Abstract
Purpose
To test if patients with age-related macular degeneration (AMD) have normal panretinal function using standardized full-field electroretinograms (ERGs).
Methods
This is a retrospective study evaluating electroretinographic studies performed in patients with AMD to assess their panretinal function. Fifty-two individuals 55 years or older had standardized ERG testing and fundus photographs.
Results
The study group was aged 57 to 93 years old with a mean of 75.7, and the controls ranged from 79 to 87 years with a mean of 81.4. On average, the photopic, scotopic, dark-adapted bright-flash, and flicker function response amplitudes are lower with longer implicit times in the study group than the controls. The most pronounced differences were seen with the bright-flash dark-adapted a-waves and the photopic b-wave amplitudes. Forty-three of 104 eyes had abnormal photopic b-wave ERGs of more than 2 SD compared to controls. The mean of the photopic b-wave amplitudes for the study group was 76.7 ± 36.2 μV (1 SD) compared to 91.4 ± 16.9 μV (1 SD) for the control group. This finding was statistically significant with P = .0269 by the Student t test and P = .0336 by the Wilcoxon test.
Conclusions
There is a subgroup of AMD patients with a panretinal cone dysfunction on ERG in association with their macular degeneration. Previous studies have shown varied results when looking at ERG changes in AMD, likely reflecting the underlying complexity of this disorder. Using standardized ERG to identify a more homogeneous subgroup of AMD patients with panretinal dysfunction will aid in better characterizing subtypes clinically and is likely to be valuable in identifying new genes contributing to AMD.
INTRODUCTION
Age-related macular degeneration (AMD) is a heterogeneous, complex disorder with numerous genetic and environmental factors contributing to its pathogenesis. AMD can be characterized as a progressive regionalized degeneration of the photoreceptors in the macula. The pathogenesis of this disorder has been attributed to basic aging processes causing dysfunction, typically represented by a thickening of Bruch’s membrane with the formation of drusen. Environmental factors that have been identified include chronic solar radiation to the macula, smoking, hypertension, and diet.1–3 Both family and twin studies have shown that there is also a strong genetic component to disease.4,5
There is good evidence that numerous genes contribute to the AMD phenotype, but to date no primary cause for AMD has been determined.6 Searches for monogenic causes of AMD have demonstrated some families with maculopathies, which typically do not have the same characteristics as AMD. Pattern dystrophies, which are mainly autosomal dominant, have an earlier onset, subretinal pigmentary deposits, and over the long term better vision than found in AMD patients. ELOVL4, also known as dominant Stargardt’s disease, is a disorder of a protein that is a photoreceptor-specific component of the fatty acid elongation system. Patients with this disorder present with macular disruption and yellow macular lesions in their 30s and 40s, but some have a later onset. Stargardt’s disease (fundus flavimaculatus or ABC4A) occasionally can have a late onset in the 40s and 50s; these cases are often mistaken for AMD but are found to have an ABCA4 mutation on later analysis. The late phenotype in these patients shows atrophic macular lesions with missing RPE and bare choroid. Sometimes there is a tiny island of RPE centrally connected to the paramacular region by an isthmus of intact RPE. These patients often are diagnosed as having choroidal sclerosis, but on the fluorescein angiogram a dark choroid effect will be seen in surrounding retina.
The strongest mimicry of AMD among the monogenic maculopathies occurs in selected cases with autosomal dominant CRX mutations. Such individuals have family histories of macular degeneration with onset in the mid-40s to 50s and are told they have dry AMD by their ophthalmologists. CRX mutations can have a wide variety of expression depending on the type and location of the mutation. CRX mutations cause Leber’s congenital amaurosis, retinitis pigmentosa, cone-rod dystrophy, and macular degeneration.7,8 The electroretinogram (ERG) and family history are very useful in identifying macular degeneration patients with CRX mutations whose diagnosis can be confirmed by mutational analysis.
The ability to find genes in family and association studies depends on having reliable markers of disease that can be followed through generations, along with other genetic markers in linkage family studies, or in association with single nucleotide polymorphisms (SNPs) in association studies. The markers in association linkage studies of AMD have typically been drusen, choroidal neovascularization (CNV), regional macular atrophy, and pigment epithelial detachments. Study of AMD compared to normal age-matched patients has led to identification of genes reported to be associated with macular degeneration.9 These include ABCA4,10 ARMD1,11 C2,12 CFB,12 CFH,13 FBLN5,14 LOC387715,15 and TLR4.16 None of these genes show any indication of being primary contributors to AMD, but are more likely susceptibility variants that influence the expression and onset of the disease. It is generally believed that there are other genetic contributors to AMD, and it would be helpful to have objective criteria and other genetically based markers to use in AMD SNP analyses. Abnormal ERG signals compared to normal signaling from age-matched controls would be good marker candidates, because they are derived from physiologic processes and thus genetically controlled.
