Abstract
Purpose
To ascertain if methicillin-resistant Staphyloccocus aureus (MRSA) ophthalmic infections are increasing.
Methods
A retrospective review of all patients with a culture positive for MRSA in the Parkland Health and Hospital System, the urban public healthcare system for Dallas County, Texas, for the years 2000 through 2004 was performed. Patients with ocular, orbital, and ocular adnexal infection were identified, and isolates were categorized as nosocomial or community-acquired (CA).
Results
A total of 3,640 patients with a culture positive for MRSA were identified, with 1,088 patients (30%) considered to have acquired the isolate via nosocomial transmission and 2,552 patients (70%) considered to have CA-MRSA. Forty-nine patients (1.3%) had ophthalmic MRSA involvement. For both ophthalmic and nonophthalmic cases, the number of CA-MRSA patients increased each year, whereas the numbers of nosocomial patients remained fairly constant. Patients with ophthalmic MRSA tended to be younger than other MRSA patients (P = .023). The most common manifestation of ophthalmic MRSA infection was preseptal cellulitis and/or lid abscess followed by conjunctivitis, but sight-threatening infections, including corneal ulcers, endophthalmitis, orbital cellulitis, and blebitis, also occurred. Empirical antibiotic coverage was initially prescribed in 48 (98%) of ophthalmic cases and did not adequately cover for the MRSA isolate in 24 (50%).
Conclusions
CA-MRSA is becoming increasingly prevalent, and ophthalmologists will see more ophthalmic MRSA infections. Although ophthalmic CA-MRSA commonly presents as preseptal lid infection and conjunctivitis, sight-threatening infections also occur. Ophthalmologists must identify MRSA patients, adjust empirical treatment regimens where MRSA is endemic, and take steps to control emergence of resistant organisms in both inpatient and outpatient practices.
INTRODUCTION
Staphylococcus aureus is a versatile and dangerous bacterial pathogen with a genome consisting of a circular chromosome of approximately 2,800 base pairs and additional prophages, plasmids, and transposons.1 Genes governing virulence and antibiotic resistance reside on both the chromosome and extrachromosomal elements,2 and these genes are transferred between staphylococcal strains or other bacterial species via extrachromosomal elements.3 Humans are a natural reservoir of S aureus, with 30% to 50% of healthy adults colonized, 10% to 20% persistently so.4,5 Persons colonized with S aureus are at increased risk for subsequent infections.6
S aureus quickly developed resistance to early antibiotics. In 1942, the introduction of benzylpenicillin (penicillin G) temporarily addressed staphylococcal infections, but continued use caused the selection of resistant strains that produced penicillinase (β-lactamase).7 Kirby8 first described penicillinase-producing strains of S aureus in 1944, and most hospital isolates were resistant to penicillin within a few years.9 There are a wide variety of β-lactamases that hydrolytically inactivate β-lactam antibiotics like penicillin; β-lactamases can be either plasmid or chromosomally mediated.10 By 1948, the prevalence of resistant strains had seriously reduced the value of benzylpenicillin in the treatment of S aureus infections,11 and by the end of the 1950s, S aureus had acquired resistance to virtually all available systemic antibiotics, including erythromycin, streptomycin, and the tetracyclines.7 By the 1970s, penicillinase-producing strains were almost as common in the community, but where nosocomial strains were usually resistant to multiple antibiotics, community-acquired isolates were often resistant solely to penicillin.12–15 Methicillin, introduced in 1960,16 is not inactivated by β-lactamase. A host of other β-lactam antibiotics, including oxacillin, nafcillin, dicloxacillin, cephalothin, cephaloridine, and cefazolin, soon followed. Strains of methicillin-resistant S aureus (MRSA) were first detected in 196117,18 but initially occurred sporadically and were only resistant to β-lactam antibiotics. Resistant nosocomial strains appeared in Australia in the late 1970s that differed from earlier strains, were resistant to multiple other antibiotics in addition to β-lactam compounds,19,20 and subsequently spread to hospitals worldwide. MRSA is now one of the most common causes of bacterial nosocomial infections, responsible for 40% to 70% of S aureus infections in intensive care units (ICUs).21,22 In the past decade new strains of MRSA have emerged in the community, causing suppurative infection in young, otherwise healthy people,23–28 and the prevalence of community-acquired (CA) MRSA is increasing.29
MRSA constitutes a significant healthcare problem. Because methicillin is rarely used today, the term “MRSA” is used now to describe strains of S aureus resistant to all β-lactam antibiotics.30 Colonization with MRSA is more likely to result in infection than colonization with methicillin-sensitive S aureus (MSSA).31,32 MRSA bacteremia is associated with significantly higher mortality rate33 and cost to treat34 than MSSA bacteremia. Higher mortality and costs have also been found with MRSA surgical site infections.35 Vancomycin, a glycopeptide antibiotic that inhibits the polymerization of peptidoglycan (an essential component of the bacterial cell wall), is the drug of choice for MRSA isolates. Whereas there has been no evidence to suggest that MRSA strains are more virulent than MSSA strains, there is evidence that vancomycin may be inferior to semisynthetic penicillins for treatment of deep-seated S aureus infections.33,36–42 Vancomycin is less bactericidal against MSSA than are β-lactam agents, and vancomycin has been associated with clinical failure in treatment of MSSA infections.35
Vancomycin was first approved by the Food and Drug Administration in 1958, and resistance first emerged in coagulase-negative staphylococci in 1987.43 In 1996, the first clinical isolate of S aureus with reduced susceptibility to vancomycin was identified in Japan.44 Risk factors for infection with S aureus with reduced vancomycin susceptibility include antecedent vancomycin use and prior MRSA infection.45 In June 2002, a strain of S aureus fully resistant to vancomycin was isolated from a patient in Michigan.46 The DNA sequence of the vanA gene responsible for vancomycin resistance in this patient’s S aureus isolate was identical to the vanA sequence from her vancomycin-resistant Enterococcus faecalis (VRE) isolate.47 Conjugate transfer for the vanA gene from enterococci to S aureus had previously been demonstrated in vitro.48
PREVIOUS REPORTS OF OPHTHALMIC MRSA INFECTION
Reports of MRSA ophthalmic infections are increasing in the literature. Conjunctivitis is the most commonly reported manifestation49–51 and has been associated with long-term care units,30 especially in patients with neurologic impairment52–55; nurseries55–57 and neonatal ICUs58,59; and healthcare workers.60 Maskin61 reported a 63-year-old diabetic man with previous MRSA chronic foot ulcer who developed MRSA infectious scleritis. MRSA keratitis has been described in nursing home patients without prior surgery.30,62,63 Sotozono and associates64 reported two patients with Stevens-Johnson syndrome with corneal intraepithelial infiltrations with conjunctival swabs that grew MRSA. The infiltrates persisted until treated with topical 1% arbekacin or 1% vancomycin. Inoue65 observed cases of MRSA keratitis in keratoconus patients with atopic dermatitis as well as blepharoconjunctivitis with severe erosion of the eyelid skin. Labit and colleagues66 reported that two of 13 S aureus isolates from patients undergoing vitrectomy for endophthalmitis were methicillin-resistant, but they did not indicate if those isolates were from patients who had recently had intraocular surgery. Saitoh and associates67 described an otherwise healthy 62-year-old woman with a history of nasolacrimal duct obstruction who developed MRSA dacryocystitis and who was treated with intravenous vancomycin and minocycline infusions and subsequent dacryocystectomy. Kubo and associates68 described an additional four healthy patients with dacryocystitis caused by MRSA treated successfully with dacryocystorhinostomy, and Shanmuganathan and colleagues30 reported three other cases with comorbidities. MRSA orbital cellulitis is rarely reported in the literature. Mehra and colleagues69 described a 44-year-old man with odontogenic MRSA sinusitis with orbital extension. Anari and associates70 reported a 4-week-old neonate born prematurely at 34 weeks gestation that presented with an orbital abscess.70
Of special concern to ophthalmic surgeons are increasing reports of postoperative MRSA infection. Kato and Hayasaka71 found that 13 of 978 eyes (1.3%) swabbed preoperatively grew MRSA, and patients with nasolacrimal duct obstruction had a higher incidence of harboring MRSA. Fukuda and associates51 found 6.6% of 1,000 asymptomatic eyes swabbed grew MRSA. In addition, they found that elderly conjunctival MRSA carriers were more likely to have anemia, cancer, liver dysfunction, or dementia; to be status post surgery; or to be chronically bedridden. In a 1991 report, Insler and colleagues63 described central MRSA keratitis after recent uncomplicated phacoemulsification procedure with posterior chamber intraocular lens insertion. More recently, MRSA wound infections have been reported with clear corneal phacoemulsification wounds.72,73 One of two patients with MRSA clear corneal wound infections described by Cosar and associates72 also had scleral extension of infection and endophthalmitis. Two other cases of postoperative MRSA endophthalmitis following cataract surgery have also been reported.67,74 In the most recently reported case, the patient had received moxifloxacin, a fourth-generation fluoroquinolone, for prophylaxis both before and after surgery.74 Fukuda and associates51 reported a case of MRSA endophthalmitis following vitrectomy in a patient with atopic dermatitis.
Refractive surgery has not escaped complications from MRSA infection. MRSA keratitis has been reported after laser in situ keratomileusis75,76 and photorefractive keratectomy (PRK),77 including a bilateral case after bilateral PRK in a medical resident.78,79 Sotozono and associates64 reported three cases of MRSA keratitis following penetrating keratoplasty, one case following lamellar keratoplasty, and four cases following epithelial transplantation for Stevens-Johnson syndrome. They felt MRSA keratitis is characterized by superficial keratitis, minimal melting, and minimal scarring. Patients with atopic dermatitis are often colonized with S aureus in their skin lesions80–82 as well as having conjunctival and eyelid colonization.65 Because these patients frequently use antibiotics for long periods in combination with corticosteroids, the prevalence of MRSA colonization in that population is increasing. Oshima and colleagues83 reviewed their experience of scleral buckle infections after retinal detachment repair and found that six (19%) of 32 eyes of patients with atopic dermatitis undergoing repair developed MRSA buckle infections compared to only one (0.4%) of 261 repairs in patients without atopic dermatitis (P < .001). Prompt removal of the infected buckle in combination with vancomycin administration was curative in four eyes; three went on to develop endophthalmitis requiring intravitreal injections or emergent vitrectomies. Osawa and colleagues84 reported another two cases of MRSA scleral buckle infection after retinal detachment repair in atopic dermatitis patients who presented with exudative retinal detachment without endophthalmitis after surgery. Resolution occurred after buckle removal and antibiotic administration. Shanmuganathan and associates30 reported an additional case of MRSA infection of a buckle segment with associated preseptal cellulitis. Finally, MRSA socket infections have been reported after enucleation and exenteration.30
To my knowledge, a comprehensive review of all ocular, orbital, and ocular adnexal MRSA infections in a healthcare system has not been reported. A review of all such infections was completed over a 5-year period in an urban county hospital system.
METHODS
The study was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Parkland Health and Hospital System. All patients with a culture positive for MRSA performed in the Parkland Health and Hospital System during the years 2000 through 2004 were identified. The chart was reviewed in detail if the culture source was identified as eye, sinus, head, or cerebrospinal fluid. The Ophthalmology Service’s electronic medical record was searched for the terms “methicillin resistant” and “MRSA” to identify other cases with ophthalmic (defined as ocular, orbital, or ocular adnexal) involvement. To capture patients who may not have had ophthalmology consultation, all hospital admissions with a diagnosis related group covering orbital cellulitis or preseptal cellulitis were also identified. Charts were reviewed in detail for patients uncovered by these methods. Finally, patients seen by the Ophthalmology Service within 30 days before or after a positive culture for MRSA were identified through search of billing records. No additional cases of ophthalmic MRSA infection were discovered.
Data collected included age at time of culture, gender, race, culture source, diagnosis, and laterality of infection. Possible risk factors investigated included recent stay at a long-term care facility, hospital, or jail; dormitory living; homelessness; hemodialysis, diabetes, hypertension, HIV infection, chronic obstructive pulmonary disease, asthma, pregnancy, cancer, liver disease, intravenous drug use, or alcohol abuse; and chemotherapy, systemic or ocular corticosteroid use, or use of other ocular medications. Data were also collected on the following: antibiotics initially prescribed; whether antibiotics were begun empirically prior to culture results; if antibiotics were changed after culture results were known; and any procedures performed, including incision and drainage. Sensitivity (or resistance) of isolates of MRSA to antibiotics tested, including minimal inhibitory concentrations (MICs), was also reviewed. The Parkland Microbiology Laboratory identifies gram-positive cocci as S aureus based on response to catalase and coagulase testing. Oxacillin is used instead of methicillin to test for β-lactam antibiotic resistance because oxacillin is more stable in vitro. The MicroScan Dried Gram Positive Panels (Dade Behring Inc, West Sacramento, California) are used to test for oxacillin resistance of S aureus isolates, and resistance is confirmed using Mueller Hinton agar with 4% NaCl and 6 μg/mL oxacillin (Remel, Lenexa, Kansas). Minimum inhibitory concentration breakpoints for susceptibility and resistance of isolates to antibiotics are those published by the Clinical and Laboratory Standards Institute.85 For example, S aureus isolates are considered to be resistant to oxacillin if the MIC is ≥ 4 μg/mL.
