Abstract
Purpose
The authors previously presented the results of their 2001 field investigation to rural Brazil to investigate a 336-member pedigree of Leber hereditary optic neuropathy (LHON). The present work describes the yearly field investigations 2001 to 2005, utilizing a variety of highly sophisticated psychophysical and electrophysiologic procedures, in asymptomatic LHON carriers, some of whom converted to affected status.
Methods
Careful, repeated examinations of 75 carriers of homoplasmic 11778 LHON mtDNA J-haplogroup mutants were performed as part of the field investigation of this pedigree. All subjects underwent a detailed neuro-ophthalmologic investigation, including formal visual fields (Humphrey; HVF) and fundus photography. In addition, many subjects underwent rigorous psychophysical examination, including Cambridge Research Systems color vision and contrast sensitivity testing, OCT, GDx, and multifocal visual evoked response (mfVER) and multifocal electroretinogram (mfERG). Two patients followed as nonsymptomatic LHON carriers converted to affected status.
Results
Many LHON carriers did, in fact, show subclinical or occult abnormalities. Focal edema was often seen involving the arcuate nerve fiber bundles, and this corresponded with areas of relative paracentral or arcuate scotomas on HVF testing. Compared to controls, LHON carriers had significant losses in color vision affecting mostly the red-green system and reduction in spatial but not temporal contrast sensitivity. The mfVER and mfERG data showed that most carriers had depressed central responses and abnormal interocular asymmetries.
Conclusions
In this very large pedigree of 11778 LHON, the carriers frequently showed manifestations of optic nerve impairments. Their occult disease reflected low-grade compromise that waxed and waned. In two cases, these changes led to a crescendo of dramatic impairments that characterize conversion to affected status.
INTRODUCTION
Leber hereditary optic neuropathy (LHON) is a maternally inherited genetic disorder, which ultimately may result in large and absolute bilateral centrocecal scotomas in young adults.1,2 In a subpopulation of visually affected individuals, it produces retinal ganglion cell degeneration and axonal loss in the optic nerve.3,4 LHON typically manifests as a subacute bilateral loss of vision that affects predominantly young adult males.5,6 LHON is usually due to one of three pathogenic mitochondrial DNA (mtDNA) point mutations.6 These mutations affect nucleotide positions 11778, 3460, and 14484, respectively, in the ND4, ND1, and ND6 subunit genes of complex I; this affects oxidative phosphorylation in mitochondria.7–11 Other, rarer mutations continue to be described.12
Penetrance is highly variable, even within the same family carrying a pathogenic mutation in homoplasmic fashion (all mtDNA copies mutated).13 The rate of penetrance varies with the mutation and pedigree, though it always is much greater in males. Hence, in a typical family with 11778 mtDNA, 8% of the women and 40% of the men may suffer devastating and sudden visual loss in young adulthood. Why one sibling might go blind when the other, who from the point of view of mitochondrial genetics is an identical twin, maintains good vision throughout life, is a mystery. Attempts have been made to explain this by environmental5,6 or supplementary genetic factors. For example, there is increasing evidence that tobacco and alcohol consumption plays a role.14,15 There is also an active search for nuclear genes16 that might modify the penetrance.
Clinically, some fundus changes, such as microangiopathy and nerve fiber layer swelling, have been described to immediately precede or accompany the onset of visual loss. This process, though usually bilateral, occurs asynchronously over the course of several weeks to months and eventually evolves to severe optic atrophy and irreversible impairments of vision.1,17–19 The smaller-caliber fibers of the papillomacular bundle (PMB) are selectively lost at a very early stage of the pathological process, which eventually extends to most of the rest of the nerve leading to optic atrophy.6 The acute stage usually lasts a few weeks. The affected eye characteristically demonstrates an early dropout of the PMB; an edematous appearance of the rest of the nerve fiber layer, especially in the arcuate bundles; and enlarged or telangiectatic and tortuous peripapillary vessels (microangiopathy).
These main features are seen on fundus examination, just before or subsequent to the onset of visual loss.17 Examination reveals decreased visual acuity, dyschromatopsia, and cecocentral scotoma on visual field examination.1,17–19 There are a few reports of spontaneous recovery,5 especially with the 14484/ND6 mutation,10 but generally the visual loss progresses and then stabilizes within a year, with the common fundus features being loss of the PMB and optic atrophy worse on the temporal side.1,17–19
There are many remarkable features in LHON, such as the question of why retinal ganglion cells are particularly vulnerable, and why, in particular, the small fibers of the PMB are involved first.6,12 Why should a subject do well until his 20s and then suddenly lose vision in both eyes within weeks? Several epidemiologic studies, including ours in Brazil, have implicated precipitating factors such as the use of antibiotics,20,21 smoking, and ethanol consumption.19,22 However, several fundamental questions remain unresolved: Why is there incomplete penetrance? Why are men much more likely to become affected than women? Why is there tissue specificity? Why do subjects do well and then suddenly, as young adults, decompensate?
In an effort to address these questions, the authors have been conducting, since 2001, large-scale, yearly field investigations to rural Brazil to study a seven-generation pedigree, with more than 336 members, of a family with LHON harboring the 11778 mtDNA mutation, homoplasmic J-haplogroup.
METHODS
The authors became aware of this extremely large pedigree when contacted by the mother of the index case in the summer of 2001. Her 14-year-old son had suddenly lost vision in one eye, and she reached us through the International Foundation for Optic Nerve Disease. She and her nuclear family were examined, photographed, and evaluated by psychophysical instruments. Blood samples confirmed the clinical impression that they were homoplasmic for 11778 J-haplotype and that all three male family members had optic neuropathy.