Historically, the ERG has not been used in the diagnosis or management of AMD. Cone photoreceptors in the macula account for only a fraction of the total number of cones in the retina, and a retina with an atrophic macula can still have a normal cone ERG.17 A poor panretinal cone signal (photopic ERG) strongly suggests that the entire cone system is involved in the retinal degeneration process. The inability to test macular function with the regular ERG has stimulated the development of multifocal electroretinography, in which macular function selectively is measured. In the past, most studies of AMD using small numbers of patients have shown unremarkable findings on full-field ERGs,18 with some showing nonspecific global dysfunction.19 More recently, Ladewig and colleagues,20 using full-field ERGs, demonstrated decreased cone function in 20 individuals over 50 years of age with AMD.
The purpose of this study is to show that there is a distinct subgroup of AMD that demonstrates decreased photoreceptor function, compared to age-matched controls, on full-field ERG. Phenotypically distinct subgroups of AMD can be used in genetic studies to further elucidate the etiology and pathogenesis of this disease. In addition, some elderly AMD patients with strong complaints of poor vision may have unsuspected panretinal disease, which can be confirmed by ERGs.
METHODS
This study is a comparative retrospective case series of AMD patients evaluated by the senior author (J.H.) in his practice at the University of California, Los Angeles. Institutional review board approval was sought and granted in advance of this study at the University of Michigan, the present employer of the authors.
The number of individuals in this study was 52 with the inclusion criteria of documentation of AMD with verification on color fundus photographs, full-field ERGs, and age ≥55 years old. The research records spanning a 12-year period (1990–2002) of the senior author were reviewed for the diagnosis of AMD. The earliest ERG was used in individuals who had multiple ERGs. The diagnosis of AMD was confirmed on review of the fundus photographs by the presence of drusen, geographic atrophy, or evidence of CNV by the two authors (S.N., J.H.). The photographs were also graded for the presence of soft and hard drusen, geographic atrophy, and evidence of CNV.
Sixteen individuals served as control subjects for this study. They were elderly and drawn from the same population over the same time period as study subjects to serve as ERG laboratory controls. Controls were documented as having no significant fundus findings on examination.
A standardized ERG was performed in all study and control subjects.21 The amplitudes and latencies of the photopic a-and b-waves, flicker function, scotopic b-waves, and dark-adapted maximum stimulation a-and b-waves were measured.
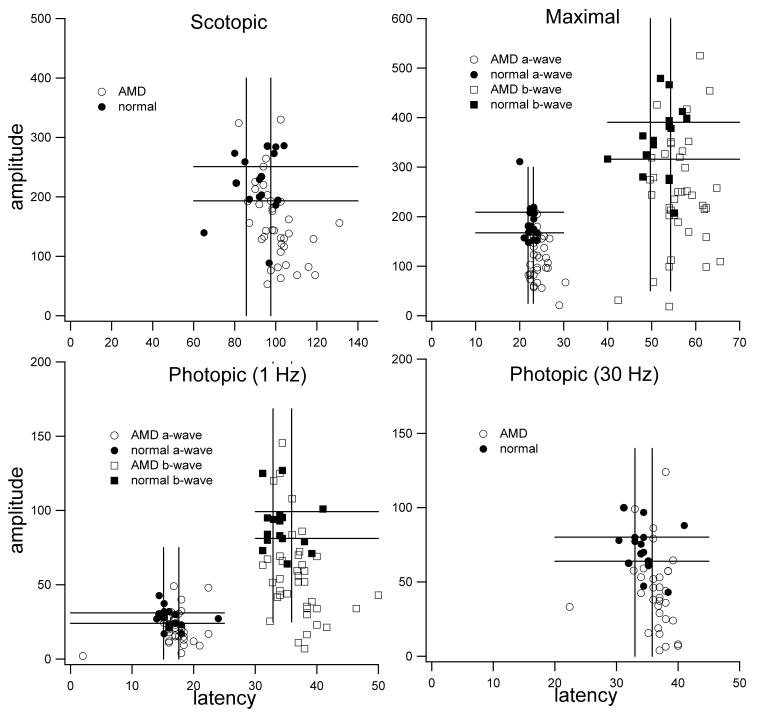
Initially, scatter plots of the ERG data were generated for a subset of the study group (age ≥70) to examine how the data points compared to the normal controls. It was clear that there was a clustering of photopic b-wave and dark-adapted bright-flash a-wave amplitudes in the AMD patients (Figure 1).
FIGURE 1.
Scatter plots of patients with age-related macular degeneration (AMD) and controls ≥70 years old; a subgroup of 36 AMD patients is compared to 16 age-matched controls without AMD. Electroretinogram amplitudes are plotted on the y-axis and latencies on the x-axis. To help reference data points, standard error lines for high and low values from mean have been drawn into graph. Scotopic responses, a-and b-wave maximal responses, a-and b-wave photopic responses, and photopic flicker are shown. The distribution of the data suggests significantly lower photopic b-wave amplitudes and a-wave maximal response amplitudes in the study group as compared to the control group.