Patients were defined as having nosocomial MRSA infection or colonization if they had been hospitalized for 48 hours prior to obtaining the culture, if they had been hospitalized at Parkland Memorial Hospital in the 6 months prior to the date of the culture, or if they were clearly exposed to contaminated medical waste as a result of trauma. Comparison of age distributions to determine statistical significance was performed using the two-tailed Student t test. Other comparisons used the one-sided Fisher exact test or chi-square test as appropriate. For comparisons regarding sensitivity to antibiotics, ciprofloxacin and levofloxacin were considered as a single class of drug, and intermediate resistance (reduced susceptibility) on antibiotic testing was treated the same as frank resistance for comparison purposes.
RESULTS
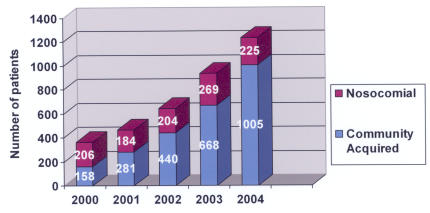
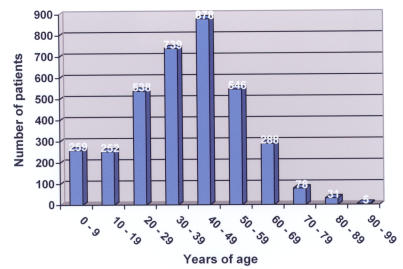
The Parkland Health and Hospital System experiences over 1,000,000 patient visits per year (Table 1). For the years 2000 through 2004, 3,640 patients with a culture positive for MRSA were identified, with 1,088 patients (30%) considered to have acquired the isolate via nosocomial transmission and 2,552 patients (70%) considered to have CA-MRSA. The increase in MRSA patients over the period 2000 through 2004 proportionally exceeds the increase in volume in the Parkland system (P < .0001). Whereas the absolute number of nosocomial MRSA patients remained fairly constant each year, the number of CA-MRSA patients steadily increased (Figure 1). The average age of MRSA patients was 38.5 years (SD = 17.8 years) (Figure 2). Age was unknown for 26 patients. For nosocomial MRSA patients the average age was 40.9 years (SD = 19.9 years), and for CA-MRSA patients it was 37.4 years (SD = 16.7 years) (P < .0001).
TABLE 1.
PATIENT VISITS IN THE PARKLAND HEALTH AND HOSPITAL SYSTEM, 2000 – 2004, BY YEAR
| VISITS | 2000 | 2001 | 2002 | 2003 | 2004 |
|---|---|---|---|---|---|
| Admissions, PMH | 41,679 | 42,426 | 41,260 | 41,081 | 41,425 |
| Emergency department visits, PMH | 144,510 | 160,650 | 156,870 | 155,536 | 148,215 |
| Outpatient visits, PMH | 401,669 | 419,507 | 447,248 | 443,639 | 427,606 |
| COPC visits | 308,409 | 367,230 | 363,294 | 384,647 | 455,927 |
COPC = community-oriented primary care clinics; PMH = Parkland Memorial Hospital.
FIGURE 1.
Number of MRSA patients in the Parkland Health and Hospital System by year, 2000 – 2004.
FIGURE 2.
Age distribution of MRSA patients in the Parkland Health and Hospital System, 2000 – 2004, by decade of life.
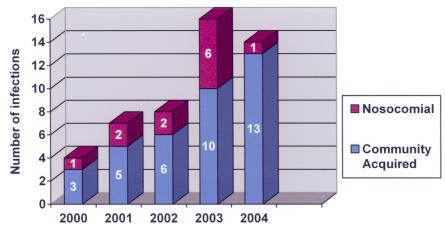
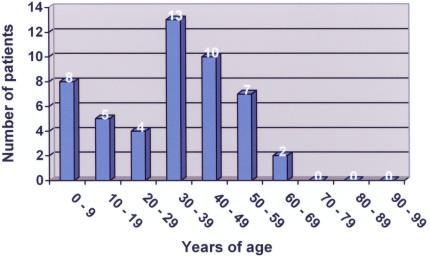
Forty-nine patients (1.3%) had ophthalmic MRSA involvement (Table 2), and the ratio of ophthalmic MRSA to all MRSA patients remained fairly constant each year (Table 3). Twenty-eight ophthalmic MRSA patients were men, and 21 were women. Eighteen were Hispanic, 15 were black, 14 were white, and two were Asian in racial origin. The disease process was bilateral in six cases, affected the left side only in 23, and affected the right side only in 18; laterality could not be determined from the chart in two cases. Twelve ophthalmic cases (24%) were nosocomial, and 37 (76%) were community-acquired; this distribution between nosocomial and community-acquired ophthalmic isolates mirrored the distribution for nonophthalmic MRSA patients. Again, as for all MRSA patients, the number of ophthalmic CA-MRSA patients increased each year, whereas the numbers of nosocomial patients remained fairly constant (Figure 3). The average age of patients with ophthalmic MRSA was 32.7 years (SD = 18.1 years) (Figure 4). Patients with ophthalmic MRSA tended to be younger than other MRSA patients (P = .023). Unlike nonophthalmic MRSA patients, patients with ophthalmic nosocomial MRSA tended to be younger (average age, 24.0 years with SD of 20.8 years) than patients with ophthalmic CA-MRSA (average age, 35.6 years with SD of 16.2 years) (P = .056).
TABLE 2.
OPHTHALMIC MRSA INFECTIONS IN THE PARKLAND HEALTH AND HOSPITAL SYSTEM, 2000 – 2004
| SUBJECT NO. | CULTURE DATE | AGE / RACE / GENDER | DIAGNOSIS | NOSOCOMIAL VS COMMUNITY- ACQUIRED | SOCIAL HISTORY | COMORBIDITIES | PRIOR IMMUNOSUPPRESSIVE TREATMENT | SURGICAL TREATMENT |
|---|---|---|---|---|---|---|---|---|
| 1 | 3/6/00 | 15BF | Blebitis | C | Systemic & topical steroids | |||
| 2 | 3/16/00 | 32BM | Corneal ulcer | C | Asthma, atopic dermatitis | |||
| 3 | 4/18/00 | 27WF | Preseptal cellulitis with abscess | N | IVDA | HIV | I&D | |
| 4 | 12/14/00 | 66HM | Endogenous endophthalmitis | C | LTCF, EtOH | DM, HTN | Vitreous tap / inject | |
| 5 | 5/14/01 | 46BF | Conjunctivitis | C | Asthma | Systemic steroids | ||
| 6 | 6/12/01 | <1HF | Conjunctivitis | N | ||||
| 7 | 7/10/01 | 51WM | Preseptal cellulitis | N | SCC medial canthus & orbit, HTN, Hep C | Chemotherapy | I&D | |
| 8 | 7/13/01 | 46HF | Preseptal cellulitis | C | DM, HTN | |||
| 9 | 9/16/01 | 35WM | Conjunctivitis | C | ||||
| 10 | 10/14/01 | 38BM | Endogenous endophthalmitis | C | DM, HTN, osteomyelitis | PPV | ||
| 11 | 11/6/01 | <1HM | Conjunctivitis | C | ||||
| 12 | 2/9/02 | 54AM | Orbital cellulitis with endophthalmitis | N | LTCF | T cell lymphoma | Enucleation and orbital biopsy | |
| 13 | 3/21/02 | 20BM | Preseptal cellulitis | C | Asthma | Systemic steroids | ||
| 14 | 4/11/02 | <1HM | Conjunctivitis | N | Systemic steroids | |||
| 15 | 5/13/02 | 55WF | Conjunctivitis | C | ||||
| 16 | 5/21/02 | 46BM | Preseptal cellulitis with abscess | C | IVDA | Hep B, Hep C | ||
| 17 | 8/21/02 | 40HM | Endogenous endophthalmitis | C | EtOH | Cirrhosis, endocarditis | PPV, repeat intravitreal injection | |
| 18 | 9/27/02 | 33WM | Preseptal cellulitis | C | DM, Hep C | |||
| 19 | 11/5/02 | <1HF | Conjunctivitis | C | ||||
| 20 | 1/3/03 | 30AM | Corneal ulcer | N | Herpes zoster | |||
| 21 | 1/25/03 | <1HM | Conjunctivitis | N | ||||
| 22 | 1/27/03 | 43BM | Conjunctivitis | C | HIV | |||
| 23 | 2/26/03 | 19BF | Preseptal cellulitis with abscess | C | I&D | |||
| 24 | 3/4/03 | 39WF | Dacryocystitis | C | I&D, DCR | |||
| 25 | 4/9/03 | 18HF | Preseptal cellulitis | N | ALL | Chemotherapy | ||
| 26 | 4/9/03 | 23HM | Conjunctivitis | C | ||||
| 27 | 4/26/03 | 31HM | Preseptal cellulitis with abscess | C | I&D | |||
| 28 | 5/7/03 | 15BM | Lid abscess | C | ||||
| 29 | 8/1/03 | 16BM | Corneal ulcer | N | ||||
| 30 | 8/13/03 | 8BM | Dacryocystitis | C | Sickle C disease | |||
| 31 | 9/12/03 | <1HF | Conjunctivitis | N | ||||
| 32 | 9/12/03 | 54BF | Blebitis | N | ||||
| 33 | 9/22/03 | 52BM | Preseptal cellulitis with abscess | C | EtOH | Laryngeal cancer, cirrhosis | ||
| 34 | 10/8/03 | 39WM | Preseptal cellulitis with abscess | C | IVDA | Hep C | I&D | |
| 35 | 12/18/03 | 8HF | Preseptal cellulitis | C | ||||
| 36 | 1/26/04 | 38WF | Corneal ulcer | N | Cocaine | PK | ||
| 37 | 2/1/04 | 64HF | Preseptal cellulitis | C | DM, HTN | |||
| 38 | 2/28/04 | 39HF | Preseptal cellulitis | C | DM, HTN | |||
| 39 | 3/6/04 | 43WF | Orbital abscess | C | IVDA | DM, Hep C | Orbitotomy with drainage of abscess × 3 | |
| 40 | 4/6/04 | 53HF | Preseptal cellulitis with abscess | C | DM, HTN, asthma | I&D | ||
| 41 | 4/19/04 | 37BM | Lid abscess | C | I&D | |||
| 42 | 5/1/04 | 37WM | Lid abscess | C | I&D | |||
| 43 | 5/27/04 | 26BF | Preseptal cellulitis with abscess | C | ||||
| 44 | 6/17/04 | 58WF | Preseptal cellulitis | C | I&D | |||
| 45 | 7/1/04 | 45BF | Corneal ulcer | C | IVDA (crack) | HTN | ||
| 46 | 7/8/04 | 31WM | Preseptal cellulitis | C | DM, Hep C | |||
| 47 | 7/29/04 | 43WM | Endogenous endophthalmitis | C | IVDA, EtOH | Hep C | Evisceration | |
| 48 | 11/11/04 | 45WM | Orbital cellulitis with endophthalmi | C | Orbitotomy, PPV | |||
| 49 | 12/27/04 | 46HM | Preseptal cellulitis with abscess | C | Jail, EtOH | DM, HTN | I&D |
A = Asian; ALL = acute lymphocytic leukemia; B = black; C = community-acquired; DCR = dacryocystorhinostomy; DM = diabetes; EtOH = alcohol abuse; H= Hispanic; Hep = hepatitis; HIV = human immunodeficiency virus infection; HTN = hypertension; I&D = incision and drainage; IVDA = intravenous drug abuse; LTCF = long-term care facility; N = nosocomial; PK = penetrating keratoplasty; PPV = pars plana vitrectomy; SCC = squamous cell carcinoma; W = white.
TABLE 3.
NUMBER OF MRSA PATIENTS IN THE PARKLAND HEALTH AND HOSPITAL SYSTEM BY YEAR, 2000 – 2004
| YEAR | ALL MRSA PATIENTS | OPHTHALMIC MRSA PATIENTS | % OPHTHALMIC |
|---|---|---|---|
| 2000 | 364 | 4 | 1.1 |
| 2001 | 465 | 7 | 1.5 |
| 2002 | 644 | 8 | 1.2 |
| 2003 | 937 | 16 | 1.7 |
| 2004 | 1230 | 14 | 1.1 |
MRSA = methicillin-resistant Staphylococcus aureus.
FIGURE 3.
Ophthalmic MRSA infections in the Parkland Health and Hospital System, 2000 – 2004, by year.
FIGURE 4.
Age distribution of ophthalmic MRSA infections in the Parkland Health and Hospital System, 2000 – 2004, by decade of life.
The most common manifestation of ophthalmic MRSA infection was preseptal cellulitis and/or lid abscess (Table 4). Conjunctivitis was next, with six (55%) of the 11 patients with conjunctivitis being newborns; the other five were adults. There were five cases of MRSA keratitis, four cases of endogenous endophthalmitis, two cases of orbital cellulitis associated with endophthalmitis, two cases of blebitis, two cases of dacryocystitis, and an orbital abscess.
TABLE 4.