The seven-generation maternal lineage we reconstructed descended from an Italian female ancestor born in 1861.12 Starting in 2001, we assembled a large team of international and Brazilian investigators and made a yearly field investigation to Colatina, Espirito Santo state, approximately 650 km from Rio de Janeiro, Brazil.12,19,23,24 Five hundred seventy eyes from about 285 of the 328 living family members of this very large LHON pedigree were evaluated. This prospective investigation included asymptomatic LHON carriers as well as those affected with symptomatic visual loss. Epidemiologic interviews were conducted that emphasized possible environmental risk factors, comprehensive neuro-ophthalmologic examinations, psychophysical tests, Humphrey visual field (HVF) studies, GDx analysis, fundus photography, electrophysiology and blood testing for mitochondrial genetic analysis, and serologic measures of oxidative stress and neurologic distress. All maternally related family members were invariably homoplasmic for 11778/ND4 mutation with a haplogroup-J mtDNA background, 33 being affected, of which 22 are still living.
This study population allowed us to prospectively examine carriers as well as affected members of this large pedigree with extensive testing. In addition, two unaffected carriers were followed for years and then underwent fairly classic conversion, offering the unparalleled opportunity of documenting the natural history of this conversion with a variety of very sophisticated measures. Both cases revealed several changes that preceded the first symptoms of visual loss.
A full neuro-ophthalmologic examination was performed on each patient. Best-corrected visual acuity was assessed with the ETDRS visual acuity chart. Ophthalmoscopy was performed with high-intensity red-free light, and fundus pictures were captured on 35-mm color slides with a 30° fundus camera. The visual field examination was performed with the SITA threshold strategy for program 24.2 on the Humphrey Field Analyzer (Humphrey Systems, Inc, Dublin, California). Patients with cataract and/or other ocular diseases not related to LHON were not considered for visual field examination. For evaluation and classification of each visual field, the methods reported by the Optic Neuritis Treatment Trial were used.25 Optic disc photographs were independently reviewed by at least two neuro-ophthalmologists; they commented on presence or absence of six itemized fundus features (optic disc pallor, optic disc hyperemia, microangiopathy, nerve fiber layer swelling, nerve fiber layer deficit, peripapillary atrophy) and indicated a grade of severity (0=absent, 1=mild, 2=moderate, 3=severe). The observers were masked regarding the patients’ data and the opinions of the other reviewers.
Many (but not all) of the subjects underwent sophisticated psychophysical examination, including Cambridge Systems colour vision and contrast sensitivity testing,24 optical coherence tomography,26 GDx, and electrophysiologic tests such as multifocal visual evoked responses (mfVERs) and electroretinograms (ERGs).27, 28 Serologic testing was also performed to look for evidence of oxidative stress or neuronal distress by neuron-specific enolase.
Quantitative measurements were obtained by the Optical Coherence Tomographer (Stratus OCT, Carl Zeiss Ophthalmic Systems Inc). This instrument employs low-coherence interferometry to generate cross-sectional images of the retina, the optic disc, and retinal nerve fiber layer (RNFL) with ≤10 μm axial resolution and transverse resolution of 20 μm. With each scan pass, the Stratus OCT captures from 128 to 768 axial A-scans. The RNFL thickness algorithm works in a two-pass process: it looks first for highest rates of changes in reflectivity at the vitreoretinal interface and then for reflectivity above a threshold value, from which nerve fiber layer thickness is calculated.
All data were eventually organized in a fully comprehensive database and subjected to statistical analysis. We computed standard deviations for numerical data and tested by chi-square test or Fisher exact test. Four groups of patients were independently evaluated: (1) carriers (those carrying the 11778 mutation and with no visual complains), (2) affected (those with the 11778 mutation and the optic neuropathy), (3) controls (spouses of the maternally related individuals having neither the mtDNA mutation nor any significant visual problems), and (4) male descendents (offspring of affected or carrier males). For the present purposes, we concentrated on the 75 carriers, 48 of whom were available for all of the testing.
RESULTS
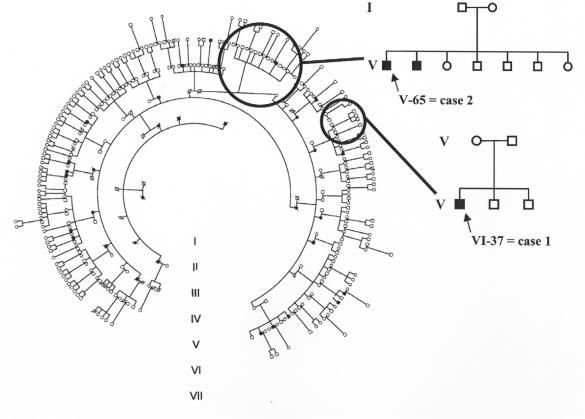
The purpose of this paper is to review the results of the authors’ yearly field investigations on an extensive LHON pedigree to rural Brazil during the period of 2001 to 2005. The initial evaluation, in the fall of 2001, gave us opportunity to examine almost all of the 295 living in the area and belonging to the pedigree. Blood testing allowed us to determine that all were homoplasmic for the G11778A mtDNA mutation with J-haplogroup. Also, a pedigree of seven generations beginning with an immigrant from Veneto, Italy,19 was assembled (Figure 1). This family and the resultant generations settled in a small region bounded by three cities west of Vitoria, Brazil.
FIGURE 1.
Seven-generation pedigree of 11778/ND4 J-haplogroup Leber hereditary optic neuropathy (LHON). This Brazilian family descends from a female Italian founder, born in Verona in 1861. The two newly affected cases are identified in their nuclear families by arrows.
The first reported19 findings were on 20 affected patients, 74 carriers, and 68 controls. Further field expeditions allowed us to see a few more subjects in each category. Several general finding can be summarized for the first two groups.