Then all patients 55 years of age or older were included in the analysis comparing the amplitudes and latencies of photopic and bright-flash scotopic a-and b-waves to the controls. Subsequent analysis involved all 12 ERG parameters mentioned above, which were compared between the study group and the control group. The values from both right and left eyes of each individual in the study groups were averaged. The means and standard deviations were compared, and the P values were calculated by using the Student t (independent, two sided) and Wilcoxon tests. The 12 ERG parameters were also compared on the basis of the severity of AMD as was determined by review of the fundus photographs. Ordered in increasing severity, the categories were soft and hard drusen alone, drusen with geographic atrophy, drusen, geographic atrophy and CNV, and CNV alone. The means and standard deviations of the ERG values for each of these groups were compared and P values calculated. The Bonferroni adjustment for multiple tests was used in both of the analyses that involved all 12 ERG parameters, though this adjustment is controversial.22
RESULTS
Two hundred ninety-five charts were identified in which the patient had AMD, of which 59 patients had full-field electroretinographic studies and color fundus photography. Five of these individuals were excluded on account of age (<55 years old). Another two individuals were excluded for a lack of fundus photographic findings consistent with the diagnosis of AMD. The study participants ranged from 57 to 94 years old, with a mean of 75.7 and a standard deviation of 8.6 years. There were 38 women and 14 men. The control group ranged from 79 to 87 years old, with a mean of 81.4 and standard deviation of 2.3 years. The controls consisted of 10 women and six men who were recruited to establish normal control values for the electrophysiology laboratory.
The scatter plots seen in Figure 1 suggested a possible significant decrease in the photopic b-wave amplitudes in the study patients as compared to the control group. The lines in the graph are the standard error values from mean for the control subjects that help to reference all values. There is a tight grouping of the controls, whereas the study group can be seen to have a broader range with a possible bimodal distribution. There was also a suggestion of a significant decrease in the maximal a-wave amplitude in the AMD group as compared to the controls. Once again, a tight range for the control group but a broader distribution of the AMD group can be seen.
The results of the initial analysis involving individuals ≥55 years of age in a subset of ERG parameters demonstrated significance for the b-wave photopic amplitude by t test and Wilcoxon test with P = .03 and P =.05, respectively. Subsequent analysis comparing all of the ERG parameters from the study and control groups confirmed the significance with P = .0296 and P = .0336 for the b-wave photopic amplitude (Table 1). The other ERG variables showed no additional statistical significance. Because the initial scattergram with individuals ≥70 years of age seemed to demonstrate subgroups, all the ERG parameters were also included in the analysis for significance with the higher age range. These data are presented in Table 2 and suggest that the photopic b-wave and bright-flash scotopic a-wave amplitudes are significantly affected in many AMD patients. The P values no longer reach the threshold of significance when an adjustment for multiple tests by the Bonferroni method was made on this latter analysis.22
TABLE 1.
COMPARISON OF ELECTRORETINOGRAPHIC VALUES IN PATIENTS WITH AGE-RELATED MACULAR DEGENERATION AND AGE-MATCHED CONTROL SUBJECTS
| CASES (N=52) | CONTROLS(N=16) | PVALUES | ||
|---|---|---|---|---|
| DEPENDENT VARIABLE | MEAN ± STANDARD DEVIATION | TTEST | WILCOXON | |
| Wave A | ||||
| Photopic – amplitude | 26.7 ± 10.7 | 28 ± 6.4 | .5492 | .4478 |
| Photopic – latency | 16 ± 2.5 | 16.4 ± 2.2 | .5564 | .9077 |
| Max Stim – amplitude | 163.3 ± 58.5 | 187.6 ± 39.7 | .0657 | .1565 |
| Max Stim – latency | 22.9 ± 2.1 | 22.6 ± 1.1 | .5262 | .7277 |
| Wave B | ||||
| Photopic – amplitude | 76.7 ± 36.2 | 91.4 ± 16.9 | .0296* | .0336* |
| Photopic – latency | 34.8 ± 3.2 | 34.3 ± 2.4 | .5141 | .6177 |
| Scotopic – amplitude | 216.9 ± 83.5 | 233.5 ± 40.1 | .6718 | .5863 |
| Scotopic – latency | 92.3 ± 10.5 | 92.5 ± 8.1 | .9364 | .9414 |
| Max Stim – amplitude | 321 ± 120 | 359.3 ± 63 | .1010 | .1982 |
| Max Stim – latency | 54.2 ± 4.8 | 52.6 ± 4.1 | .2208 | .3326 |
| Flicker fusion | ||||
| Amplitude | 62.2 ± 30.1 | 73.8 ± 16.4 | .0518 | .0602 |
| Latency | 34.8 ± 2.4 | 34.6 ± 2.3 | .7953 | .7317 |
Photopic b-wave amplitudes demonstrate significance prior to adjustment for multiple variables.