OPHTHALMIC MRSA INFECTIONS IN THE PARKLAND HEALTH AND HOSPITAL SYSTEM, 2000 – 2004, BY DIAGNOSIS
| DIAGNOSIS | NO. OF CASES (%) |
|---|---|
| Preseptal cellulitis and/or lid abscess | 22 (42) |
| Preseptal cellulitis with lid abscess | 10 |
| Preseptal cellulitis without abscess | 9 |
| Lid abscess without mention of cellulitis | 3 |
| Conjunctivitis | 11 (21) |
| Corneal ulcer | 5 (10) |
| Endogenous endophthalmitis | 4 (8) |
| Orbital cellulitis with endophthalmitis | 2 (4) |
| Blebitis | 2 (4) |
| Dacryocystitis | 2 (4) |
| Orbital abscess | 1 (2) |
MRSA = methicillin-resistant Staphylococcus aureus.
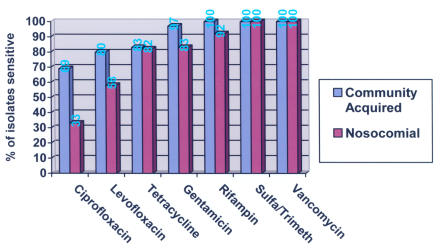
In all but one of the 49 infected ophthalmic MRSA cases, patients were placed on antibiotics after the culture was performed. Inadequate antibiotic coverage for the MRSA isolate was initially prescribed in 24 cases (50%) (Table 5), and the antibiotic was subsequently changed to one the isolate was sensitive to in only 13 cases (54%). Nosocomial isolates tended to be resistant to more antibiotics than community-acquired isolates (Figure 5). For the six major antibiotics tested (fluoroquinolones, tetracycline, aminoglycosides, rifampin, sulfamethoxazole-trimethoprim, vancomycin), half of nosocomial isolates were resistant to one or more antibiotics. Whereas 11 (30%) of 37 community-acquired isolates were resistant to one or more antibiotics tested, six (50%) of 12 nosocomial isolates were (P = .175) (Table 6). Nosocomial isolates were resistant on average to 0.83 antibiotics and community-acquired isolates were resistant to 0.32.
TABLE 5.
ANTIBIOTICS USED IN OPHTHALMIC MRSA INFECTIONS WITH INITIAL SUBOPTIMAL COVERAGE
| ANTIBIOTIC CLASS | NUMBER* |
|---|---|
| Penicillin derivatives | 12 |
| Cephalosporins | 11 |
| Fluoroquinolones | 6 |
| Clindamycin | 5 |
| Erythromycin | 2 |
| Aminoglycoside | 1 |
Although there were 24 cases of initial suboptimal antibiotic coverage, because combinations of antibiotics were used, there are more than 24 antibiotics in this column.
FIGURE 5.
Sensitivity of ophthalmic MRSA isolates to antibiotics.
TABLE 6.
SENSITIVITIES TO ANTIBIOTICS TESTED* FOR EACH OPHTHALMIC MRSA ISOLATE
| SUBJECT NO. | NOSOCOMIAL VS COMMUNITY-ACQUIRED | CIPRO | LEVO | TET | GENT | AMIK | RIF | SULFA/TRIM | VANC |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C | S | S | S | S | S | S | ||
| 2 | C | R | R | R | S | S | S | S | |
| 3 | N | S | S | S | S | S | S | S | |
| 4 | C | R | R | R | R | S | S | S | |
| 5 | C | S | S | S | S | S | S | S | S |
| 6 | N | S | S | R | R | S | R | S | S |
| 7 | N | R | I | S | S | S | S | S | S |
| 8 | C | S | S | S | S | S | S | S | S |
| 9 | C | S | S | S | S | S | S | S | S |
| 10 | C | S | S | S | S | S | S | S | S |
| 11 | C | R | R | S | S | S | S | S | S |
| 12 | N | R | R | S | S | S | S | S | S |
| 13 | C | S | S | S | S | S | S | S | S |
| 14 | N | R | R | S | S | S | S | S | S |
| 15 | C | S | S | S | S | S | S | S | S |
| 16 | C | S | S | S | S | S | S | S | S |
| 17 | C | S | S | R | S | S | S | S | S |
| 18 | C | S | S | S | S | S | S | S | S |
| 19 | C | R | R | S | S | S | S | ||
| 20 | N | S | S | S | S | S | S | ||
| 21 | N | R | R | R | S | S | S | ||
| 22 | C | R | S | S | S | S | |||
| 23 | C | S | S | S | S | S | S | ||
| 24 | C | S | S | S | S | S | |||
| 25 | N | S | S | S | S | S | S | ||
| 26 | C | S | S | S | S | S | S | ||
| 27 | C | R | S | S | S | S | S | ||
| 28 | C | S | S | S | S | S | S | ||
| 29 | N | I | R | S | S | S | S | ||
| 30 | C | S | S | S | S | S | S | ||
| 31 | N | S | S | S | S | S | S | ||
| 32 | N | S | S | S | S | S | S | ||
| 33 | C | S | S | S | S | S | S | ||
| 34 | C | S | S | S | S | S | S | ||
| 35 | C | R | S | S | S | S | S | ||
| 36 | N | S | S | S | S | S | S | ||
| 37 | C | S | R | S | S | S | S | ||
| 38 | C | S | S | S | S | S | S | ||
| 39 | C | S | S | S | S | S | S | ||
| 40 | C | S | S | S | S | S | S | ||
| 41 | C | S | S | S | S | S | S | ||
| 42 | C | S | R | S | S | S | S | ||
| 43 | C | S | S | S | S | S | S | ||
| 44 | C | S | S | S | S | S | S | ||
| 45 | C | S | S | S | S | S | S | ||
| 46 | C | S | S | S | S | S | S | ||
| 47 | C | S | R | S | S | S | S | ||
| 48 | C | S | S | S | S | S | |||
| 49 | C | S | S | S | S | S |
Amik = amikacin; C = community-acquired; Cipro = ciprofloxacin; Gent = gentamicin; I = intermediate (reduced) sensitivity; Levo = levofloxacin; MRSA = methicillin-resistant Staphylococcus aureus; N= nosocomial; R = resistant; Rif = rifampin; S = sensitive; Sulfa/Trim = sulfamethoxazole/trimethoprim; Tet = tetracycline; Vanc = vancomycin.
Not all antibiotics were tested for each isolate.
PRESEPTAL CELLULITIS AND/OR LID ABSCESS
Nineteen (86%) of the 22 patients with preseptal cellulitis and/or lid abscess had CA-MRSA. Seven (32%) of the 22 had diabetes compared to only three (10%) of the other 30 patients with nonlid disease MRSA infection or colonization (P = .054). Three (14%) of the 22 patients with lid disease had cancer, compared to only one (3%) of the other 30 patients (P = .198). Six (27%) with lid disease had liver disease (usually hepatitis C) compared to three (10%) without lid disease (P = .105). An antibiotic regimen that did not cover MRSA was begun empirically in 16 (73%) of the 22 cases with lid disease, and incision and drainage or debridement was performed in 10 cases (45%). In another case the patient lanced the lesion himself, whereas spontaneous drainage occurred in most other cases. Seven cases (32%) resolved despite receiving only β-lactam antibiotics or cephalosporins.
Two patients were initially misdiagnosed as having insect bites. Two other patients developed preseptal cellulitis after surgical procedures. One had MRSA infection after inferior forniceal reconstruction of a constricted orbital socket with alloderm graft 2 weeks previously. The other developed nosocomial MRSA infection after craniofacial resection of a medial canthal basal cell carcinoma with orbital extension.
CONJUNCTIVITIS
Six (54%) of the 11 patients with MRSA conjunctivitis were infants under 1 month of age, and the four cases (36%) of nosocomial infection were in infants; the seven other cases (64%) were community-acquired. In one of the infants classified as having community-acquired infection, culture was performed within 48 hours of birth, and there was no evidence of transplacental transmission. In the other infant, culture was obtained on an outpatient basis, and evidence of prior hospitalization was lacking.
CORNEAL ULCER
Three of the five patients with MRSA keratitis had nosocomial infection. All but one was begun on effective antibiotic coverage empirically. That patient had atopic dermatitis with a history of shield ulcers bilaterally and was started on ciprofloxacin ophthalmic drops. The isolate proved resistant to both levofloxacin and ciprofloxacin. Of the other patients, two had a history of cocaine use (one with definite crack keratopathy), one had preceding herpes zoster ophthalmicus after decompressive craniotomy for intraparenchymal hematoma due to motor vehicle collision, and one suffered trauma to the eye when a container of medical waste exploded. The latter patient was considered to have nosocomial infection, and the MRSA isolate had reduced susceptibility to levofloxacin and resistance to tetracycline.
ENDOGENOUS ENDOPHTHALMITIS
All four patients with endogenous endophthalmitis had CA-MRSA by our definition; however, one patient presenting with osteomyelitis had been an inpatient in another hospital for 3 days 1 month previously on account of an acute ankle fracture due to a motor vehicle collision. Another patient lived in a long-term nursing home facility, so healthcare–associated infection cannot be ruled out. One had a history of intravenous drug abuse, two were diabetic, two were hypertensive, and three were alcoholic. All four patients had positive blood cultures for MRSA. Three patients received vancomycin and ceftazidime intravitreally after cultures were performed either by means of pars plana vitrectomy (two cases) or vitreous tap (one case). The fourth patient was started on vancomycin and gentamicin intravenously for his bacteremia, and the eye was eviscerated.
ORBITAL CELLULITIS
There were three cases of orbital cellulitis, two accompanied by endophthalmitis. One of these cases was nosocomial involving a 54-year-old man with T-cell lymphoma living in a long-term nursing home facility. Cefuroxime and clindamycin were begun intravenously prior to the culture results, and the eye was enucleated and the orbit biopsied. The other 45-year-old male patient had CA-MRSA, was begun on vancomycin and ceftazidime intravenously prior to culture results, and underwent orbitotomy and pars plana vitrectomy.
A third case of MRSA orbital cellulitis, community-acquired, occurred in a 43-year-old diabetic female intravenous drug abuser with hepatitis C. She had an orbital abscess not involving the globe, and empirical therapy with intravenous ceftriaxone and clindamycin was begun prior to culture results. She ultimately underwent a total of three orbital drainage procedures before resolution.
BLEBITIS
In both patients with MRSA blebitis, topical vancomycin and tobramycin therapy was begun prior to culture results. In one patient, a nosocomial infection developed 12 weeks after bleb revision, and in the other, MRSA blebitis began 4.5 months after trabeculectomy.
DACROCYSTITIS
Both dacryocystitis cases were community-acquired infections. In a 39-year-old woman, empirical therapy with dicloxacillin was begun, and incision and drainage was required prior to dacryocystorhinostomy. In an 8-year-old boy with a history of dacryostenosis and sickle C disease, empirical therapy with cefdinir was begun, and the patient did not require surgery.
DISCUSSION
Penicillin is inactivated by β-lactamase, a serine protease that hydrolyzes the β-lactam ring. Production of β-lactamase is widespread in S aureus and may be conferred by the blaZ gene.29 Less than 5% of all S aureus isolates remain sensitve to penicillin.1 Methicillin resistance results from one of three known mechanisms86: hyperproduction of β-lactamases; modification of normal penicillin binding proteins; or, most commonly, production of an altered penicillin binding protein, PBP2a, which has decreased affinity for most β-lactam antibiotics, including penicillins, cephalosporins, β-lactam/β-lactamase inhibitor combinations, monobactams, and carbapenems.86–90 PBP2a is encoded by the gene mecA on a mobile genetic element, the staphylococcal cassette chromosome (SCC) mec.87,91 De novo development of MRSA occurs when a strain of MSSA acquires SCCmec.92,93 The mec genes likely originated from a different species of staphylococci.94,95 Although antibiotic therapy does not cause the genetic mutation, it rewards the mutation with a selective advantage to survive.96 At least five types of SCCmec elements have been identified, numbered I to V.97,98 Type I SCCmec contains the mecA gene as the sole resistance determinant, whereas SCCmec types II and III also contain determinants for resistance to non-β-lactam antibiotics.29 Because of their larger size, horizontal transfer of SCCmec types II and III occurs less easily than with type IV,87,99–103 so spread of MRSA strains with SCCmec types II and III mainly occurs due to selective pressure of antibiotic exposure over time (vertical spread).97 SCCmec types II and III are commonly found in multidrug-resistant nosocomial MRSA isolates.29
Genetic analysis of MRSA strains from around the world revealed that transfer of SECmec to a MSSA strain has occurred only a few times, so the emergence of MRSA has resulted from dissemination of only a few clonal types rather than the development of new MRSA clones.92,93,95,104–109 Thus virtually all patients with MRSA infection or colonization acquire their strain from an external source.93,107,109 The success reported in controlling MRSA with rigorous infection control practices supports the premise that transmission is the major factor contributing to the increasing prevalence of MRSA.96 Whereas antibiotic therapy is an important risk factor for MRSA by providing a selective advantage for its survival and spread,109 there have been no published reports of the eradication of MRSA through antibiotic control programs alone.96
DISRUPTING THE TRANSMISSION OF MRSA
The spread of infection from patient to patient by contaminated healthcare workers was noted over 150 years ago by Holmes110 and Semmelweis.111 Most transmission of MRSA in hospital inpatients is thought to be via transiently colonized healthcare workers.56,112 Environmental contamination may be the source for some spread. Boyce and coworkers113 found that 73% of hospital rooms with patients infected with MRSA and 69% of rooms with patients colonized with MRSA had some environmental contamination. In the same study, nurses contaminated their gloves 42% of the time despite touching only environmental surfaces in rooms of MRSA patients, whereas another report showed similar contamination of bare hands from surfaces.114 Other potential environmental reservoirs reported include computer keyboards used by clinicians,115 blood pressure cuffs,116 showers,116 and bathtubs.56 MRSA may survive for weeks or months on various surfaces.117 Dietz and coworkers118 found that MRSA could survive on the external surfaces of sterile goods packages for over 38 weeks. Hand hygiene may be the single most important measure for controlling transmission of MRSA and other multidrug-resistant organisms.96 Overcrowding and understaffing in hospitals are also risk factors for the spread of MRSA.119,120 Herr and colleagues121 found that the construction of more single-bed hospital rooms not only would help prevent spread of MRSA but would eliminate a major source of cost of isolation: the cost of an unoccupied bed when a single patient is isolated in a two-bed room.
