, Ross-Degnan D. Blindness in Saudi Arabia. JAMA. 1986;255:3378–3384. [PubMed] [Google Scholar]
- 93.Tuppurainen K, Herrgard E, Martikainen A, et al. Ocular findings in prematurely born children at 5 years of age. Graefes Arch Clin Exp Ophthalmol. 1993;231:261–266. doi: 10.1007/BF00919102. [DOI] [PubMed] [Google Scholar]
- 94.Laws D, Shaw DE, Robinson J, et al. Retinopathy of prematurity: a prospective study. Review at six months. Eye. 1992;6:477–483. doi: 10.1038/eye.1992.101. [DOI] [PubMed] [Google Scholar]
- 95.Schalij-Delfos NE, de Graaf MEL, Treffers WF, et al. Long term follow up of premature infants: detection of strabismus, amblyopia, and refractive errors. Br J Ophthalmol. 2000;84:963–967. doi: 10.1136/bjo.84.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dowdeswell HJ, Slater AM, Broomhall J, Tripp J. Visual deficits in children born at less than 32 weeks’ gestation with and without ocular pathology and cerebral damage. Br J Ophthalmol. 1995;79:447–452. doi: 10.1136/bjo.79.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jacobson L, Lundin S, Flodmark O, Ellstrom KG. Periventricular leukomalacia causes visual impairment in preterm children. A study on the aetiologies of visual impairment in a population-based group of preterm children born 1989–95 in the county of Varmland, Sweden. Acta Ophthalmol Scand. 1998;76:593–598. doi: 10.1034/j.1600-0420.1998.760516.x. [DOI] [PubMed] [Google Scholar]
- 98.The International Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Int Ophthalmol. 1985;8:3–10. doi: 10.1007/BF00136454. [DOI] [PubMed] [Google Scholar]
- 99.The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. II. The classification of retinal detachment. Arch Ophthalmol. 1987;105:906–912. [PubMed] [Google Scholar]
- 100.Birch EE, Hale LA. Criteria for monocular acuity deficit in infancy and early childhood. IOVS. 1988;29:636–643. [PubMed] [Google Scholar]
- 101.Salomao SR, Ventura DF. Large sample population age norms for visual acuities obtained with Vistech-Teller Acuity Cards. IOVS. 1995;36:657–670. [PubMed] [Google Scholar]
- 102.Moke P, Turpin A, Beck R, et al. Development of a computerized method of visual acuity testing: adaptation of the Amblyopia Treatment Study visual acuity testing protocol for children. Am J Ophthalmol. 2001;132:903–909. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 103.Holmes J, Beck R, Repka MX, et al. The Amblyopia Treatment Study visual acuity protocol. Arch Ophthalmol. 2001;119:1345–1353. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 104.Pediatric Eye Disease Investigator Group. The clinical spectrum of early-onset esotropia experience of the congenital esotropia observational study. Am J Ophthalmol. 2002;133:109–118. doi: 10.1016/s0002-9394(01)01317-4. [DOI] [PubMed] [Google Scholar]
- 105.Pediatric Eye Disease Investigator Group. Spontaneous resolution of early-onset esotropia: experience of the congenital esotropia observational study. Am J Ophthalmol. 2002;133:109–118. doi: 10.1016/s0002-9394(01)01316-2. [DOI] [PubMed] [Google Scholar]
- 106.Pediatric Eye Disease Investigator Group. The clinical profile of moderate amblyopia in three to six year olds: experience of the Amblyopia Treatment Study 1. Arch Ophthalmol. 2002;120:281–287. [PubMed] [Google Scholar]
- 107.Pediatric Eye Disease Investigator Group. Treatment of moderate amblyopia in children: Results of the Amblyopia Treatment Study 1. Arch Ophthalmol. 2002;120:268–278. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 108.Kivlin JD. A 12-year ophthalmologic experience with the shaken baby syndrome at a regional children’s hospital. Trans Am Ophthalmol Soc. 1999;97:545–581. [PMC free article] [PubMed] [Google Scholar]
- 109.Kivlin JD, Simons KB, Lazoritz S, Ruttum MS. Shaken baby syndrome. Ophthalmology. 2000;107:1246–1254. doi: 10.1016/s0161-6420(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 110.McCabe CF, Donahue SP. Prognostic indicators for vision and mortality in shaken baby syndrome. Arch Ophthalmol. 2000;118:373–377. doi: 10.1001/archopht.118.3.373. [DOI] [PubMed] [Google Scholar]