TABLE 2.
COMPARISON OF ELECTRORETINOGRAPHIC VALUES IN A SUBGROUP OF PATIENTS ≥70 YEARS OLD WITH AGE-RELATED MACULAR DEGENERATION AND AGE-MATCHED CONTROL SUBJECTS
| CASES (N=36) | CONTROLS(N=16) | PVALUES | ||
|---|---|---|---|---|
| DEPENDENT VARIABLE | MEAN ± STANDARD DEVIATION | TTEST | WILCOXON | |
| WAVE A | ||||
| Photopic – amplitude | 24.4 ± 10.2 | 28.0 ± 6.4 | .1253 | .1083 |
| Photopic – latency | 16.1 ± 2.8 | 16.4 ± 2.2 | .7350 | .5117 |
| Max Stim – amplitude | 149.2 ± 59.9 | 187.6 ± 39.7 | .0093* | .0132* |
| Max Stim – latency | 23.1 ± 2.2 | 22.6 ± 1.1 | .2508 | .2064 |
| WAVE B | ||||
| Photopic – amplitude | 72.7 ± 37.3 | 91.4 ± 16.9 | .0162* | .0156* |
| Photopic – latency | 35.5 ± 3.4 | 34.3 ± 2.4 | .1722 | .2261 |
| Scotopic – amplitude | 202.5 ± 87.4 | 223.5 ± 40.1 | .2470 | .1738 |
| Scotopic – latency | 93.8 ± 11.0 | 92.5 ± 8.1 | .6330 | .6118 |
| Max Stim – amplitude | 304.0 ± 130.6 | 359.3 ± 63.0 | .0448 | .0652 |
| Max Stim – latency | 54.5 ± 5.1 | 52.6 ± 4.1 | .1637 | .2459 |
| FLICKER FUSION | ||||
| Amplitude | 60.5 ± 33.5 | 73.8 ± 16.4 | .0592 | .0652 |
| Latency | 35.0 ± 2.6 | 34.6 ± 2.3 | .6423 | .5994 |
A-wave maximal response and b-wave photopic amplitude demonstrate significance prior to adjustment for multiple variables.
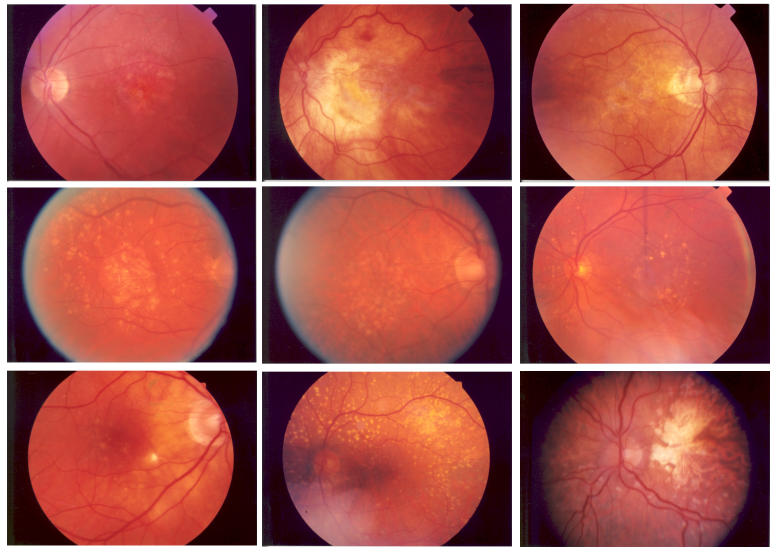
The breakdown of the severity of AMD in the study group can be seen in Table 3. The comparison of these different groups to ERG values demonstrated significance (Table 4). The a-wave maximal stimulus latency between the different groups demonstrates P = .0082. Comparisons between each group individually by posthoc pairwise methodology with Bonferroni adjustment show significant differences between geographic atrophy compared to geographic atrophy with CNV (P = .03) and drusen (soft or hard) compared to geographic atrophy with CNV (P = .004). These results suggest that disciform events may cause more damage to the retina than commonly thought, or that these eyes may have more severe disease. In a review of the fundus photographs of the AMD patients, no correlations were seen between severity of the maculopathy and the photopic b-wave amplitudes. The clinical examination does not predict which patients will have poor ERGs; examples of representative fundus photos are shown in Figure 2, demonstrating low, medium, and high photopic b-wave amplitudes with their corresponding maculae.
TABLE 3.