Although the high usage of antibiotics in the healthcare environment provides a selective advantage that facilitates spread of MRSA, successful eradication programs have relied primarily on preventing person-to-person spread with less emphasis on antibiotic control.58,96,120,122,123 Success in controlling nosocomial MRSA has been greatest in countries that adhere to rigorous infection control policies that include surveillance cultures to identify colonized patients and strict application of barrier precautions for patients colonized or infected with MRSA.124–126 Isolation only of patients detected by routine clinical microbiologic cultures provides little benefit in high-risk areas.122 A French study involving 14 ICUs found that over half of patients colonized with MRSA did not have a positive clinical culture and would not have been detected without screening cultures.127 The study concluded that it was more cost effective to isolate all ICU patients on admission and remove them from isolation only if admission screening cultures were negative. At Parkland Memorial Hospital, MRSA was endemic in the neonatal ICU from 1987 through 1991, but MRSA has been controlled since then by strict adherence to hand hygiene, contact isolation, and cohorting of culture-positive infants.
Isolation measures are intended to disrupt transmission of MRSA and include isolation wards for known or suspected carriers, nurse cohorting (designated staff assigned to care for MRSA patients segregated from the rest of the ward), single-bed rooms, and barrier precautions (gowns, gloves, and even masks used by healthcare workers to prevent transmission). Cooper and associates112 found that despite the lack of well-designed studies, there was evidence that efforts that include isolation can reduce MRSA even in endemic settings. However, healthcare workers may spend less time with isolation patients, and isolation patients may have higher rates of pressure sores, falls, and fluid or electrolyte disorders than other patients.128
EMERGENCE OF MRSA IN THE COMMUNITY
The prevalence rate of MRSA among S aureus isolates in US hospitals is now over 50%,129 and is greater than 80% in some.109 MRSA is increasingly prevalent in the Parkland Health and Hospital System. Although Parkland’s hospital admissions and emergency department visits have remained relatively constant during the period 2000 through 2004, outpatient visits at the main campus, as well as at community-based outpatient primary care clinics in the system, have increased, yet the increase in MRSA is proportionally greater than the increase in visit volume in the system (P < .0001). Whereas the prevalence of MRSA has increased in hospitals worldwide, until recently strains were uncommon in the community. The absence of antibiotic selective pressure favoring their survival and attenuated virulence were thought to make resistant organisms unable to compete in the community.130 In the mid-1990s, though, reports began to appear in the United States, particularly among children, of MRSA infection in patients without identifiable risk factors,23,131 reminiscent of the appearance of penicillinase-producing strains of MRSA in the community two decades previously.129 Since then, CA-MRSA has been on the rise. At Harbor-UCLA Medical Center, 62% of community-associated S aureus infections are due to MRSA.132 Seventy-six percent of all S aureus isolates are methicillin-resistant at San Francisco General Hospital’s Integrated Soft Tissue Infection Clinic.133 Among patients presenting to an Oakland county hospital emergency room with community-acquired skin and soft tissue infections, 77% of S aureus isolates were methicillin-resistant.134 It is not feasible to determine the true background rate of MRSA carriage in the community.86,129 Unlike nosocomial strains, CA-MRSA strains usually have the smaller SCCmec type IV cassette, suggesting the possibility of increased mobility and easier transfer via plasmids or bacteriophages.135–137
The two MRSA clones in the United States most closely associated with community outbreaks are pulsed field types USA300 (multilocus sequence type ST8) and USA400 (ST1 lineage).132,138–140 These CA-MRSA strains are only distantly related to nosocomial strains and represent novel acquisitions of SCCmec.141 There is some evidence that the acquisition of Panton-Valentine leukocidin, a staphylococcal membrane toxin that targets leukocytes, is the principal virulence factor responsible for the spread of CA-MRSA.130,142–144 Panton-Valentine leukocidin causes tissue necrosis and leukocyte destruction and has been linked to recurrent, often severe primary skin infections as well as necrotizing pneumonia.145 The pvl genes that encode for Panton-Valentine leukocidin are rarely found in MSSA or nosocomial MRSA isolates.130 These genes, however, are found not only in successful community-acquired clones in the United States138,140 but also around the world.144 It is possible that the pvl locus is only a marker for other virulence or fitness determinants.130
The most common manifestations of CA-MRSA are skin and soft tissue infections,26,132–134,138,140,146 and the lesion may be mistaken for a spider bite.26,147 Two of the current series’ patients with CA-MRSA preseptal cellulitis were originally diagnosed as having insect bites. Patients with CA-MRSA are often younger than patients with nosocomial infection.138 Prior exposure to antibiotics has been implicated as a risk factor for development of CA-MRSA infection.140,148 Other risk factors may include homelessness,133,134 low socioeconomic status,138,149,150 injection drug use,132–134,151,152 previous MRSA infection, diabetes, chronic hepatitis C, cancer, and HIV infection.132 Outbreaks have been reported among jail inmates25,26,28,153 and among athletes who share equipment.27,140 In one study of necrotizing fasciitis caused by CA-MRSA, four of 14 patients had no identifiable risk factors.132 Unlike nosocomial MRSA isolates, which are more more likely to be resistant to multiple other antibiotics, CA-MRSA isolates are more likely to be sensitive to other antibiotics (other than β-lactams).138 For many types of CA-MRSA infections, there is little track record for the use of oral antibiotics to which isolates show in vitro susceptibility, so vancomycin remains, for now, the preferred antibiotic for empirical coverage of suspected CA-MRSA infections.130
The lack of standardized criteria for classifying MRSA infection as community-acquired makes it difficult to compare published studies.86 Parkland considers MRSA colonization or infection to be nosocomial if the patient had been hospitalized for 48 hours prior to obtaining the culture or if the patient had been hospitalized at Parkland Memorial Hospital in the previous 6 months. All other instances are considered community-acquired. These definitions are based on the Centers for Disease Control and Prevention’s definitions of nosocomial infection154 and serve to help Parkland identify if the hospital has new outbreaks of MRSA but ignore the possibility that the patient acquired MRSA in a healthcare environment beyond the traditional healthcare setting. Salgado and colleagues155 proposed the term “community onset” for infections seemingly acquired outside the hospital setting. They found a MRSA colonization rate in the community of 1.3% when several studies were pooled. However, among studies that excluded subjects with healthcare contacts, the pooled community MRSA colonization rate was only 0.2%. Friedman and associates156 suggested that bloodstream infections be classified as hospital-acquired, healthcare–associated, or community-acquired. They labeled an infection healthcare–associated if the patient received intravenous therapy at home, wound care or specialized nursing through a healthcare agency, family, or friends, or self-administered intravenous medical therapy within 30 days before infection; was hospitalized in an acute care hospital for 2 or more days in the preceding 3 months; or resided in a nursing home or long-term care facility. One of the 37 patients considered to have ophthalmic CA-MRSA by the study definition definitely would be considered to have healthcare–associated MRSA infection by the above definition (the patient resided in a long-term care facility and developed bacteremia and endogenous endophthalmitis). Like the healthcare–associated MRSA isolates in a recent report by Lesens and coworkers,157 the isolate in this study from a nursing home patient with endogenous endophthalmitis behaved more like a nosocomial MRSA isolate in that it was resistant to fluoroquinolones and aminoglycosides. Another case of endogenous endophthalmitis was classified as CA-MRSA by the study definition despite the patient having been hospitalized elsewhere 1 month previously. Other cases possibly could have been missed owing to inadequate documentation in the medical record. Two patients with preseptal cellulitis had undergone day surgery prior to infection (one 2 weeks prior, the other 2 months) and one case of CA-MRSA blebitis occurred 4.5 months after trabeculectomy. These three cases could possibly be considered to have healthcare–associated MRSA infection, though all three isolates were sensitive to the non-β-lactam antibiotics tested.
Because CA-MRSA has a predilection for causing skin and soft tissue infections, it is not surprising that most MRSA infections in our series involved the lids with preseptal cellulitis, lid abscess, or both. Whether initial therapy with an antibiotic active against MRSA even affects the outcome of skin and soft tissue infections is uncertain.130 Appropriate drainage is the definitive management of many skin and soft tissue infections and is an important adjunct to antibiotic therapy in deep, closed-space infections.29 Abscesses left without drainage in the setting of antibiotic resistance may promote the emergence of resistance.158 Data from the surgical literature suggest that, at least with skin and soft tissue infections, adequate surgical drainage allows many CA-MRSA infections to resolve regardless of whether the isolate is susceptible in vitro to the antibiotic chosen.133,134 It has been suggested that infections caused by CA-MRSA may be less virulent and have a more subacute onset than similar infections caused by other organisms.132 In the current series, 16 (73%) of 22 cases of lid infections had empirical antibiotics started that did not cover MRSA, and seven cases (32%) resolved despite receiving only β-lactam antibiotics or cephalosporins. Thirteen (59%) of the 22 cases formed lid abscesses, and most were incised or spontaneously drained (11 of 13, or 85%). We recently reviewed all cases of preseptal cellulitis seen at Parkland Memorial Hospital (excluding cases only seen in the community outpatient primary care clinics) for the years 2000 through 2004. Although half of the 48 cases were culture-negative, 18 (38%) of the culture-positive cases grew S aureus, and 16 of those isolates (89%) were methicillin-resistant (Witherspoon SR, ARVO Meeting, 2006, Abstract). This high prevalence of methicillin resistance combined with the 86% rate of MRSA lid infections being community-acquired in the present study would indicate a high prevalence of methicillin resistance in S aureus strains in the Dallas community. I am now more apt to treat community lid infections in Dallas with antibiotics other than with β-lactams or cephalosporins and to recommend incision and drainage of lesions. Zetola and colleagues29 recommend against the use of vancomycin as empirical treatment of furuncles and nonsevere skin and soft tissue infections.
IMPLICATIONS FOR THE PRACTICING OPHTHALMOLOGIST
Ophthalmic MRSA infection appears to be increasingly prevalent in the Parkland System. Although it is difficult to identify all other non-MRSA ophthalmic infections over the 5-year study period, I recently reviewed all culture-positive corneal ulcers, endophthalmitis, and blebitis seen at Parkland (P.H. Blomquist, MD, unpublished data, 2006). All MRSA corneal ulcers, endophthalmitis, and blebitis during the study period had been seen by the Ophthalmology Service. In addition, we had reviewed all preseptal cellulitis and orbital cellulitis seen at Parkland Memorial Hospital (Witherspoon SR, ARVO Meeting, 2006, Abstract). Combining the two data sets provided the information in Table 7. Whereas non-MRSA ophthalmic infections reviewed remained relatively level, numbers of MRSA ophthalmic infections were generally higher in more recent years (P = .042).
TABLE 7.
CULTURE-POSITIVE OPHTHALMIC INFECTIONS* AT PARKLAND MEMORIAL HOSPITAL, 2000 – 2004, BY YEAR
| Infection | 2000 | 2001 | 2002 | 2003 | 2004 |
|---|---|---|---|---|---|
| MRSA | 3 | 2 | 8 | 3 | 14 |
| Non- MRSA | 23 | 20 | 24 | 27 | 28 |
Includes all corneal ulcers, endophthalmitis, and blebitis seen by the Ophthalmology service at Parkland as well as all preseptal and orbital cellulitis seen at Parkland Memorial Hospital.
To my knowledge, this study includes the first cases reported of MRSA blebitis. Traditionally, streptococci and Haemophilus influenzae are the most common pathogens cultured in bleb-associated endophthalmitis.159–161 Whereas S aureus has been described as a causative agent in blebitis162 and bleb-associated endophthalmitis,163 methicillin resistance was not reported by the authors. Of seven total cases of blebitis at Parkland during the years 2000 through 2004, two were due to MRSA, two grew MSSA and streptococci, and three were culture-negative. Similarly, although MRSA has been reported as a cause of endophthalmitis after surgery,67,72,74 the current series is the first to clearly identify MRSA as a cause of endogenous endophthalmitis. That MRSA can cause such serious ocular infections is worrisome. We recently reviewed all orbital cellulitis cases seen at Parkland Memorial Hospital for the years 2000 through 2004. Of the 29 cases seen during that time, 10 (34%) were culture-negative, and four (14%) grew MRSA (Witherspoon SR, ARVO Meeting, 2006, Abstract). In the fourth case of MRSA orbital cellulitis, the patient had been transferred to Parkland. Because the cultures were done at the outside hospital and not at Parkland, this case was not included in the MRSA infection control database.