- 111.Salomao SR, Ventura DF. Large sample population age norms for visual acuities obtained with Vistech-Teller acuity cards. IOVS. 1995;36:657–670. [PubMed] [Google Scholar]
- 112.Multicenter trial of cryotherapy for retinopathy of prematurity: natural history ROP: ocular outcome at 5 1/2 years in premature infants with birth weights less than 1251 g. Arch Ophthalmol. 2002;120:595–599. doi: 10.1001/archopht.120.5.595. [DOI] [PubMed] [Google Scholar]
- 113.Gallo JE, Lennerstrand G. A population based study of ocular abnormalities in premature children aged 5 to 10 years. Am J Ophthalmol. 1991;111:539–547. doi: 10.1016/s0002-9394(14)73695-5. [DOI] [PubMed] [Google Scholar]
- 114.Holmstrom G, el Azzazi M, Kugelberg U. Ophthalmological follow up of preterm infants: a population based, prospective study of visual acuity and strabismus. Br J Ophthalmol. 1999;83:143–150. doi: 10.1136/bjo.83.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McGinnity FG, Bryars JH. Controlled study of ocular morbidity in school children born preterm. Br J Ophthalmol. 1992;76:520–524. doi: 10.1136/bjo.76.9.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O’Connor AR, Stephenson TJ, Johnson A, et al. Visual function in low birthweight children. Br J Ophthalmol. 2004;88:1149–1153. doi: 10.1136/bjo.2003.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohlsson J, Villarreal G, Sjostrom A, et al. Visual acuity, residual amblyopia and ocular pathology in a screened population of 12–13 year old children in Sweden. Acta Ophthalmol Scand. 2001;79:589–595. doi: 10.1034/j.1600-0420.2001.790609.x. [DOI] [PubMed] [Google Scholar]
- 118.Courage ML, Adams RJ. Visual acuity in extremely low birth weight infants. J Dev Behav Pediatr. 1997;18:4–12. doi: 10.1097/00004703-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 119.Zubcov AA, Rossillion BM, Kacer B, et al. [Predictive value of teller Acuity Card Test (TACT) and comparison of recognition and grating acuities in premature children with and without residua of Retinopathy of Prematurity (ROP)] Klin Monatsbl Augenheilkd. 2002;219:722–727. doi: 10.1055/s-2002-35699. [DOI] [PubMed] [Google Scholar]
- 120.Mash C, Dobson V. Long-term reliability and predictive validity of the Teller acuity card procedure. Vis Res. 1998;38:619–626. doi: 10.1016/s0042-6989(97)88335-6. [DOI] [PubMed] [Google Scholar]
- 121.CRYO-ROP group. Three and a half year outcome: structure and function. Arch Ophthalmol. 1993;111:339–344. [PubMed] [Google Scholar]
- 122.CRYO-ROP group. The natural outcome of premature birth and retinopathy. Arch Ophthalmol. 1994;112:903–912. doi: 10.1001/archopht.1994.01090190051021. [DOI] [PubMed] [Google Scholar]
- 123.Hardy RJ, Palmer EA, Dobson V, et al. Risk analysis of prethreshold retinopathy of prematurity. Arch Ophthalmol. 2003;121:1697–1701. doi: 10.1001/archopht.121.12.1697. [DOI] [PubMed] [Google Scholar]
- 124.Tommiska V, Heinonen K, Kero P, et al. A national two year follow up study of extremely low birthweight infants born in 1996–1997. Arch Dis Child Fetal Neonatal Ed. 2003;88:F29–35. doi: 10.1136/fn.88.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fielder AR, Reynolds JD. Retinopathy of prematurity: clinical aspects. Semin Neonatol. 2001;6:461–475. doi: 10.1053/siny.2001.0091. [DOI] [PubMed] [Google Scholar]
- 126.Jacobson L, Fernell E, Broberger U, et al. Children with blindness due to retinopathy of prematurity: a population-based study. Perinatal data, neurological and ophthalmological outcome. Dev Med Child Neurol. 1998;40:155–159. doi: 10.1111/j.1469-8749.1998.tb15439.x. [DOI] [PubMed] [Google Scholar]
- 127.Rahi JS, Dezateux C. Epidemiology of visual impairment in Britain. Arch Dis Child. 1998;78:381–386. doi: 10.1136/adc.78.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rogers M. Vision impairment in Liverpool: prevalence and morbidity. Arch Dis Child. 1996;74:299–303. doi: 10.1136/adc.74.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001;108:809–811. doi: 10.1542/peds.108.3.809. [DOI] [PubMed] [Google Scholar]
- 130.Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol. 2002;120:1470–1476. doi: 10.1001/archopht.120.11.1470. [DOI] [PubMed] [Google Scholar]