SEVERITY OF AGE-RELATED MACULAR DEGENERATION CATEGORIZED BY MACULAR CHANGES BASED ON FUNDUS PHOTOGRAPHS
| PREDOMINANT FUNDUS FINDING | NO. OF EYES |
|---|---|
| Soft and/or hard drusen | 27 (26%) |
| Drusen and geographic atrophy | 61 (59%) |
| Drusen, geographic atrophy, and CNV | 7 (7%) |
| CNV | 9 (9%) |
| Total | 104 |
CNV = choroidal neovascularization.
TABLE 4.
ELECTRORETINOGRAM ANALYZED BY FUNDUS APPEARANCE IN STUDY GROUP OF PATIENTS WITH AGE-RELATED MACULAR DEGENERATION
| GROUP 1 | GROUP 2 | GROUP 3 | GROUP 4 | ||
|---|---|---|---|---|---|
| DEPENDENT VARIABLE | GA & CNV (N = 7 EYES) | GA (N = 61 EYES) | CNV (N = 9 EYES) | HD OR SD (N = 27 EYES) | PVALUE |
| MEAN ± STANDARD DEVIATION | |||||
| WAVE A | |||||
| Photopic – amplitude | 27.7 ± 13.8 | 24.7± 10.7 | 27.2 ±8.3 | 30.6 ± 12.6 | .6148 |
| Photopic – latency | 18.5 ±3.0 | 15.9±3.0 | 15.8 ±1.0 | 15.7 ±1.8 | .0082* |
| Max Stim – amplitude | 128.0 ± 68.1 | 155.1± 56.7 | 192.5 ± 66.2 | 187.1 ± 53.1 | .2672 |
| Max Stim – latency | 23.4 ±2.0 | 22.9±2.5 | 22.4 ±0.7 | 22.7 ±1.5 | .9915 |
| WAVE B | |||||
| Photopic – amplitude | 63.2 ± 27.3 | 72.0±37.7 | 77.7 ± 28.3 | 89.5 ±37.7 | .5565 |
| Photopic – latency | 36.2 ±3.2 | 35.3±3.7 | 32.9 ±3.1 | 34.0 ±2.8 | .4216 |
| Scotopic – amplitude | 175.7 ± 79.3 | 208.2±92.3 | 225.7 ± 53.4 | 245.5 ±77.9 | .3144 |
| Scotopic – latency | 95.4 ± 18.9 | 93.2±10.7 | 92.9 ±5.1 | 90.2 ±10.7 | .6938 |
| Max Stim – amplitude | 278.7 ± 93.3 | 308.3± 131.6 | 363.4 ±99.9 | 359.0 ± 107.4 | .9267 |
| Max Stim – latency | 54.6 ±4.5 | 54.7±5.5 | 54.4 ±6.0 | 52.9 ±4.3 | .8930 |
| FLICKER FUSION | |||||
| Amplitude | 50.9 ± 24.9 | 57.6 ±32.8 | 68.9 ± 25.1 | 72.1 ± 27.9 | .3094 |
| Latency | 36.0 ±1.7 | 35.3 ±3.2 | 34.4 ±1.4 | 33.7 ±2.2 | .5337 |
CNV = choroidal neovascularization; GA = geographic atrophy; HD = hard drusen; SD = soft drusen.
A-wave photopic latencies in eyes that have evidence of CNV demonstrate significance prior to adjustment for multiple variables.
FIGURE 2.
Representative fundus photographs from patients with age-related macular degeneration with low, normal, or high photopic b-wave amplitudes demonstrating the clinical findings across groups. The electroretinogram has the ability to find a subgroup of patients with poor photoreceptor function despite similar macular findings. Top row: left, 65-year-old with an amplitude of 11.0 μV; center, 84-year-old with an amplitude of 21.4 μV; right, 88-year-old with an amplitude of 23.0 μV. Middle row: left, 82-year-old with an amplitude of 94.0 μV; center, 79-year-old with an amplitude of 95.3 μV; right, 71-year-old with an amplitude of 97.4 μV. Bottom row: left, 77-year-old with an amplitude of 147.0 μV; center, 62-year-old with an amplitude of 156.0 μV; right, 71-year-old with an amplitude of 191.0 μV.
DISCUSSION
The panretinal cone dysfunction as measured by the decreased b-wave photopic amplitudes is not generally recognized as a finding associated with AMD.17 Severe panretinal cone dysfunction with a normal or near-normal rod-isolated tracing is a hallmark of the cone dystrophies, which ultimately may have a bearing on this subgroup of AMD patients when the involved genes are found.