What are the implications of an increasing prevalence of MRSA in the community for the practicing ophthalmologist? Just as in the hospital setting, the healthcare worker can unknowingly spread MRSA from patient to patient. Lam and coworkers164 looked at microbial hand contamination among ophthalmologists in a tertiary care outpatient practice and found an increase in prevalence of transient flora, including MRSA, after patient contact. Hand washing reduced the resident and transient flora load by at least an order of magnitude. Yet, historically, studies assessing compliance with hand hygiene have shown low compliance rates, averaging approximately 40% and ranging as low as 10%.96 Chlorhexidine gluconate is frequently recommended for hand washing on account of low toxicity and broad antimicrobial spectrum.165,166
Standard antibiotic regimens that assume that local strains of S aureus are sensitive to β-lactams need to be reevaluated in MRSA endemic areas.167 Vancomycin eyedrops can be prepared by mixing an ampule intended for intravenous use with phosphate-buffered artificial tears. The solution retains in vitro antistaphylococcal activity for at least 2 weeks without refrigeration168 and is usually used at a concentration of 50 mg/mL,168–170 though a concentration of 31 mg/mL has been used.50 Although vancomycin is certainly the standard for MRSA infections at present, widescale use is a known risk factor for the development of VRE,53 and long-term use is a risk factor for reduced susceptibility and frank resistance in S aureus.44,46,47 The Hospital Infection Control Practices Advisory Committee strongly advises against the routine use of vancomycin for prophylaxis.171 Yet vancomycin is used, including at Parkland Memorial Hospital, for the prophylaxis of endophthalmitis after open globe injury.172 Even more patients in the United States routinely receive prophylactic intracameral vancomycin during cataract surgery,173 an operation where endophthalmitis is a rare event, occurring in only 2.15 cases per 1,000, according to a recent review of Medicare data from 1994 through 2001.174 The prophylactic efficacy of intracameral vancomycin has been questioned.175–177 Studies have failed to demonstrate that vancomycin prophylaxis significantly reduced residual bacteria in the anterior chamber,178,179 and vancomycin may not remain in the anterior chamber long enough to have a bactericidal effect.180 Indeed, a significantly lower rate (0.84 cases per 10,000) of endophthalmitis after cataract surgery performed at the affiliated hospitals of the University of Texas Southwestern Medical Center (including Parkland Memorial Hospital) from 1995 to 2002 has been reported despite not using intracameral antibiotics.181
However, even though there have been worries about the use of vancomycin prophylaxis at the time of cataract surgery on account of cost,175 potential for dilution error,182 and adverse effects on the eye (including cystoid macular edema),183 Gordon175 believes that ophthalmic use of vancomycin is unlikely to be a serious selection factor for promoting resistance. Libre and associates184 believe that the intraocular use of antibiotics is extremely unlikely to promote the development of drug-resistant organisms, because the intraocular environment rarely harbors colonizing organisms. However, it is known that topical antibiotics can cause bacterial resistance at the site of application in the conjunctiva, cornea, and lids,185 and recently the first report of topical ocular antibiotics inducing bacterial resistance at extraocular sites was reported.186 Perhaps intraocular surgeons should reevaluate the practice of using vancomycin for prophylaxis.
What changes are necessary in the ophthalmologist’s practice in view of increasing bacterial resistance in the community? First, healthcare workers, including those in outpatient settings, must remove transient microorganisms from hands by using hand washing or hand antisepsis between all patient contacts and after contact with inanimate objects in the immediate vicinity of patients.187 Eye lane surfaces and hand instruments should be cleaned periodically. Contact isolation measures should be instituted for patients known to be harboring resistant organisms like MRSA. Protective gloves and gowns are used in the Parkland ophthalmology outpatient clinics, a dedicated isolation eye lane is used to examine MRSA and VRE patients, and all surfaces and instruments are cleaned after the patient is discharged from the room.96 Although gloves greatly reduce the risk of hand contamination, they do not obviate the need for hand washing after their removal.188,189 Use of a mask may be of some benefit in preventing nasal acquisition of MRSA by healthcare workers.96 Indeed, the Centers for Disease Control and Prevention has recommended the use of a mask when caring for patients with S aureus with reduced susceptibility or resistance to vancomycin.46 It is difficult in many outpatient practices to identify MRSA and VRE patients; the establishment of electronic medical record systems may aid in this endeavor. For example, Parkland uses a tracking flag in the electronic patient management system to identify MRSA and VRE patients, thus alerting the healthcare team to take contact isolation precautions. Currently, there are no data to suggest that treatment to eradicate colonized patients with CA-MRSA is necessary or effective in the long term,7,138,190 and resistance to antibiotics used for decolonization has evolved rapidly when attempted.191–193
In the absence of a history of MRSA or VRE, a high level of suspicion is needed when patients have risk factors for nosocomial or healthcare-associated infections. In areas with endemic CA-MRSA, incision, drainage, and culturing of simple lid abscesses should be considered.26 Consider the possibility of CA-MRSA for cases with the appearance of a spider or insect bite; if there is a history of contact with a correctional facility (either as a prisoner or a visitor); if there is a history of playing contact sports or association with a sports facility; or if a skin or soft tissue infection is recurrent.147 In addition to draining suppurative collections, consider removing foreign devices such as infected scleral buckles,83 because the likelihood of sterilizing an infected site in the presence of a foreign device is low.1
Empirical antibiotic therapy should include coverage for MRSA in endemic areas; β-lactam antibiotics may not be appropriate for empirical treatment of suspected staphylococcal infections.138 It may not be necessary to use vancomycin for coverage, because most CA-MRSA strains are susceptible to other antibiotics, such as tetracycline and aminoglycosides.138,167 Resistance to older quinolones is common among MRSA and occurs in as many as 90% of isolates in some hospitals.22 Resistance was found to ciprofloxacin and levofloxacin also in the current study of ophthalmic isolates, especially from nosocomial infections. Chloramphenicol eyedrops have been used to treat MRSA external ocular infections,30,51 but chloramphenicol is not widely used on account of the risk of developing aplastic anemia with its use.194,195 I tend to avoid clindamycin because of the risk of development of inducible clindamycin resistance in CA-MRSA strains that are erythromycin-resistant by virtue of carrying the erm (erythromycin ribosome methylase) gene.147,196,197 Inducible resistance to clindamycin may not be apparent on routine susceptibility testing.198–200 Frazee and associates134 recommended using the combination of trimethoprim-sulfamethoxazole and cephalexin for oral therapy of moderate to severe skin and soft tissue infections, with cephalexin used to cover for Streptococcus pyogenes. For minor infections in adults, they recommended doxycycline.
Prescribed treatment regimens should be appropriate in both dose and duration, because inadequate dose or inadequate or excessive duration of therapy may make development of resistance more likely.96,201 Steps should be taken to decrease the occurrence of new resistant strains by adhering to guidelines advocating against the routine prophylactic use of vancomycin in the hospital setting,171,177 and strong consideration should be given to eliminating the practice in other healthcare settings. Preoperative povidone-iodine antisepsis of the ocular surface has the most support in the literature for efficacy in preventing bacterial endophthalmitis after intraocular surgery and should be adopted. Although preoperative and postoperative topical application of broad-spectrum antibiotics and postoperative use of subconjunctival antibiotics remain common, the evidence is less clear regarding their efficacy,176,202 and prolonged topical postoperative antibiotic administration may predispose to development of antimicrobial resistance.177,202 Some investigators suggest avoiding simultaneous bilateral treatment for elective ocular procedures (eg, refractive surgery) on patients colonized with MRSA or who live or work in a healthcare environment,78,79 in addition to using a fourth-generation fluoroquinolone or bacitracin for preoperative prophylaxis.78 In vitro studies have identified MRSA strains, however, that are resistant to fourth-generation fluoroquinolone antibiotic.203,204
As MRSA increases in prevalence, early and accurate detection of colonized individuals will be important to prevent spread. Traditional antimicrobial susceptibility test methods require at least 24 hours to perform.86 Polymerase chain reaction (PCR) analysis of donor eye preservation media for spa and mecA genes has been used to detect contamination with MRSA more rapidly than conventional culture.205 However, PCR is not routinely used by most clinical diagnostic laboratories.86 Newer detection methods for MRSA have been developed, including the BBL Crystal MRSA ID System (Becton Dickinson, Sparks, Maryland), which uses a fluorescent indicator of dissolved oxygen in broth as a marker for bacterial growth in wells containing oxygen; Velogene Rapid MRSA Identification Assay (Alexon-Trend, Inc, Ramsey, Minnesota), a colorimetric enzyme immunoassay utilizing a mecA gene probe; and the MRSA-Screen (Denk Deiken Co, Tokyo, Japan), a slide latex agglutination test utilizing a monoclonal antibody against PBP2a.86 These tests promise results within 4 hours to as little as 15 minutes.86
New antibiotic classes with good activity against gram-positive organisms (such as ketolides and oxazolidinones) show promise in the treatment of MRSA,206 as may new versions of older compounds like streptogramins and quinolones.167,196 However, antibiotic resistance even to newer antibiotics like linezolid has already been reported.207 Teicoplanin, another glycopeptide antibiotic, is intrinsically less effective against S aureus than vancomycin and is unlikely to be effective in strains with reduced vancomycin susceptibility or frank resistance.208,209 In view of increasing antimicrobial resistance worldwide, will physicians need to turn to alternative agents to treat bacterial infections as they did prior to the discovery of antibiotics? MRSA conjunctivitis has been treated with the antiseptic benzethonium chloride 0.02%.55 Lysostaphin is a zinc metalloproteinase extracted from Staphylococcus simulans that lyses S aureus by disrupting its peptidoglycan layer.210,211 Lysostaphin has been shown to be effective in treating experimental MRSA keratitis and endophthalmitis.212,213 Another potentially useful enzyme, LasA protease (also called staphylolysin), is a staphylolytic endopeptidase secreted by Pseudomonas aeruginosa that also targets the peptidoglycan of S aureus.214–216 LasA protease was comparable to vancomycin in experimental MRSA keratitis when treatment was started early and was more effective than vancomycin when treatment was started late.217 This suggests that LasA protease can lyse bacterial cell walls during the bacteria’s stationary phase of growth, unlike vancomycin.212,218 A vaccine for S aureus has been evaluated experimentally and clinically.219–221
Antimicrobial resistance to penicillin, methicillin, and vancomycin is an inevitable consequence of the selective pressure of antibiotic exposure.129 Ophthalmologists need to be prepared to identify and treat resistant strains and take steps to control the emergence and spread of resistant strains, both in the hospital and in the community.
REFERENCES
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Novick RP. The staphylococcus as a molecular genetic system. In: Novick RP, ed. Molecular Biology of the Staphylococci New York: VCH; 1990:1–37.