The affected group consisted of 17 males and three females. The average age at onset of visual loss was 26 ± 10 years (range, 10 to 41 years). The age at onset did not change significantly over the generations. The average visual acuity of those affected was 0.0125 (5/400; range, light perception to 20/400). Three quarters of the patients recalled their visual loss as occurring either simultaneously in both eyes or occurring in the second eye within a couple of weeks from the event in the first eye. The carrier group consisted of 27 males and 49 females, although one was excluded on account of unrelated retinal disease.
Very extensive epidemiologic investigations were conducted in attempts to correlate exposures, risk factors, and lifestyle to the affected status. Generally, two risk factors stood out19: 65% of these 20 affected patients smoked, and 60% drank heavily. This was statistically significant in comparison to the carriers (14% and 34%, respectively; P < .01) or controls (26% and 38%, respectively; P < .05).
Many of the carriers demonstrated changes on at least some of the neuro-ophthalmologic and psychophysical testing. None of the carriers were aware of any visual impairment. All specifically denied problems with visual acuity, visual field, and color vision. Nonetheless, many had abnormalities in these and other psychophysical areas as well as signs on clinical examination.
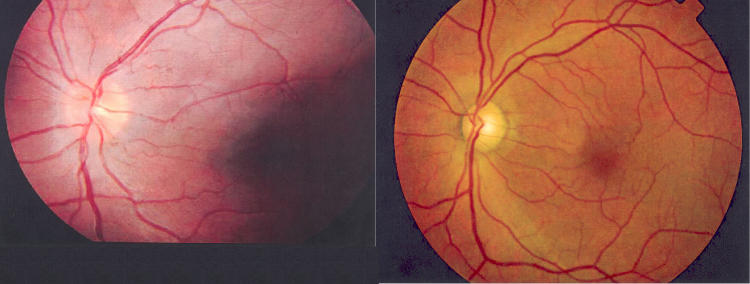
In regard to ophthalmologic examination, we included 75 carriers, of which 26 were male and 49 were female.22 The mean age of this group was 31, and the mean visual acuity (excluding five with cataracts) was 20/20-. Most remarkably, of these 75 carriers, microangiopathy of the optic disc was seen in 13% of the eyes and in 21% of the subjects (Figure 2). Two of these carriers with abnormal telangiectatic vessels of the optic disc were less than 12 years old. Focal nerve fiber layer swelling was also found in about 14% of the eyes and 21% of the subjects (Figure 2). Once again, a few of these patients were under 12 years of age. Eighty-six percent of the eyes with focal nerve fiber layer swelling also had microangiopathy. Optic disc pallor was much less common (3% of the eyes).
FIGURE 2.
Leber hereditary optic neuropathy. Left, Case 1. Fundus photograph taken during year 2 (2002) as part of the routine examinations of carriers. At the time, the patient had no visual complaints and visual acuities of 20/20 OU but had borderline deficiencies on FM-100 color vision testing OS. Fundus examination showed mild nerve fiber layer swelling and subtle microangiopathy both superior and inferior temporally. The patient first complained of visual loss in this eye 5 months later in 2003. Right, Case 2. Fundus photograph OS taken at year 3 (2003), about 8 months after onset of visual loss in this eye. This shows some generalized nerve fiber layer loss but relative preservation of the inferior arcuate bundle by comparison to profound papillomacular bundle loss. At this date, the patient’s visual acuity was reduced to counting fingers in this eye.
Most of the eyes with abnormal fundus findings also had abnormalities on HVF testing.22 Although 30% of the carriers had abnormalities on HVF, reliability was not very good. However, those patients with abnormal fundus findings most often showed visual field defects on HVF testing that conformed to the very same abnormalities noted on funduscopy. Further corroboration of such changes could be observed on serial GDx studies29 (Figure 3).
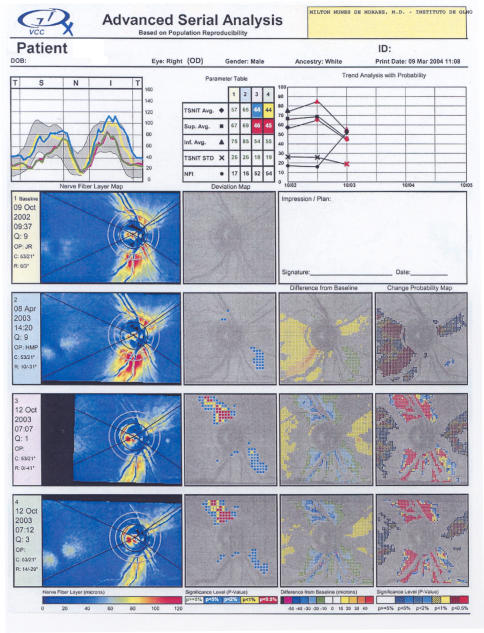
FIGURE 3.
Leber hereditary optic neuropathy (LHON). An example of GDx changes in the right eye of case 2. Advanced Serial Analysis allows comparison of GDx VCC scans over time. Both decreases in thickness and increases in thickness are documented. The TSNIT curves (upper left of printout) are color coded for each examination. The Trend Analysis with Probability (upper right) is depicted by lines connecting the results listed in the Parameter Table. Bright colors depict increases in birefringence at the noted location and dark colors depict decreases in birefringence. Increases in birefringence occur in the acute stage of LHON, perhaps on account of an increase in the number of microtubules of mitochondria.
Studies of color vision began (2001) with use of pseudoisochromatic color test plates. The following year, FM-100 studies were added. Subsequently, the color vision testing was improved by utilizing the Cambridge Colour Test (Cambridge Research Systems) and also contrast sensitivity was tested with the PSYCHO software (Cambridge Research Systems).