Cone dystrophies are a group of inherited disorders that may manifest early, such as in achromatopsia, and appear stationary over time, or in later childhood to adulthood, which are typically progressive.23 The stationary cone dystrophies are present at birth, whereas progressive cone dystrophies present in childhood or in early adult life.24 The diagnosis of a cone dystrophy after the age of 50 has been reported but is unusual.25 The clinical features of cone dystrophies include reduced visual acuity, small central scotomata, variable color vision, photosensitivity abnormalities, and retinal pigment irregularities that may manifest as a bull’s-eye maculopathy,24 but because cone dystrophies have an earlier onset, this disease process is not thought to be related to AMD.
There has been no uniform recognition that some AMD patients have panretinal abnormalities of cone and rod function, and the profound effects of central visual loss have overshadowed the significance that some patients have panretinal functional loss as part of their AMD process. This may explain why some patients vehemently insist their AMD is worse even though their visual acuity has not changed. If peripheral retinal testing was performed, a worsening of their condition might be found. Many macular degeneration patients have poor rod function26 and functional night blindness that contribute to their disability.
In this study and in an earlier report (“Senile panretinal cone degeneration,” Heckenlively J., ARVO Meeting, 1995, Abstract), we demonstrated a subgroup of AMD patients that have significant cone dysfunction as one of its primary characteristics. Such a distinct phenotype suggests a distinct genetic physiologic mechanism contributing to the AMD. Ladewig and associates,20 in a similar study, describe a late-onset cone dystrophy in patients with the diagnosis of AMD. In contrast to our proposal, the authors conclude that there is a late-onset cone dystrophy that is a distinctly different diagnosis from AMD. The suggestion is that in this group, AMD was either misdiagnosed or is a coincidental finding because of its prevalence in this age-group. This differs from our interpretation that the cone degeneration represents a subtype within AMD, which has a high likelihood of a genetic correlation.
Rating the severity of the AMD in this study had dual purposes. First, this review allowed for the confirmation of the diagnosis of AMD. Second, with this aspect of the study, we are able to show that the decrease in cone function is not simply secondary to advanced morphologic change in the retina, such as geographic atrophy. The significant association between the cases of more advanced morphology (CNV AMD) and the a-wave maximal stimulus implicit time is interesting, because the ERG is measuring from the entire retina, not just the macula. Generally, increased implicit times from normal are regarded as a sign of illness in the retina and suggest that the hemorrhagic event may have caused more extensive damage to the retina than would be apparent after the blood resolves. No correlation was found between AMD findings and the b-wave photopic amplitude. An increase in scotopic b-wave implicit times and a decrease in photopic amplitudes in geographic atrophy have been reported previously by Walter and associates19 in 66 patients with AMD.
This study has limitations on account of its retrospective methodology. We were limited to the data that were documented in the chart. Patients were not systematically checked for color vision or asked about light sensitivity, as would be the protocol for a prospective study. We also had to rely on controls that were collected to establish control ERG values, which is a less desirable methodology than use of prospective age-matched controls. We were also limited by both the size of the control group and the age variation.
The findings in this study have research implications for the investigation into the genetics underlying AMD. The single most important aspect of any genetic study is to start with a well-defined clinical entity. If the definition is overly broad, then it will likely be represented by an assortment of underlying genes, making traditional linkage analysis difficult. One obvious way around this problem is to collect large multigenerational families. This approach makes the assumption that families are more likely to carry a specific genetic mutation or set of mutations. Collecting families with enough affected patients over several generations in a late-onset disease such as AMD is nearly impossible. Finding a marker such as cone dysfunction that is unique to a subgroup of the AMD population makes it possible to look for genes associated with this finding using association linkage techniques.6
Using distinct phenotypes in complex disease such as AMD along with SNP association studies has proven to be a powerful combination for identifying genetic variation in disease. SNPs are persistent substitutions found in at least one population with a frequency of more than 1%. These genetic changes have the advantages of being abundant, stable, and easily identified6 and are used for identifying contributing genes in complex diseases. Identifying the genetics underlying the described AMD subgroup will help to elucidate the complex interaction of genes contributing to the clinical entity of AMD.
PEER DISCUSSION
DR MARK W. JOHNSON
The clinical phenotype that we identify as classic age-related macular degeneration (AMD) is characterized by macular drusen with or without components of retinal pigment epithelial hyperplasia, geographic atrophy, and choroidal neovascularization. Numerous environmental and genetic factors contribute pathogenically to the heterogeneous group of diseases that share this phenotype. Demographic and environmental risk factors for the development of AMD may include age, sex, race, chronic light exposure, diet, smoking, hypertension, and underlying cardiovascular disease.1,2 AMD is thought to be a polygenic disorder, with multiple genes conferring susceptibility to and protection from the disease.1,2 Characterization of the genetic defects associated with AMD may lead to the identification of disease subtypes that differ with respect to pathogenesis and may provide opportunities to develop novel therapeutic and prevention strategies.