- 3.Schaberg DR, Zervos MJ. Intergeneric and interspecies gene exchange in gram-positive cocci. Antimicrob Agents Chemother. 1986;30:817–822. doi: 10.1128/aac.30.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble WC, Valkenburg HA, Wolters CHL. Carriage of Staphylococcus aureus in random samples of a normal population. J Hyg (Lond) 1967;65:567–573. doi: 10.1017/s002217240004609x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casewell MW, Hill RLR. The carrier state: methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1986;18(Suppl A):1–12. doi: 10.1093/jac/18.supplement_a.1. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect. 1995;31:13–24. doi: 10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 7.Brumfitt W, Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989;320:1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- 8.Kirby WM. Extraction of a high potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 9.Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;1:641–644. doi: 10.1016/s0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaye KS, Engemann JJ, Fraimow HS, et al. Pathogens resistant to antimicrobial agents: epidemiology, molecular mechanisms, and clinical management. Infect Dis Clin North Am. 2004;18:467–511. doi: 10.1016/j.idc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;2:641–644. doi: 10.1016/s0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- 12.Jessen O, Rosendal K, Bulow P, et al. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med. 1969;281:627–635. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- 13.Ross S, Rodriguez W, Controni G, et al. Staphylococcal susceptibility to penicillin G. The changing pattern among community strains. JAMA. 1974;229:1075–1077. [PubMed] [Google Scholar]
- 14.Hughes GB, Chidi CC, Macon WL. Staphylococci in community-acquired infections: increased resistance to penicillin. Ann Surg. 1976;183:355–357. doi: 10.1097/00000658-197604000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn DL, Baker WA. Penicillin G susceptibility of “rural” Staphylococcus aureus. J Fam Pract. 1980;11:43–46. [PubMed] [Google Scholar]
- 16.Robinson GN, Stevens S, Batchelor FR, et al. Bacteriological studies on a new penicillin—BRL.1241. Lancet. 1960;2:564–567. doi: 10.1016/s0140-6736(60)91642-1. [DOI] [PubMed] [Google Scholar]
- 17.Jevons MP. “Celbenin”-resistant staphylococci”. Br J Med. 1961;1:124–125. [Google Scholar]
- 18.Knox R. “Celbenin”-resistant staphylococci”. Br J Med. 1961;1:126. [Google Scholar]
- 19.Gedney J, Lacey RW. Properties of methicillin-resistant staphylococci now endemic in Australia. Med J Aust. 1982;1:448–450. doi: 10.5694/j.1326-5377.1982.tb132412.x. [DOI] [PubMed] [Google Scholar]
- 20.Pavillard R, Harvey K, Douglas D, et al. Epidemic of hospital-acquired infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust. 1982;1:451–454. [PubMed] [Google Scholar]
- 21.Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database—USA. Clin Infect Dis. 1999;29:259–263. doi: 10.1086/520195. [DOI] [PubMed] [Google Scholar]
- 22.Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphyoccocal species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997 – 1999. Clin Infect Dis. 2001;32(suppl 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 23.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Four pediatric deaths from community acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997—1999. MMWR Morb Mortal Wkly Rep. 1999;48:707–710. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi 2000. MMWR Morb Mortal Wkly Rep. 2001;50:919–922. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Outbreaks of community-acquired methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002—2003. MMWR Morb Mortal Wkly Rep. 2003;52:88. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants—Colorado, Indiana, Pennsylvania and Los Angeles County, 2000 – 2003. MMWR Morb Mortal Wkly Rep. 2003;52:793–795. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California and Texas, 2001 – 2003. MMWR Morb Mortal Wkly Rep. 2003;52:992–996. [PubMed] [Google Scholar]
- 29.Zetola N, Francis JS, Nuermberger EL, et al. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 30.Shanmuganathan VA, Armstrong M, Buller A, et al. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA) Eye. 2005;19:284–291. doi: 10.1038/sj.eye.6701465. [DOI] [PubMed] [Google Scholar]
- 31.Muder RR, Brennen C, Wagener MM, et al. Methicillin-resistant staphylococcal colonization and infection in a long-term care facility. Ann Intern Med. 1991;114:107–112. doi: 10.7326/0003-4819-114-2-1-107. [DOI] [PubMed] [Google Scholar]
- 32.Leman R, Alvarado-Ramy F, Pocock S, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus in an American Indian population. Infect Hosp Control Epidemiol. 2004;25:121–125. doi: 10.1086/502361. [DOI] [PubMed] [Google Scholar]
- 33.Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 34.Abramson MA, Sexton DJ. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: At what costs? Infect Control Hosp Epidemiol. 1999;20:408–411. doi: 10.1086/501641. [DOI] [PubMed] [Google Scholar]
- 35.Engemann JJ, Carmeli Y, Cosgrove SE, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36:592–598. doi: 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- 36.Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in non-addicts. Ann Intern Med. 1982;97:496–503. doi: 10.7326/0003-4819-97-4-496. [DOI] [PubMed] [Google Scholar]
- 37.Chambers HF, Miller RT, Newman MD. Right-sided Staphylococcus aureus endocarditis in intravenous drug abusers: two-week combination therapy. Ann Intern Med. 1988;109:619–624. doi: 10.7326/0003-4819-109-8-619. [DOI] [PubMed] [Google Scholar]
- 38.Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34:1227–1231. doi: 10.1128/aac.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 40.Hartstein AI, Mulligan ME, Morthland VH, et al. Recurrent Staphylococcus aureus bacteremia. J Clin Microbiol. 1992;30:670–674. doi: 10.1128/jcm.30.3.670-674.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson WR, Karchmer AW, Dajani AS, et al. Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci, and HACEK microorganisms. American Heart Association. JAMA. 1995;274:1706–1713. [PubMed] [Google Scholar]
- 42.Gonzalez C, Rubio M, Romero-Vivas J, et al. Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis. 1999;29:1171–1177. doi: 10.1086/313440. [DOI] [PubMed] [Google Scholar]
- 43.Schwalbe RS, Stapleton JT, Gilligan PH. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987;316:927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- 44.Hiramatsu K, Hanaki H, Ino T, et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 45.Fridkin SK, Hageman J, McDougal LK, et al. Epidemiologic and microbiologic characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997 – 2001. Clin Infect Dis. 2003;36:429–439. doi: 10.1086/346207. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:565–567. [PubMed] [Google Scholar]
- 47.Chang S, Sievert DM, Hagerman JC, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 48.Noble WC, Virani Z, Cree RG. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 49.Ribner BS. Endemic, multiply resistant Staphylococcus aureus in a pediatric population. Am J Dis Child. 1987;141:1183–1187. doi: 10.1001/archpedi.1987.04460110053021. [DOI] [PubMed] [Google Scholar]
- 50.Ross J, Abate MA. Topical vancomycin for the treatment of Staphylococcus epidermidis and methicillin-resistant Staphylococcus aureus conjunctivitis. DICP. 1990;24:1050. doi: 10.1177/106002809002401104. 1053. [DOI] [PubMed] [Google Scholar]
- 51.Fukuda M, Ohashi H, Matsumoto C, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphulococcus ocular surface infection: efficacy of chloramphenicol eye drops. Cornea. 2002;21(7 suppl):S86–S89. doi: 10.1097/01.ico.0000263125.99262.42. [DOI] [PubMed] [Google Scholar]
- 52.Brennen C, Muder RR. Conjunctivitis associated with methicillin-resistant Staphylococcus aureus in a long-term-care facility. Am J Med. 1990;88(5N):14N–17N. [PubMed] [Google Scholar]
- 53.Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol. 1992;13:700–705. doi: 10.1086/648342. [DOI] [PubMed] [Google Scholar]
- 54.Spindel SJ, Strausbaugh LJ, Jacobson C. Infections caused by Staphylococcus aureus in a veterans’ affairs nursing home care unit: a 5-year experience. Infect Control Hosp Epidemiol. 1995;16:217–223. doi: 10.1086/647093. [DOI] [PubMed] [Google Scholar]
- 55.Horil T, Futamura N, Suzuki Y. Antiseptic treatment of methicillin-resistant Staphylococcus aureus conjunctivitis. J Infect. 2001;42:166–169. doi: 10.1053/jinf.2001.0804. [DOI] [PubMed] [Google Scholar]
- 56.Mitsuda T, Arai K, Fujita S, et al. Epidemiological analysis of strains of methicillin-resistant Staphylococcus aureus (MRSA) infection in the nursery; prognosis of MRSA carrier infants. J Hosp Infect. 1995;31:123–134. doi: 10.1016/0195-6701(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 57.Hayakawa T, Hayashidera T, Yoneda K, et al. Unexpectedly prolonged colonization of exfoliative toxin A-producing methicillin-resistant Staphylococcus aureus in infants. Eur J Pediatr. 1998;157:781. doi: 10.1007/s004310050935. [DOI] [PubMed] [Google Scholar]
- 58.Jernigan JA, Titus MG, Groschel DH, et al. Effectiveness of contact isolation during a hospital outbreak of methicillin-resistant Staphylococcus aureus. Am J Epidemiol. 1996;143:496–504. doi: 10.1093/oxfordjournals.aje.a008770. [DOI] [PubMed] [Google Scholar]
- 59.Saiman L, Cronquist A, Wu F, et al. An outbreak of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2003;24:317–321. doi: 10.1086/502217. [DOI] [PubMed] [Google Scholar]
- 60.Muder RR, Brennen C, Goetz AM. Infection with methicillin-resistant Staphylococcus aureus among hospital employees. Infect Control Hosp Epidemiol. 1993;14:576–578. doi: 10.1086/646640. [DOI] [PubMed] [Google Scholar]
- 61.Maskin SL. Infectious scleritis after a diabetic foot ulcer. Am J Ophthalmol. 1993;115:254–255. doi: 10.1016/s0002-9394(14)73934-0. [DOI] [PubMed] [Google Scholar]
- 62.Eiferman RA, O’Neill KP, Morrison NA. Methicillin-resistant Staphylococcus aureus corneal ulcers. Ann Ophthalmol. 1991;23:414–415. [PubMed] [Google Scholar]
- 63.Insler MS, Fish LA, Silbernagel J, et al. Successful treatment of methicillin-resistant Staphylococcus aureus keratitis with topical ciprofloxacin. Ophthalmology. 1991;98:1690–1692. doi: 10.1016/s0161-6420(91)32067-0. [DOI] [PubMed] [Google Scholar]
- 64.Sotozono C, Inagaki K, Fujita A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylcoccus aureus infections in the cornea. Cornea. 2002;21(7 suppl):S94–S101. doi: 10.1097/01.ico.0000263127.84015.3f. [DOI] [PubMed] [Google Scholar]
- 65.Inoue Y. Ocular infections in patients with atopic dermatitis. Int Ophthalmol Clin. 2002;42:55–69. doi: 10.1097/00004397-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Labit CM, Claeys GW, Verbraeken HE, et al. Methicillin resistance of bacteria isolated from vitreous fluid from patients undergoing vitrectomy. Eur J Ophthalmol. 2001;11:160–165. doi: 10.1177/112067210101100210. [DOI] [PubMed] [Google Scholar]
- 67.Saitoh A, Jinbayashi H, Saitoh AK, et al. Parameter estimation and dosage adjustment in the treatment with vancomycin of methicillin-resistant Staphylococcus aureus ocular infections. Ophthalmologica. 1997;211:232–235. doi: 10.1159/000310797. [DOI] [PubMed] [Google Scholar]
- 68.Kubo M, Sakuraba T, Arai Y, et al. Dacryocystorhinostomy for dacryocystitis caused by methicillin-resistant Staphylococcus aureus: report of four cases. Jpn J Ophthalmol. 2002;46:177–182. doi: 10.1016/s0021-5155(01)00496-8. [DOI] [PubMed] [Google Scholar]
- 69.Mehra P, Caiazzo A, Bestgen S. Odontogenic sinusitis causing orbital cellulitis. JADA. 1999;130:1086–1092. doi: 10.14219/jada.archive.1999.0340. [DOI] [PubMed] [Google Scholar]
- 70.Anari S, Karagama YG, Fulton B, et al. Neonatal disseminated methicillin-resistant Staphylococcus aureus presenting as orbital cellulitis. J Laryngol Otol. 2005;119:64–67. doi: 10.1258/0022215053223003. [DOI] [PubMed] [Google Scholar]
- 71.Kato T, Hayasaka S. Methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococci from conjunctivas of preoperative patients. Jpn J Ophthalmol. 1988;42:461–465. doi: 10.1016/s0021-5155(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 72.Cosar CB, Cohen EJ, Rapuano CJ, et al. Clear corneal wound infection after phacoemulsification. Arch Ophthalmol. 2001;119:1755–1759. doi: 10.1001/archopht.119.12.1755. [DOI] [PubMed] [Google Scholar]
- 73.Chiang RK, Rapuano CJ. Recurrent methicillin-resistant Staphylococcus aureus wound ulcer after clear-cornea cataract surgery. CLAO J. 2002;28:109–110. [PubMed] [Google Scholar]
- 74.Moshirfar M, Marx DP, Mirzaian G. Endophthalmitis in patients using fourth-generation fluoroquinolones following phacoemulsification. J Cataract Refract Surg. 2005;31:1669–1670. doi: 10.1016/j.jcrs.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 75.Rudd JC, Moshirfar M. Methicillin-resistant Staphylococcus aureus keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:471–473. doi: 10.1016/s0886-3350(00)00882-8. [DOI] [PubMed] [Google Scholar]
- 76.Rubenfeld RS, Negvesky GJ. Methicillin-resistant Staphylococcus aureus ulcerative keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1523–1525. doi: 10.1016/s0886-3350(00)00783-5. [DOI] [PubMed] [Google Scholar]
- 77.Förster W, Becker K, Hungermann D, et al. Methicillin-resistant Staphylococcus aureus after excimer laser photorefractive keratectomy. J Cataract Refract Surg. 2002;28:722–724. doi: 10.1016/s0886-3350(01)01076-8. [DOI] [PubMed] [Google Scholar]
- 78.Solomon R, Donnenfeld ED, Perry HD, et al. Bilateral methicillin-resistant Staphylococcus aureus keratitis in a medical resident following an uneventful bilateral photorefractive keratectomy. Eye Contact Lens. 2003;29:187–189. doi: 10.1097/01.ICL.0000072826.38354.31. [DOI] [PubMed] [Google Scholar]
- 79.Donnenfeld ED, O’Brien TP, Solomon R, et al. Infectious keratitis after photorefractive keratectomy. Ophthalmology. 2003;110:743–747. doi: 10.1016/S0161-6420(02)01936-X. [DOI] [PubMed] [Google Scholar]
- 80.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 81.Hanifin JM, Rogge JL. Staphylococcal infections in patients with atopic dermatitis. Arch Dermatol. 1993;103:1315–1322. [PubMed] [Google Scholar]
- 82.Hoeger PH, Lenz W, Boutonnier A, et al. Staphylococcal skin colonization in children with atopic dermatitis: prevalence, persistence, and transmission of toxigenic and nontoxigenic strains. J Infect Dis. 1992;165:1064–1068. doi: 10.1093/infdis/165.6.1064. [DOI] [PubMed] [Google Scholar]
- 83.Oshima Y, Ohji M, Inoue Y, et al. Methicillin-resistant Staphylococcus aureus infections after scleral buckling procedures for retinal detachments associated with atopoic dermatitis. Ophthalmology. 1999;106:142–147. doi: 10.1016/S0161-6420(99)90025-8. [DOI] [PubMed] [Google Scholar]
- 84.Osawa S, Sasoh M, Ito K, et al. Exudative retinal detachment due to methicillin-resistant Staphylococcal aureus after scleral buckling in the treatment of retinal detachment accompanied by atopic dermatitis. Retina. 2002;22:649–651. doi: 10.1097/00006982-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 85.Clinical and Laboratory Standards Institute/NCCLS. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement. CLSI/NCCLS Document M100-S15. Wayne, Pennsylvania: CLSI; 2005.