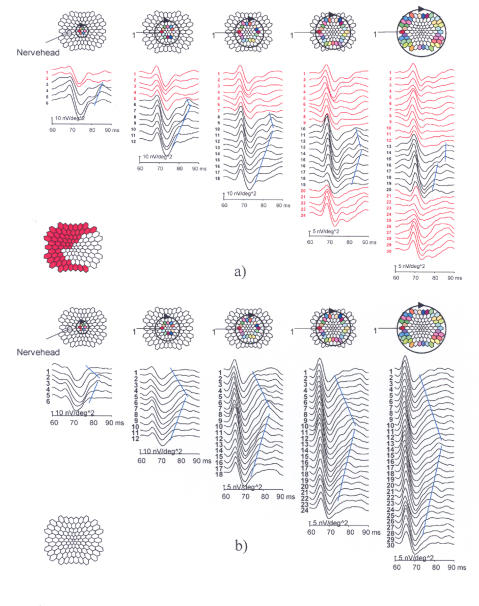
In general, statistically significant differences were found between the LHON carriers and controls.24 This was true for all spatial frequencies of chromatic and luminance spatial contrast sensitivities (P < .001). However, the fundus findings described above did not correlate with these abnormalities in color vision and contrast sensitivity. mfERG changes that were deemed highly abnormal were also seen in several carriers, including those noted to have abnormalities on color and contrast sensitivity testing. These mfERG changes included abnormal dynamics of inner retinal responses and deficiencies of the optic nerve head component in the paracentral regions (Figure 4).
FIGURE 4.
Example of a highly abnormal unaffected male Leber hereditary optic neuropathy (LHON) carrier mfERG in comparison with a carrier whose responses are indistinguishable from those of normal subjects. The optic nerve head component (ONHC) is recognized by its varying implicit time that increases in proportion to the fiber length connecting the stimulus patch with the optic disc. It is generated near the lamina cribrosa, at the transition where the fibers become myelinated. The traces on concentric circles around the fovea are plotted in columns, always starting from the trace closest to the nerve head, proceeding through the upper visual field, and returning through the lower field. The blue lines connect a feature contributed by the ONHC. In the red traces and corresponding red stimulus areas, the ONHC is considered below normal or missing.
In regard to OCT, LHON-affected patients showed extensive thinning of the RNFL, as would be expected in cases of optic atrophy. LHON carriers sometimes showed significant RNFL thickening of the arcuate bundles, especially in correlation with similar changes noted by funduscopy or GDx. Less often, we saw thinning in the temporal and inferior quadrants, as well as in the 360° average measurement.
This study population also allowed us to prospectively examine carriers as well as affected members of this large pedigree with this extensive testing. The subclinical findings described in several of our carriers can be best demonstrated in the summarization of two unaffected carriers who were followed for years and then underwent fairly classic conversion. This also offers an unparalleled opportunity of documenting the natural history of this conversion with a variety of very sophisticated measures. Both cases revealed several subclinical abnormalities that preceded the first symptoms of visual loss.
CASE REPORTS
CASE 1
The patient, a 12-year-old boy, was first seen in 2001 together with almost 300 members of his extended family. He was the sixth cousin of the proband (Figure 1). On presentation, he had no visual complaints and was in good general health without significant exposure to any risk factors. His diet was good.
Best-corrected visual acuities were 20/20 OU, and results of optic nerve functions studies, including color, were normal. Slit-lamp examination, intraocular pressure measurements, and ocular motility examinations were normal.
Dilated fundus examination of the optic discs showed no swelling or pallor, and the cup-to-disc ratio was 0.1 OU. However, there was a subtle hint of microangiopathy (a few telangiectatic vessels) temporally OD. Nonetheless, the ocular examination was deemed to be normal. Contrast sensitivity studies were normal across all spatial frequencies; however, HVFs showed three missed central points out of 76 OD and no missed points OS.
YEAR 2
One year later, the patient returned for routine follow-up examination with no subjective complaints. His visual acuities remained normal as did all optic nerve functions. Dilated fundus examination showed no changes compared to the previous examination, although there was still mild nerve fiber layer swelling and subtle microangiopathy superiorly and inferiorly, worse OS than OD (Figure 2, left). The optic nerve fiber layer was also analyzed by GDx (VCC/SLO), and the baseline values were deemed as normal OU.
HVFs had improved to completely normal OD, although there was a slight nonspecific general depression centrally OS, which was seen on frequency-doubling technology.
YEAR 3
The patient returned for the third yearly comprehensive follow-up examination, and visual acuities and most of the examination remained normal. However, fundus examination showed temporal pallor, and there was obvious inferior NFL swelling and microangiopathy OD. There was also NFL swelling inferiorly OS. HVFs again demonstrated slight nonspecific general depression centrally OU. GDx testing confirmed the funduscopic findings of focal areas of NFL swelling at the superior and inferior poles OU. However, at the same time, there was thinning noted in the temporal NFL bundles OU.
YEAR 4
Six months later, the patient presented with complaints of subacute onset of progressive blurred vision OS, of a few weeks duration. His visual acuities were impaired to 20/20 OD and 20/100 OS, and there was an afferent papillary defect (APD) OS. HVFs were normal OD but demonstrated a small and dense central scotoma OS. GDx testing in the affected eye confirmed dramatic thinning of the NFL. However, surprisingly, in the nonaffected eye (OD), GDx also showed that temporally there was marked thinning of the NFL.
Subsequent examinations in the same year demonstrated further deterioration of vision OS with visual acuities 3 months after the first symptoms at 20/20 OD and 20/400 OS. Furthermore, HVF showed slight central depression OD as well as a large central scotoma OS. Both eyes, after an initial GDx thickening of some NFL, now showed areas of thinning.