Because AMD is generally considered to be a regional degenerative condition limited to the macular area, significant changes in full-field electroretinography (ERG) responses would not be expected with this disorder. However, Heckenlively and colleagues have presented intriguing pilot data suggesting that ERG testing may be able to identify a subgroup of AMD patients with generalized cone dysfunction that is not explained by macular abnormalities or other clinically-apparent fundus alterations. Previous studies of AMD patients using full-field ERG evaluations have yielded inconsistent results, although one study on a small group of AMD patients found panretinal cone dysfunction similar to that seen by Heckenlively and co-authors.3 In addition, a recent study of patients with early AMD found progressive loss in the cone-driven multifocal ERG response that extended beyond the visible drusen area.4
As acknowledged by the authors, the current study has several limitations due in large part to its retrospective design. There is a potential bias in patient selection, since 59 AMD patients that had full-field ERG testing and fundus photography for unspecified reasons were selected from a pool of 295 AMD patients. No statistical analysis is provided to verify that the study participants and controls are adequately age-matched. Information is also lacking regarding other variables that may affect ERG responses, such as fundus pigmentation, lens status, refractive error, and pupil size. Finally, it would be useful for the reader to know whether the graders of fundus photographs were masked to the ERG results.
If confirmed in a prospective study, the identification of an AMD subtype with generalized retinal dysfunction would be of significant importance. A more homogeneous phenotypic subgroup would likely facilitate the detection of new AMD genes that could eventually lead to new treatments. Furthermore, it might explain the relatively common clinical observation that some AMD patients complain year after year of progressive visual dimming and decline, despite stable macular lesions and visual acuities. Finally, it would imply that at least for a subgroup of AMD patients, long-term visual preservation will require interventions in addition to those required to stabilize the macular pathology. The authors are to be congratulated on this innovative and important contribution.
REFERENCES
- 1.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 2.Donoso LA, Kim D, Frost A, et al. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladewig M, Kraus H, Foerster MH, et al. Cone dysfunction in patients with late-onset cone dystrophy and age-related macular degeneration. Arch Ophthalmol. 2003;121:1557–1561. doi: 10.1001/archopht.121.11.1557. [DOI] [PubMed] [Google Scholar]
- 4.Gerth C, Delahunt PB, Alam S, et al. Cone-mediated multifocal electroretinogram in age-related macular degeneration: Progression over a long-term follow-up. Arch Ophthalmol. 2006;124:345–352. doi: 10.1001/archopht.124.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
DR MALCOLM R. ING
The association between sunlight exposure and macular degeneration is still controversial. What is your feeling about the use of sunglasses in general population? Will it help to retard development of AMD?
DR ALLAN J. FLACH
There are a group of patients who have drusen in the periphery only. Dr Joe Hollyfield has compared these peripheral drusen to macular drusen and has discovered some interesting things about the chemistry of the two. I wonder what would happen if, in addition to the controls you used, you used controls that had peripheral drusen only and whether this might be a important sub-group.
DR MICHAEL H. GOLDBAUM
Could some of these findings that you’ve described be an epi-phenomenon, and is it possible that examining family members who don’t have macular degeneration might find some sort of similar ERG response?
DR JOHN R. HECKENLIVELY
We certainly agree that a retrospective study is never as good as a prospective study and that analyzing data that it would been much better if everything had be uniformly collected. However, the simplicity of this study also is a positive factor, in that these were patients who had AMD, that had ERGs, and there was no other factor involved, other than they had to be over 55 years of age. So, we think the preliminary data is highly suggestive that there is a real phenomenon that we are measuring.
The use of sunglasses can be looked at in two ways in this is AMD group. Certainly we may worry about photo toxicity and an aspect of solar retinopathy. But also these patients that have poor photopic functions may actually see better with a tint. It’s a standard procedure to use sunglasses in cone dystrophies and achromatopsia to try to improve their function. In many cases it substantially helps children see better in school. I had some success in patients with senile cone degeneration where their photopic ERGs may be one-third of normal and they improve with a light tint. The patients note the improvement and use the tint all the times, some even thinking they should drive at night, when perhaps they should not.
Yes, there was a statistical check on the age match between the controlled and the tested patients and there was no significant difference. We were masked to the ERG values. During review of fundus photographs, we did not know who had high or low values. At the time, the photos were also scrambled, so that the left and right eyes were evaluated differently, in different places.
The examination of peripheral drusen was not done, but would be an interesting group to look at, to see if there are differences compared to age-matched controls.
I seriously doubt this is an epi-phenomenon. I agree that a family study is appropriate in this group. There is some suggestion that the subgroup is autosomal dominant. When you’re taking family histories, we frequently found that one of the parents had macular degeneration in this group. But that was not systematically looked at, that also needs to be done prospectively.
ACKNOWLEDGMENTS
The authors thank David Musch, PhD, of the Department of Ophthalmology and Visual Science, Kellogg Eye Center, University of Michigan, for providing the initial statistical analysis used in this study.