- 86.Palavecino E. Community-acquired methicillin-resistant Staphyloccus aureus infections. Clin Lab Med. 2004;24:403–418. doi: 10.1016/j.cll.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and chemical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katayama Y, Zhang HZ, Hong D, et al. Jumping the barrier to beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 2003;185:5465–5472. doi: 10.1128/JB.185.18.5465-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asheshov EH, Dyke KG. Regulation of the synthesis of penicillinase in diploids of Staphylococcus aureus. Biochem Biophys Res Commun. 1968;30:213–218. doi: 10.1016/0006-291x(68)90437-3. [DOI] [PubMed] [Google Scholar]
- 90.Sabath LD. Mechanisms of resistance of beta-lactam antibiotics in strains of Staphylococcus aureus. Ann Intern Med. 1982;97:339–344. doi: 10.7326/0003-4819-97-3-339. [DOI] [PubMed] [Google Scholar]
- 91.Ito T, Katayama Y, Asada K, et al. Structural comparison of three types of staphylococcal cassette chromosome mec in the chromosome of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–1326. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hiramatsu K. Molecular evolution of MRSA. Microbiol Immunol. 1995;39:531–543. doi: 10.1111/j.1348-0421.1995.tb02239.x. [DOI] [PubMed] [Google Scholar]
- 93.Hiramatsu K, Cui L, Kuroda M, et al. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9:486–493. doi: 10.1016/s0966-842x(01)02175-8. [DOI] [PubMed] [Google Scholar]
- 94.Archer GL, Niemeyer DM. Origin and evolution of DNA associated with resistance to methicillin in staphylococci. Trends Microbiol. 1994;2:343–347. doi: 10.1016/0966-842x(94)90608-4. [DOI] [PubMed] [Google Scholar]
- 95.Enright MC, Robinson DA, Randle G, et al. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci USA. 2002;99:7687–7692. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of mulitdrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 97.Ito T, Okuma K, Ma XX, et al. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist Updates. 2003;6:41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 98.Ito T, Ma XX, Takeuchi F, et al. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–2651. doi: 10.1128/AAC.48.7.2637-2651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Enright MC, Day NP, Davies CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma XX, Ito T, Tiensasitorn C, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–1152. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fey PD, Said-Salim B, Rupp ME, et al. Comparative molecular analysis of community or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oliveira DC, Tomasz A, de Lencastre H. Secrets of success of a human pathogen: molecular evolution of pandemic clones of methicillin-resistant Staphylococcus aureus. Lancet Infect Dis. 2002;2:180–189. doi: 10.1016/s1473-3099(02)00227-x. [DOI] [PubMed] [Google Scholar]
- 103.Charlebois ED, Perdreau-Remington F, Kreiswirth B, et al. Origins of community strains of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2004;39:47–54. doi: 10.1086/421090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kreiswirth B, Kornblum J, Arbeit RD, et al. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993;259:227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- 105.Oliveira DC, Tomasz A, de Lancastre H. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb Drug Resist. 2001;7:349–361. doi: 10.1089/10766290152773365. [DOI] [PubMed] [Google Scholar]
- 106.Musser J, Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992;30:2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Givney R, Vickery A, Holliday A, et al. Evolution of an endemic methicillin-resistant Staphylococcus aureus population in an Australian hospital from 1967 – 1996. J Clin Microbiol. 1998;36:552–556. doi: 10.1128/jcm.36.2.552-556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crisostomo MI, Westh H, Tomasz A, et al. The evolution of methicillin resistance in Staphylococcus: similarity of genetic backgrounds in historically early methicillin-susceptible and resistant and contemporary epidemic clones. Proc Natl Acad Sci USA. 2001;98:9865–9870. doi: 10.1073/pnas.161272898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farr BM. Prevention and control of methicillin-resistant Staphylococcus aureus infections. Curr Opin Infect Dis. 2004;17:317–322. doi: 10.1097/01.qco.0000136926.52673.cd. [DOI] [PubMed] [Google Scholar]
- 110.Holmes O. The contagiousness of puerperal fever. N Engl Q J Med Surg. 1842–1843;1:501–530. [Google Scholar]
- 111.Semmelweis I. The Etiology, the Concept and the Prophylaxis of Childbed Fever Pest, Hungary: CA Hartleben’s Verlag-Expedition, 1861.
- 112.Cooper BS, Stone SP, Kibbler CC, et al. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004;329:533–538. doi: 10.1136/bmj.329.7465.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boyce JM, Potter-Bynoe G, Chenevert C, et al. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18:622–627. [PubMed] [Google Scholar]
- 114.Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004;25:164–167. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 115.Devine J, Cooke RP, Wright EP. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance? J Hosp Infect. 2001;48:72–75. doi: 10.1053/jhin.2001.0955. [DOI] [PubMed] [Google Scholar]
- 116.Layton MC, Perez M, Heald P, et al. An outbreak of mupirocin-resistant Staphylococcus aureus on a dermatology ward associated with an environmental reservoir. Infect Control Hosp Epidemiol. 1993;14:369–375. doi: 10.1086/646764. [DOI] [PubMed] [Google Scholar]
- 117.Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastics. J Clin Microbiol. 2000;38:724–726. doi: 10.1128/jcm.38.2.724-726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dietz B, Raht A, Wendt C, et al. Survival of MRSA on sterile goods packaging. J Hosp Infect. 2001;49:255–261. doi: 10.1053/jhin.2001.1094. [DOI] [PubMed] [Google Scholar]
- 119.Borg MA. Bed occupancy and overcrowding as determinant factors in the incidence of MRSA infections within general ward settings. J Hosp Infect. 2003;54:316–318. doi: 10.1016/s0195-6701(03)00153-1. [DOI] [PubMed] [Google Scholar]
- 120.Haley RW, Cushion NB, Tenover FC, et al. Eradication of endemic methicillin-resistant Staphylococcus aureus infections from a neonatal intensive care unit. J Infect Dis. 1995;171:614–624. doi: 10.1093/infdis/171.3.614. [DOI] [PubMed] [Google Scholar]
- 121.Herr CE, Heckrodt TH, Hofmann FA, et al. Additional costs for preventing the spread of methicillin-resistant Staphylococcus aureus and a strategy for reducing these costs on a surgical ward. Infect Control Hosp Epidemiol. 2003;24:673–678. doi: 10.1086/502274. [DOI] [PubMed] [Google Scholar]
- 122.Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982;97:309–317. doi: 10.7326/0003-4819-97-3-309. [DOI] [PubMed] [Google Scholar]
- 123.Back NA, Linnemann CC, Jr, Staneck JL, et al. Control of methicillin-resistant Staphylococcus aureus in a neonatal intensive-care unit: use of intensive microbiologic surveillance and mupirocin. Infect Control Hosp Epidemiol. 1996;17:227–231. doi: 10.1086/647285. [DOI] [PubMed] [Google Scholar]
- 124.Salmenlinna S, Lyytikainen O, Kotilaninen P, et al. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Finland. Eur J Clin Microbiol Infect Dis. 2000;19:101–107. doi: 10.1007/s100960050438. [DOI] [PubMed] [Google Scholar]
- 125.Verhoef J, Beaujean D, Blok H, et al. A Dutch approach to methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1999;18:461–466. doi: 10.1007/s100960050324. [DOI] [PubMed] [Google Scholar]
- 126.Kotilainen P, Routamaa M, Peltonen R, et al. Elimination of epidemic methicillin-resistant Staphylococcus aureus from a university hospital and district institutions, Finland. Emerg Infect Dis. 2003;9:169–175. doi: 10.3201/eid0902.020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lucet JC, Chevret S, Durand-Zaleske I, et al. Prevalence and risk factors for carriage of methicillin-resistant Staphylococcus aureus at admission to the intensive care unit: results of a multicenter study. Arch Intern Med. 2003;163:181–188. doi: 10.1001/archinte.163.2.181. [DOI] [PubMed] [Google Scholar]
- 128.Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 129.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chambers HF. Community-associated MRSA—resistance and virulence converge. N Engl J Med. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 131.Frank AL, Marcinak JF, Mangat PD, et al. Increase in community-acquired methicillin-resistant Staphylococcus aureus in children. Clin Infect Dis. 1999;29:935–936. doi: 10.1086/520463. [DOI] [PubMed] [Google Scholar]
- 132.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 133.Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissues infections among medically underserved patients. Arch Surg. 2004;139:947–953. doi: 10.1001/archsurg.139.9.947. [DOI] [PubMed] [Google Scholar]
- 134.Frazee BW, Lynn J, Charlebois ED, et al. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45:311–320. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 135.Daum RS, Ito T, Hiramatsu K, et al. A novel methicillin-resistant cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186:1344–1347. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 136.Wielders CL, Vriens MR, Brisse S, et al. In-vivo transfer of mecA DNA to Staphylococcus aureus. Lancet. 2001;357:1674–1675. doi: 10.1016/s0140-6736(00)04832-7. [DOI] [PubMed] [Google Scholar]
- 137.Baba T, Takeuchi F, Kuroda M, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 138.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-acquired and health care-associated methicillin-resistant Staphyloccus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 139.McDougal LK, Steward CD, Killgore GE, et al. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 141.Robinson DA, Enright MC. Multilocus sequence typing and the evolution of methicillin-resistant Staphylococcus aureus. Clin Microb Infect. 2004;10:92–97. doi: 10.1111/j.1469-0691.2004.00768.x. [DOI] [PubMed] [Google Scholar]
- 142.Dufour P, Gillet Y, Bes M, et al. Community-acquired methicillin-resistant Staphylococcal aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35:819–824. doi: 10.1086/342576. [DOI] [PubMed] [Google Scholar]
- 143.Ala’Aldeen D. A non-multiresistant community methicillin-resistant Staphylococcus aureus exposes its genome. Lancet. 2002;359:1791–1792. doi: 10.1016/S0140-6736(02)08727-5. [DOI] [PubMed] [Google Scholar]
- 144.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 146.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 147.Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17:220–226. doi: 10.3122/jabfm.17.3.220. [DOI] [PubMed] [Google Scholar]
- 148.Baggett HC, Hennessy TW, Leman R, et al. An outbreak of community-onset methicillin-resistant Staphylococcus aureus skin infections in southwestern Alaska. Infect Control Hosp Epidemiol. 2003;24:397–402. doi: 10.1086/502221. [DOI] [PubMed] [Google Scholar]
- 149.Charlebois ED, Bangsberg DR, Moss NJ, et al. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco. Clin Infect Dis. 2002;34:425–433. doi: 10.1086/338069. [DOI] [PubMed] [Google Scholar]
- 150.Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA. 2001;286:1201–1205. doi: 10.1001/jama.286.10.1201. [DOI] [PubMed] [Google Scholar]
- 151.Charlebois ED, Bangsberg DR, Moss NJ, et al. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco. Clin Infect Dis. 2002;34:425–433. doi: 10.1086/338069. [DOI] [PubMed] [Google Scholar]
- 152.Fleisch F, Zbinden R, Vanoli C, et al. Epidemic spread of a single clone of methicillin-resistant Staphylococcus aureus among injection drug users in Zurich, Switzerland. Clin Infect Dis. 2001;32:581–586. doi: 10.1086/318716. [DOI] [PubMed] [Google Scholar]
- 153.Pan ES, Diep BA, Carleton HA, et al. Increasing prevalence of methicillin-resistant Staphylococcus aureus infection in California jails. Clin Infect Dis. 2003;37:1384–1388. doi: 10.1086/379019. [DOI] [PubMed] [Google Scholar]
- 154.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, ed. Hospital Epidemiology and Infection Control, 3rd ed Philadelphia: Lippincott Williams & Wilkins; 2004:1659–1702.