YEAR 5
A few months later, the patient became symptomatic in the right eye as well. His visual acuities now were 20/400 OD and counting fingers 3 feet OS. Fundus examination showed temporal pallor OD and severe diffuse optic atrophy OS. GDx testing showed further thinning of the NFL OU. The other parameters of vision were typical for a recently affected patient with LHON.
CASE 2
This patient was also followed as he transitioned from the carrier to affected status. His condition paralleled that of the patient described in case 1. The patient is an uncle of the proband (and the third cousin of the patient in case 1). He was first examined with the family in 2001 and presented as an asymptomatic carrier. When he was first seen, he was 44 years old and had no visual complaints. However, unlike his cousin, he had several risk factors. He had smoked up to 20 homemade cigarettes a day for over 30 years; he drank an average of five cachacas (hard liquor comparative to rum) and five beers per day and drank at that rate for 23 years. On presentation, his visual acuities were 20/20 OU and optic nerve function studies were all normal. Dilated fundus examination showed discs, macula, and vessels to be normal. In particular, the optic discs showed no swelling or pallor, and we saw no swelling of the nerve fiber layer. The ocular examination was deemed to be completely normal. HVFs were performed and found to be normal. Contrast sensitivities were normal across all spatial frequencies.
One year later, he continued to have no subjective complaints of visual losses. His visual acuities remained 20/20 OU. Optic nerve functions were all normal. However, fundus examination showed, for the first time, abnormalities consisting of telangiectatic vessels in association with swollen fibers in the NFL at the inferior optic disc margin OD. Telangiectasias without NFL swelling were also seen temporally OS. GDx also showed borderline swelling of the NFL inferiorly OU. However, HVF and visual evoked potentials were all normal. Nine months later, the patient came in complaining of a recent loss of vision OS characterized as a darkened area in the center of his vision. A month later, he also noticed blurring OD. His visual acuities were impaired to 20/40 OD and 20/60 OS, and there was dyschromatopsia and an APD OS. Fundus examination showed swelling of the NFL in an inferior bundle at the margin of the optic discs OU associated with some fine telangiectatic vessels OU.
HVFs now became abnormal. There was a slight central depression OD and a small and dense central scotoma OS. GDx testing confirmed the fundus findings documenting increased thickening of the inferior NFL at the disc margin OD (Figure 2, right). Over the next 2 weeks, the patient noted further deterioration of vision OU, and his visual acuities deteriorated to 20/400 OD and counting fingers 5 feet OS. The GDx showed a mild reduction of the previously very swollen inferior NFL OD, and there were marked increases in NFL thickening both superiorly and inferiorly OS. Six months later, the patient had visual acuities of counting fingers 4 inches OU. Fundus examination showed diffuse and severe optic atrophy OU. HVF showed complete depression within the central 24 degrees. GDx testing now showed a marked decrease of thickening of both superior and inferior NFL OU. Viewed serially, the four GDx scans (Figure 4; right eye of case 2) in both eyes suggest that the changes that occurred as this LHON carrier converted to affected were (1) thickening of the PMB bundle, (2) thickening of RNFL in the arcuate bundles, (3) loss of most of the PMB, followed by (4) loss of most of the RNFL.
DISCUSSION
Yearly systematic and comprehensive examinations of the authors’ very large 11778 LHON pedigree allowed us to follow the natural history of this optic neuropathy with a large number of clinical, psychophysical, and laboratory measures. Three unexpected findings are described. First, affected patients demonstrated small deteriorations of their visual impairment. Second, carriers, who had no symptoms, did often demonstrate occult or subclinical disease. Third, visually unaffected carriers were observed going through the process of conversion to affected status. The classic description of LHON is as an all-or-none phenomenon whereby a few subjects suffer sudden and catastrophic monophasic visual loss.1,18 Yet, all three findings of the present study speak to the impression that LHON may represent a low-grade constant compromise of retinal ganglion cell function.
Many of the LHON carriers showed subclinical abnormalities. Focal edema was often seen involving the arcuate nerve fiber bundles, and this corresponded with areas of relative paracentral or arcuate scotomas on HVF testing. There were significant differences in color vision and contrast sensitivity between carriers and controls. The ONHC of mfERG data showed depressed paracentral responses and abnormal interocular asymmetries.
In both of the cases described here, peripapillary NFL swellings associated with mild microangiopathy at the superior and inferior poles of the optic disc were found on routine screening. GDx confirmed the NFL swelling. There were also mild central depressions as noted on HVF testing. These signs became more obvious the next year. In case 1, a paracentral scotoma was noted on frequency-doubling technology. The patient went on to have subacute loss of vision in one eye, followed months later by loss of vision in the other eye.
It is interesting to consider the progression of the subclinical signs, before the onset of acute visual loss in the conversion cases of LHON. However, it should also be noted that the same subclinical signs sometimes waxed and waned in other members of the pedigree. In the present case, we observed a march from focal nerve fiber layer swelling (especially at the superior and inferior poles) to an associated microangiopathy, then to central or paracentral mild visual field depressions, and then PMB thinning observable by fundus examination as well as by GDx. Finally, this buildup ended in severe subacute visual loss. Further NFL thinning and optic atrophy followed. These subclinical findings and their crescendo before the threshold of permanent and dramatic visual loss may provide important clues to the underlying pathophysiology.
Three intriguing disconnects are noted. The first is the temporal difference between the first subclinical signs and later visual loss. The second divide is between structure and function, as often the NFL swelling did not associate with visual loss. The third is a spatial disconnect. The most obvious NFL losses seen were in the inferior pole in the absence of arcuate scotomas but in association with relative central scotomas that anatomically should reflect PMB defects.