REFERENCES
- 1.Klein R, Tunde P, Bird A, et al. The epidemiology of age-related macula degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 2.van Leeuwen R, Klaver CW, Vingerling JR, et al. Epidemiology of age-related maculopathy: a review. Eur J Epidemiol. 2003;18:845–854. doi: 10.1023/a:1025643303914. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JM, Chen CA. The epidemiology of macular degeneration. Int Ophthalmol Clin. 2004;44:17–39. doi: 10.1097/00004397-200404440-00004. [DOI] [PubMed] [Google Scholar]
- 4.Seddon JM, Ajani UA, Mitchell ED. Familial aggregation of age-related maculopathy. Am J Ophthamol. 1997;123:199–206. doi: 10.1016/s0002-9394(14)71036-0. [DOI] [PubMed] [Google Scholar]
- 5.Meyers SM, Zalhary AA. Monozygotic twins with age-related macular degeneration. Am J Ophthamol. 1988;106:651–653. doi: 10.1001/archopht.1988.01060130705029. [DOI] [PubMed] [Google Scholar]
- 6.Tuo J, Bojanowski C, Chan C. Genetic factors in age-related macular degeneration. Prog Retinal Eye Res. 2004;23:229–249. doi: 10.1016/j.preteyeres.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaroop A, Wang Q-L, Wu W, et al. Leber congenital amaurosis caused by a homozygous mutation (R90W) in the homeodomain of the retinal transcription factor CRX: direct evidence for the involvement of CRX in the development of photoreceptor function. Hum Mol Genet. 1999;8:299–305. doi: 10.1093/hmg/8.2.299. [DOI] [PubMed] [Google Scholar]
- 8.Evans K, Duvall-Young J, Fitzke FW, et al. Chromosome 19q cone-rod retinal dystrophy. Ocular phenotype. Arch Ophthalmol. 1995;113:195–201. doi: 10.1001/archopht.1995.01100020079033. [DOI] [PubMed] [Google Scholar]
- 9.RetNet. Retinal Information Network. Available at: http://www.sph.uth.tmc.edu/Retnet/home.htm Accessed April 9, 2006.
- 10.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 11.Schultz DW, Klein ML, Humpert AJ, et al. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet. 2003;12:3315–3123. doi: 10.1093/hmg/ddg348. [DOI] [PubMed] [Google Scholar]
- 12.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards AO, Ritter R, III, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 14.Stone EM, Braun TA, Russell SR, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsdottir J, Conley YP, Weeks DE, et al. Susceptibility genes for age-related maculopathy on chromosome 10q. Am J Hum Genet. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zareparsi S, Buraczynska M, Branham KEH, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005;14:1449–14. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- 17.van Lith GHM. The macular function in the ERG. Doc Ophthalmol Proc Ser. 1976;10:405–415. [Google Scholar]
- 18.Sunness JS, Massof RW, Johnson MA, et al. Peripheral retinal function in age-related macular degeneration. Arch Ophthamol. 1985;103:811–8. doi: 10.1001/archopht.1985.01050060071029. [DOI] [PubMed] [Google Scholar]
- 19.Walter P, Widder RA, Luke C, et al. Electrophysiologic abnormalities in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237:962–968. doi: 10.1007/s004170050331. [DOI] [PubMed] [Google Scholar]
- 20.Ladewig M, Hannelore K, Foerster M, et al. Cone dysfunction in pateints with late-onset cone dystrophy and age-related macular degeneration. Arch Ophthalmol. 2003;121:1557–1561. doi: 10.1001/archopht.121.11.1557. [DOI] [PubMed] [Google Scholar]
- 21.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. International Society for Clinical Electrophysiology of Vision. Standard for clinical electroretinography (2004 update) Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 22.Perneger IV. What’s wrong with Bonferroni adjustments. Br Med J. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heckenlively JR. Cone dystrophies and degenerations. In: Heckenlively JR, Geoffrey AB, eds. Principles and Practice of Clinical Electrophysiology 2nd ed. Cambridge, Massachusetts: MIT Press; 2006:795–802.
- 24.Simunovic MP, Moore AT. The cone dystrophies. Eye. 1998;12:553–565. doi: 10.1038/eye.1998.145. [DOI] [PubMed] [Google Scholar]
- 25.Rowe SE, Trobe JD, Sieving PA. Idiopathic photoreceptor dysfunction causes unexplained visual acuity loss later in adulthood. Ophthalmology. 1990;97:1632–16. doi: 10.1016/s0161-6420(90)32366-7. [DOI] [PubMed] [Google Scholar]
- 26.Feigl B, Brown B, Lovie-Kitchin J, Swann P. Cone- and rod-mediated electroretinogram in early age-related maculopathy. Eye. 2005;19:431–441. doi: 10.1038/sj.eye.6701503. [DOI] [PubMed] [Google Scholar]