- 155.Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131–139. doi: 10.1086/345436. [DOI] [PubMed] [Google Scholar]
- 156.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 157.Lesens O, Hansmann Y, Brannigan E, et al. Healthcare-associated Staphylococcus aureus bacteremia and the risk for methicillin resistance: Is the Centers for Disease Control and Prevention definition for community-acquired bacteremia still appropriate? Infect Control Hosp Epidemiol. 2005;26:204–209. doi: 10.1086/502527. [DOI] [PubMed] [Google Scholar]
- 158.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123–127. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 159.Mandelbaum S, Forster RK, Gelender H, et al. Late onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985;92:964–972. doi: 10.1016/s0161-6420(85)33947-7. [DOI] [PubMed] [Google Scholar]
- 160.Greenfield DS, Suner IJ, Miller MP, et al. Endophthalmitis after filtering surgery with mitomycin C. Arch Ophthalmol. 1996;114:943–949. doi: 10.1001/archopht.1996.01100140151007. [DOI] [PubMed] [Google Scholar]
- 161.Kangas TA, Greenfield DS, Flynn HW, Jr, et al. Delayed-onset endophthalmitis associated with conjunctival filtering blebs. Ophthalmology. 1997;104:746–752. doi: 10.1016/s0161-6420(97)30238-3. [DOI] [PubMed] [Google Scholar]
- 162.Waheed S, Ritterband DC, Greenfield DS, et al. Bleb-related ocular infection in children after trabeculectomy with mitomycin C. Ophthalmology. 1997;104:2117–2120. doi: 10.1016/s0161-6420(97)30051-7. [DOI] [PubMed] [Google Scholar]
- 163.Akova YA, Bulut S, Dabil H, et al. Late bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmic Surg Lasers. 1999;30:146–151. [PubMed] [Google Scholar]
- 164.Lam RF, Hui M, Leung DY, et al. Extent and predictors of microbial hand contamination in a tertiary care ophthalmic outpatient practice. Invest Ophthalmol Vis Sci. 2005;46:3578–3583. doi: 10.1167/iovs.05-0216. [DOI] [PubMed] [Google Scholar]
- 165.Larson E, Leyden JJ, McGinley KJ, et al. Physiologic and microbiologic changes in skin related to frequent handwashing. Infect Control. 1986;7:59–63. doi: 10.1017/s019594170006389x. [DOI] [PubMed] [Google Scholar]
- 166.Doebbeling BN, Stanley GL, Sheetz CT, et al. Comparative efficacy of alternative hand-washing agents in reducing nosocomial infections in intensive care units. N Engl J Med. 1992;327:88–93. doi: 10.1056/NEJM199207093270205. [DOI] [PubMed] [Google Scholar]
- 167.Fraise AP. Guidelines for the control of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1998;42:287–289. doi: 10.1093/jac/42.3.287. [DOI] [PubMed] [Google Scholar]
- 168.Fleischer AB, Hoover DL, Khan JA, et al. Topical vancomycin formulation for methicillin-resistant Staphylococcus epidermidis blepharoconjunctivitis. Am J Ophthalmol. 1986;101:283–287. doi: 10.1016/0002-9394(86)90820-2. [DOI] [PubMed] [Google Scholar]
- 169.Khan JA, Hoover D, Ide CH. Methcillin-resistant Staphylococcus epidermidis blepharitis. Am J Ophthalmol. 1984;98:562–565. doi: 10.1016/0002-9394(84)90241-1. [DOI] [PubMed] [Google Scholar]
- 170.Goodman DF, Gottsch JD. Methicillin-resistant Staphylococcus epidermidis keratitis treated with vancomycin. Arch Ophthalmol. 1988;106:1570–1571. doi: 10.1001/archopht.1988.01060140738046. [DOI] [PubMed] [Google Scholar]
- 171.Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 1995;44(RR–12):1–13. [PubMed] [Google Scholar]
- 172.Mariana MD, Colby KA. Evaluation and initial management of patients with ocular and adnexal trauma. In: Albert DM, Jakobiec FA, Azar DT, et al, eds. Principles and Practice of Ophthalmology, 2nd ed Philadelphia: WB Saunders; 2000:5180–5200.
- 173.Gills JP. Filter and antibiotics in irrigating solution for cataract surgery. J Cataract Refract Surg. 1991;17:385. doi: 10.1016/s0886-3350(13)80842-5. [DOI] [PubMed] [Google Scholar]
- 174.West ES, Behrens A, McDonnell PJ, et al. The incidence of endophthalmitis after cataract surgery among the US Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112:1388–1394. doi: 10.1016/j.ophtha.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 175.Gordon YJ. Vancomycin prophylaxis and emerging resistance: Are ophthalmologists the villains? The heroes? Am J Ophthalmol. 2001;131:371–376. doi: 10.1016/s0002-9394(00)00955-7. [DOI] [PubMed] [Google Scholar]
- 176.Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery. An evidence-based update. Ophthalmology. 2002;109:13–26. doi: 10.1016/s0161-6420(01)00899-5. [DOI] [PubMed] [Google Scholar]
- 177.AAO-CDC Task Force on Vancomycin Prophylaxis in Ophthalmic Surgery. The prophylactic use of vancomycin for intraocular surgery. Quality of Care Publications, Number 515 San Francisco: American Academy of Ophthalmology; 1999.
- 178.Ferro J, De-Pablos M, Logrono M. Postoperative contamination after vancomycin and gentamicin during phacoemulsification. Arch Ophthalmol. 1997;115:165–170. doi: 10.1001/archopht.1997.01100150167003. [DOI] [PubMed] [Google Scholar]
- 179.Feys J, Salvanet-Bouccara A, Edmond JP, et al. Vancomycin prophylaxis and intraocular contamination during cataract surgery. J Cataract Refract Surg. 1997;23:894–897. doi: 10.1016/s0886-3350(97)80250-7. [DOI] [PubMed] [Google Scholar]
- 180.Kodjikian L, Renaud FN, Roques C, et al. In vitro influence of vancomycin on adhesion of a Staphylococcus epidermidis strain encoding intercellular adhesion locus ica to intraocular lenses. J Cataract Refract Surg. 2005;31:1050–1058. doi: 10.1016/j.jcrs.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 181.McCulley JP. Low acute endophthalmitis rate: possible explanations. J Cataract Refract Surg. 2005;31:1074–1075. doi: 10.1016/j.jcrs.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 182.Fry LL. Vancomycin dilution error. J Cataract Refract Surg. 2005;31:1674. doi: 10.1016/j.jcrs.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 183.Axel-Siegel R, Stiebel-Kalish H, Rosenblatt I, et al. Cystoid macular edema after cataract surgery with intraocular vancomycin. Ophthalmology. 1999;106:1660–1664. doi: 10.1016/S0161-6420(99)90339-1. [DOI] [PubMed] [Google Scholar]
- 184.Libre PE, Della-Latta P, Chin N. Intracameral antibiotic agents for endophthalmitis prophylaxis. A pharmacokinetic model. J Cataract Refract Surg. 2003;29:1791–1794. doi: 10.1016/s0886-3350(03)00134-2. [DOI] [PubMed] [Google Scholar]
- 185.Hwang DG. Fluoroquinolone resistance in ophthalmology and the potential role for newer ophthalmic fluoroquinolones. Surv Ophthalmol. 2004;49(suppl 2):S79–S83. doi: 10.1016/j.survophthal.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 186.Gaynor BD, Chidambaram JD, Cevallos V, et al. Topical ocular antibiotics induce bacterial resistance at extraocular sites. Br J Ophthalmol. 2005;89:1097–1099. doi: 10.1136/bjo.2005.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boyce JM, Pittet D. Guidelines for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23(12 suppl):S3–S40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 188.Olsen RJ, Lynch P, Coyle MB, et al. Examination gloves as barriers to hand contamination in clinical practice. JAMA. 1993;270:350–353. [PubMed] [Google Scholar]
- 189.Doebbeling BN, Pfaller MA, Houston AK, et al. Removal of nosocomial pathogens from the contaminated glove: implications for glove reuse and handwashing. Ann Intern Med. 1988;109:394–398. doi: 10.7326/0003-4819-109-5-394. [DOI] [PubMed] [Google Scholar]
- 190.Werheim HF, Vos MC, Ott A, et al. Mupirocin prophylaxis against nosocomial Staphylococcus aureus infections in nonsurgical patients: a randomized study. Ann Intern Med. 2004;140:419–425. doi: 10.7326/0003-4819-140-6-200403160-00007. [DOI] [PubMed] [Google Scholar]
- 191.Miller MA, Dascal A, Portnory J, et al. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect Control Hosp Epidemiol. 1996;17:811–813. doi: 10.1086/647242. [DOI] [PubMed] [Google Scholar]
- 192.Torvaldsen S, Roberts C, Riley TV. The continuting evolution of methicillin-resistant Staphylococcus aureus in western Australia. Infect Control Hosp Epidemiol. 1999;20:133–135. doi: 10.1086/501594. [DOI] [PubMed] [Google Scholar]
- 193.Vasquez JE, Walker ES, Franzus BW, et al. The epidemiology of mupirocin resistance among methicillin-resistant Staphylococcus aureus at a Veterans’ Affairs hospital. Infect Control Hosp Epidemiol. 2000;21:459–464. doi: 10.1086/501788. [DOI] [PubMed] [Google Scholar]
- 194.Fraunfelder FT, Bagby GC, Kelly DJ. Fatal aplastic anemia following topical administration of ophthalmic chloramphenicol. Am J Ophthalmol. 1982;93:356–360. doi: 10.1016/0002-9394(82)90540-2. [DOI] [PubMed] [Google Scholar]
- 195.Doona M, Walsh JB. Use of chloramphenicol as topical eye medication: Time to cry halt? BMJ. 1995;310:1217–1218. doi: 10.1136/bmj.310.6989.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marcinak JF, Frank AL. Treatment of community-acquired methicillin-resistant Staphylococcus aureus in children. Curr Opin Infect Dis. 2003;16:265–269. doi: 10.1097/00001432-200306000-00014. [DOI] [PubMed] [Google Scholar]
- 197.Panagea S, Perry JD, Gould FK. Should clindamycin be used as treatment of patients with infections caused by erythromycin-resistant staphylococci? J Antimicrob Chemother. 1999;44:581–582. doi: 10.1093/jac/44.4.581. [DOI] [PubMed] [Google Scholar]
- 198.Rao GG. Should clindamycin be used in treatment of patients with infections caused by erythromycin-resistant staphylococci? J Antimicrob Chemother. 2000;45:715. doi: 10.1093/jac/45.5.715. [DOI] [PubMed] [Google Scholar]
- 199.Drinkovic D, Fuller ER, Shore KP, et al. Clindamycin treatment of Staphylococcus aureus expressing inducible clindamycin resistance. J Antimicrob Chemother. 2001;48:315–316. doi: 10.1093/jac/48.2.315. [DOI] [PubMed] [Google Scholar]
- 200.Siberry GK, Tekle T, Carroll K, et al. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37:1257–1260. doi: 10.1086/377501. [DOI] [PubMed] [Google Scholar]
- 201.Forrest A, Nix DE, Ballow CH, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kowalski RP, Karenchak LM, Romanowski EG. Infectious disease: changing antibiotic susceptibility. Ophthalmol Clin North Am. 2003;16:1–9. doi: 10.1016/s0896-1549(02)00061-5. [DOI] [PubMed] [Google Scholar]
- 203.Ling ML, Tan PL. In vitro activity of moxifloxacin against local bacterial isolates. Ann Acad Med Singapore. 2001;30:607–610. [PubMed] [Google Scholar]
- 204.Abb J. In vitro activity of linezoid, quinupristin-dalfopristin, vancomycin, teicoplanin, moxifloxacin, and mupirocin against methicillin-resistant Staphylococcus aureus: comparative evaluation by the E test and a broth microdilution method. Diag Microbiol Infect Dis. 2002;43:319–321. doi: 10.1016/s0732-8893(02)00407-8. [DOI] [PubMed] [Google Scholar]
- 205.Tsuji H, Tsuru T, Okuzumi K. Detection of methicillin-resistant Staphylococcus aureus in donor eye preservation media by polymerase chain reaction. Jpn J Ophthalmol. 1998;42:352–356. doi: 10.1016/s0021-5155(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 206.Michel M, Gutmann L. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: therapeutic realities and possibilities. Lancet. 1997;349:1901–1906. doi: 10.1016/s0140-6736(96)11192-2. [DOI] [PubMed] [Google Scholar]
- 207.Pillai SK, Sakoulas G, Wennersten C, et al. Linezolid resistance in Staphylococcus aureus: characterization and stability of resistant phenotype. J Infect Dis. 2002;186:1603–1607. doi: 10.1086/345368. [DOI] [PubMed] [Google Scholar]
- 208.Hiramatsu K, Aritaka N, Hanaki H, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 209.Linden PK. Clinical implications of nosocomial gram-positive bacteremia and superimposed antimicrobial resistance. Am J Med. 1998;104(5A):24S–33S. doi: 10.1016/s0002-9343(98)00152-1. [DOI] [PubMed] [Google Scholar]
- 210.Schindler CA, Schuhart VT. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc Natl Acad Sci USA. 1965;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sloan GL, Robinson JM, Kloos WE. Identification of ‘Staphylococcus staphylolyticus’ NRRL B-2628 as biovar of Staphylococcus simulans. Int J Bacteriol. 1982;32:170–174. [Google Scholar]
- 212.Dajcs JJ, Hume EB, Moreau JM, et al. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Invest Ophthalmol Vis Sci. 2000;41:1432–1437. [PubMed] [Google Scholar]
- 213.Dajcs JJ, Thibodeaux BA, Hume EB, et al. Lysostaphin is effective in treating methicillin-resistant Staphylococcus aureus endophthalmitis in the rabbit. Curr Eye Res. 2001;22:451–457. doi: 10.1076/ceyr.22.6.451.5486. [DOI] [PubMed] [Google Scholar]
- 214.Kessler E, Safrin M, Olson JC, et al. Secreted LasA protease of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem. 1993;268:7503–7508. [PubMed] [Google Scholar]
- 215.Kessler E. β-lytic endopeptidases. Methods Enzymol. 1995;248:740–756. doi: 10.1016/0076-6879(95)48050-1. [DOI] [PubMed] [Google Scholar]
- 216.Kessler E, Ohman DE. Staphylolysin. In: Barrett AJ, Rawlings ND, Woessner JF, eds. Handbook of Proteolytic Enzymes London: Academic Press; 1998:1476–1478.
- 217.Barequet IS, Ben Simon GY, Safrin M, et al. Pseudomonas aeruginosa LasA protease in treatment of experimental staphylococcal keratitis. Antimicrob Agents Chemother. 2004;48:1681–1687. doi: 10.1128/AAC.48.5.1681-1687.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Callegan MC, Hill JM, Insler MS, et al. Methicillin-resistant Staphylococcus aureus keratitis in the rabbit: therapy with ciprofloxacin, vancomycin, and cefazolin. Curr Eye Res. 1992;11:1111–1119. doi: 10.3109/02713689209015083. [DOI] [PubMed] [Google Scholar]
- 219.Fattom AI, Sarwar J, Ortiz A, et al. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lee JC, Park JS, Shepherd SE, et al. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65:4146–4151. doi: 10.1128/iai.65.10.4146-4151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]