Recently, using OCT, a similar combination of changes in the NFL of LHON patients has been reported.30,31 The investigators describe in LHON-affected patients a stage of initial swelling of the NFL and then later a dramatic thinning of the temporal fibers. They also note that in LHON carriers, a statistically significant increase in NFL thickness can be seen by OCT.
These three disconnects suggest that structural changes need not directly reflect the primary pathology of LHON. Instead, NFL swelling may reflect compensatory effects such as the accumulation of mitochondria. Such aggregations of mitochondria have been described in several mitochondrial diseases, including LHON muscle.3 As explanation, we suggest that under duress, some of the nerve fibers adequately compensate by swelling with mitochondria, whereas others fail to do so, lose function, and eventually atrophy. Other changes, such as microangiopathy, may reflect the elaboration of trophic factors, such as vascular endothelial growth factor, by distressed neurons.
The first morphologic changes may reflect a wave of compensatory effects that may delay or even prevent the pathophysiology from reaching a critical threshold. However, once this threshold is reached, a cascade of decompensation occurs. This period of balance near the tipping point in LHON may be long, lasting many months. However, once the threshold is crossed, there may follow fairly acute losses of visual function.
This unparalleled opportunity of following over 300 patients with the same mtDNA mutation for LHON over a course of 5 years permitted us to observe subtle as well as dramatic evidence of the disease. Carriers frequently demonstrated manifestations of optic nerve impairment. The subclinical disease documented reflects a low-grade compromise that may often be compensated for. However, decompensation, often after a period of progressive worsening of visual function, may lead to dramatic impairments that mark the conversion from carrier to affected status in LHON. Further quantitative and objective measures performed in a very large number of well-characterized and homogenous cases of LHON can test the hypothesis of pathophysiology and, ultimately, lead to a rational and effective means of treatment.
PEER DISCUSSION
DR KENT W. SMALL
This paper is a snapshot of an important large longitudinal study of a single large family with a rare inherited disease, LHON. This ongoing study is important for many reasons, the most important of which is that this study holds one of our best hopes of being a successful genotype-phenotype correlation study. In other words, how does the genotype, the mutation or DNA change, affect the clinical aspects of the disease such as appearance, age of onset, severity, and penetrance? What makes this study particularly interesting is that because the primary mutation is already known, and many other variables are controlled for, other genetic modifying factors may be discovered.
Some genetic factors that are currently under investigation are nuclear genomic and mitochondrial mutations and heteroplasmia. Nuclear genomic factors modifying the expression of LHON (epistasis) has long been suspected. Because of the markedly different penetrance between males and females, a locus on the x- chromosome has been suspected but to date not found.
Once the mutation was found for LHON, many of us assumed that the variable penetrance was due to heteroplasmia of the mutation in different tissues. Most studies report only leukocyte heteroplamia/homoplasmia. To date, the degree of heteroplasmia has not been shown to predict the phenotype. However, the most important tissue to assay would be optic nerve tissue which is understandably difficult to obtain. There are three other ongoing studies of large LHON family and datasets which offer some possible clues into the variable expression of LHON. These studies are in China, Thailand and The Netherlands (1–4). Some interesting results have recently been published from these and it would be interesting to see if these same factors play a role in Dr. Sadun’s Brazilian family.
Mitochondrial factors that may be altering the expression of LHON have been studied. The halpotype or the DNA sequence of the remaining mt DNA has special interest since the mt haplotype has been of great interest in studying population migrations. A few studies suggest that this might have some role. The large Thailand study of 30 G11778A pedigrees has suggested that there are other secondary/modifying base pair changes in the mitochondrial DNA. They found that the secondary LHON mutations G3316A and C3497T had a synergistic deleterious effect with the G11778A mutation, accelerating the onset of the disease in their patients. A novel mutation, tRNAMet A4435G, has a potential modifier role in increasing the penetrance and expressivity of the primary LHON-associated G11778A mutation in a large Chinese family. The large Dutch study of 25 families with LHON that have been followed for over 40 years has found no genotype – phenotype correlation.
I applaud Dr. Sadun’s pioneering work and his comprehensive and exhaustive studies of this family. We look forward to future studies from Dr. Sadun and hope that this methodical study will continue to reveal new and important findings.
REFERENCES
- 1.Qu J, Li R, Zhou X, et al. The novel A4435G mutation in the mitochondrial tRNAMet may modulate the phenotypic expression of the LHON-associated ND4 G11778A mutation. Invest Ophthalmol Vis Sci. 2006;47:475–483. doi: 10.1167/iovs.05-0665. [DOI] [PubMed] [Google Scholar]
- 2.Zhadanov SI, Atamanov VV, Zhadanov NI, Schurr TG. De novo COX2 mutation in a LHON family of Caucasian origin: implication for the role of mtDNA polymorphism in human pathology. J Hum Genet. 2006;51:161–170. doi: 10.1007/s10038-005-0340-y. [DOI] [PubMed] [Google Scholar]
- 3.Chuenkongkaew WL, Suphavilai R, Vaeusorn L, et al. Proportion of 11778 mutant mitochondrial DNA and clinical expression in a Thai population with Leber hereditary optic neuropathy. J Neuroophthalmol. 2005;25:173–175. doi: 10.1097/01.wno.0000176631.87234.49. [DOI] [PubMed] [Google Scholar]
- 4.Spruijt L, Kolbach DN, de Coo RF, et al. Influence of mutation type on clinical expression of Leber hereditary optic neuropathy. Am J Ophthalmol. 2006;141:676–682. doi: 10.1016/j.ajo.2005.11.007. [DOI] [PubMed] [Google Scholar]
DR EDWIN M. STONE
Could you comment on your current understanding of why, with all this axonal death, there is no cupping in this disease?
DR DENNIS M. ROBERTSON
Having identified the risk factors of alcohol and smoking, have there been instances when somebody's lifestyle changed (for example, becoming a smoker or drinker at a later age) and precipitated a late onset visual loss?
DR MARILYN B. METS
In thinking about maternal inheritance, why would one brother have the disease and another brother does not? In oogenesis while the egg is being developed the mitochondria segregates randomly, so you would think that often you would get one egg with mostly affected mitochondria, and another egg with mostly unaffected mitochondria. Did you find that in your study?
DR SEAN P. DONAHUE
We think of this disease having a sudden precipitous loss. Do you still think that is the case, or could it be a slow progression that suddenly hits a crescendo, and the crescendo really just is reduced redundancy of whatever’s left and you end up finally losing those resistant primarily central fibers?
DR ALFREDO A. SADUN
In regards to Dr. Small’s question, as is the case with most of the families, these were homoplasmic, i.e. all the patients had all abnormal mitochondria, without any mixture. In fact, if I might reply to Dr Mets’ question out of order, the issue would not apply in this particular study because the mother had nothing but abnormal mitochondria. There would be no possibility of having different oocytes with different combinations of wild type and abnormal ones.
We did have an opportunity to look at the optic nerves for homoplasmy as well for, as Dr. Small pointed out, such genetic sampling is usually done on leukocytes in the blood. We can talk about this heteroplasmy issue, but in this particular family, not only is there homoplasmy across all patients, but across all tissues.
Dr. Small raised the issue as to whether there is some hot spot and indeed we found such a hot spot on the X chromosome, and there is of course this predilection for men to have the disease. The particular place on that hot spot is not clear and we do not know what nuclear modifying factor is the key player. Included in that hot spot is the MnSOD, which would a good way of dealing with the reactive oxygen-species that might accumulate as a result of this blocked up oxidative phosphorylation.
Dr. Small raised the issue of the polymorphisms seen in other studies, that is, the mitochondria DNA that is not mutated but has different versions. Different background DNA might be interesting in terms of why perhaps one family has a higher penetrance than another family. This would not, however, answer the basic question just raised of why one brother does, and the other doesn’t lose vision because all the mitochondria, in terms of background, mutation type, and polymorphism in this homoplasmic family, are absolutely identical.
Dr. Stone asked about cupping. The OCT studies have shown us is that there is a little bit of a correlation; the patients who have the smallest optic disks are most likely to lose vision at an early age. So there may not be more cupping since they started off with a more crowded disk. OCT studies have also shown that LHON children who have small optic disks often develop a typical picture of LHON affected at a young age (publication forthcoming). Those children who have a large optic disk don’t develop visual symptoms at all, are protected, or develop a much more subacute version of this disease.
Dr Robertson asked whether lifestyle changes might have provoked the loss of vision in our large pedigree. The second case that I mentioned in my presentation was a 44-year-old who had gone into clinical depression, about a year or two before his loss of vision, with associated heavy drinking and smoking. With an N of one, we don’t want to make too much of this case but it might be an example of what Dr. Robertson was proposing. More persuasive, however, is the family that Dr John Keltner and I described from Northern California wherein the mother lost vision, her 9-year-old daughter lost vision (unusual at that age and gender), and her 4-year-old daughter all became affected, i.e. all of her children at this point have lost vision all within a few months of each other. They happened to have lived downwind of a large tire fire and had been inhaling a lot of smoke from that fire.
In terms of the tempo of visual loss as Dr Donohue asks, perhaps the OCT has been our most useful instrument. The OCTs suggest that there is a curve regarding the thickness of the temporal nerve fiber layer that reflects a loss of axons and that every patient seems to have a slightly different curve. Some are on a slightly downward course and others are more steeply down. Sometime, usually about three to nine months before the clinical crash, the curve becomes very nonlinear and really drops off. So, there is, certainly, this effect of constant attrition, but there’s also some sort of a positive feedback or threshold effect, by which the curve loses linearity and there is a much greater loss of fibers in a short period of time.
ACKNOWLEDGMENTS
We thank the family of Maria Franchi, as well as our investigator colleagues, Jeffrey Roth, Tim Lam, Milton Moraes, Milton Moraes Filho, Celina Tamaki, Luana Mendieta, Josenilson Tereira, Paula Sacai, Frafael Cinoto, Rafael Andrade, Sung Watanabe, Mirella Gualtieri, Marcelo Costa, Andre Pereira, Angelo Passos, Fred Ross-Cisneros, and Hevillin Paula.
REFERENCES
- 1.Newman NJ. Leber’s optic neuropathy. In: Miller NR, Newman NJ, eds. Walsh and Hoyt’s Clinical Neuro-Ophthalmology Baltimore: Williams & Wilkins; 1998:742–753.
- 2.Carelli V. Leber’s hereditary optic neuropathy. In: Schapira AHV, DiMauro S, eds. Mitochondrial Disorders in Neurology 2nd ed. Boston: Butterworth-Heinemann; 2002;115–142.
- 3.Sadun AA, Kashima Y, Wurdeman AE, et al. Morphological findings in the visual system in a case of Leber’s hereditary optic neuropathy. Clin Neurosci. 1994;2:165–172. [Google Scholar]
- 4.Kerrison JB, Howell N, Miller NR, et al. Leber hereditary optic neuropathy. Electron microscopy and molecular genetic analysis of a case. Ophthalmology. 1995;102:1509–1516. doi: 10.1016/s0161-6420(95)30838-x. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo JF., III Adenosine triphosphate deficiency: a genre of optic neuropathy. Neurology. 1995;45:11–16. doi: 10.1212/wnl.45.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Sadun AA, Win PH, Ross-Cisneros FN, et al. Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Acad Ophthalmol. 2000;98:881–923. [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers RM, Schapira AHV. Clinical, biochemical and molecular genetic features of Leber’s hereditary optic neuropathy. Biochem Biophys Acta. 1999;1410:147–158. doi: 10.1016/s0005-2728(98)00163-7. [DOI] [PubMed] [Google Scholar]
- 8.Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 9.Huoponen K, Vilkki J, Aula P, et al. A new mtDNA mutation associated with Leber hereditary optic neuropathy. Am J Hum Genet. 1991;48:1147–1153. [PMC free article] [PubMed] [Google Scholar]
- 10.Howell N, Bindoff LA, McCullough DA, et al. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991;49:939–950. [PMC free article] [PubMed] [Google Scholar]
- 11.Mackey D, Howell N. A variant of Leber hereditary optic neuropathy characterized by recovery of vision and by an unusual mitochondrial genetic etiology. Am J Hum Genet. 1992;51:1218–1228. [PMC free article] [PubMed] [Google Scholar]
- 12.Carelli V, Ross-Cisneros F, Sadun A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retinal Eye Res. 2004;23:53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Kirby DM, Kahler SG, Freckmann ML, et al. Leigh disease caused by the mitochondrial DNA G14459A mutation in unrelated families. Ann Neurol. 2000;48:102–104. [PubMed] [Google Scholar]
- 14.Cock HR, Tabrizi SJ, Cooper JM, Schapira AHV. The influence of nuclear background on the biochemical expression of 3460 Leber’s hereditary optic neuropathy. Ann Neurol. 1998;44:187–193. doi: 10.1002/ana.410440208. [DOI] [PubMed] [Google Scholar]
- 15.Cullom ME, Heher KL, Miller NR, et al. Leber’s hereditary optic neuropathy masquerading as tobacco-alcohol amblyopia. Arch Ophthalmol. 1993;111:1482–1485. doi: 10.1001/archopht.1993.01090110048021. [DOI] [PubMed] [Google Scholar]
- 16.Howell N, Mackey DA. Low-penetrance branches in matrilinear pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1998;63:1220–1224. doi: 10.1086/302049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalmers RM, Harding AE. A case-control study of Leber’s hereditary optic neuropathy. Brain. 1996;119:1481–1486. doi: 10.1093/brain/119.5.1481. [DOI] [PubMed] [Google Scholar]
- 18.Nikoskelainen EK. Clinical picture of LHON. Clin Neurosci. 1994;2:115–120. [Google Scholar]
- 19.Sadun AA, Carelli V, Salomao SR, et al. Extensive investigation of large Brazilian pedigree of Italian ancestry (SOA-BR) with 117788/Haplogroup J Leber’s hereditary optic neuropathy (LHON) Am J Ophthalmol. 2003;136:231–238. doi: 10.1016/s0002-9394(03)00099-0. [DOI] [PubMed] [Google Scholar]
- 20.De Marinis M. Optic neuropathy after treatment with anti-tuberculous drugs in a subject with Leber’s hereditary optic neuropathy mutation. J Neurol. 2001;248:818–819. doi: 10.1007/s004150170103. [DOI] [PubMed] [Google Scholar]
- 21.Hwang JM, Kim J, Park SS. Leber’s hereditary optic neuropathy mutations in ethambutol-induced optic neuropathy. J Neurol. 2003;250:87–89. doi: 10.1007/s00415-003-0960-0. [DOI] [PubMed] [Google Scholar]
- 22.Sadun F, De Negri A, Carelli V, et al. Ophthalmologic findings in large pedigree of 11778/Haplogroup J Leber’s hereditary optic neuropathy. Am J Ophthalmol. 2004;137:271–277. doi: 10.1016/j.ajo.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Salomao SR, Berezovsky A, Andrade RE, et al. Visual electrophysiologic findings in patients from an extensive Brazilian family with Leber’s hereditary optic neuropathy. Doc Ophthalmol. 2004;108:147–155. doi: 10.1023/b:doop.0000036829.37053.31. [DOI] [PubMed] [Google Scholar]
- 24.Ventura DF, Quiros P, Carelli V, et al. Chromatic and luminance contrast sensitivities in asymptomatic carriers from a large Brazilian pedigree of 11778 Leber hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2005;46:4809–4814. doi: 10.1167/iovs.05-0455. [DOI] [PubMed] [Google Scholar]
- 25.Keltner JL, Johnson CA, Spurr JO, et al. Baseline visual field profile of optic neuritis. The experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1993;111:231–234. doi: 10.1001/archopht.1993.01090020085029. [DOI] [PubMed] [Google Scholar]
- 26.Savini G, Zanini M, Carelli V, et al. Correlation between retinal nerve fiber layer thickness and optic nerve head size: an optical coherence tomography study. Br J Ophthalmol. 2005;89:489–492. doi: 10.1136/bjo.2004.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medeiros FA, Zangwill LM, Bowd C, et al. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1008–1015. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 28.Shimada Y, Bearse MA, Jr, Sutter EE. Multifocal electroretinograms combined with periodic flashes: direct responses and induced components. Graefes Arch Clin Exp Ophthalmol. 2005;243:132–141. doi: 10.1007/s00417-004-1072-y. [DOI] [PubMed] [Google Scholar]
- 29.Roth JM, Sherman J, Salomao SR, et al. Serial retinal nerve fiber layer loss measured with the GDx VCC in a large Brazilian pedigree with 11778 mtDNA Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2004;45:1014. [Google Scholar]
- 30.Savini G, Barboni P, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology. 2005;112:127–131. doi: 10.1016/j.ophtha.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 31.Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112:120–126. doi: 10.1016/j.ophtha.2004.06.034. [DOI] [PubMed] [Google Scholar]