Abstract
Purpose
The purpose of this study was to evaluate the concept of targeting mediators of the scarring process at multiple points across the course of bleb failure, in order to prolong bleb survival.
Methods
There were three linked parts to the experiment. In the first part, a cannula glaucoma filtration surgery (GFS) was performed on 32 New Zealand White (NZW) rabbits, and bleb survival was assessed for six different regimens plus controls by grading bleb height and width. For the second part of the study, the same GFS surgery was performed on an additional 10 NZW rabbits. Two additional filtering blebs were treated with balanced saline solution (BSS), two received mitomycin-C (MMC) (0.4 mg/mL), and for the remaining six, a sequential regimen was given consisting of 200 mmol/L mannose-6-phosphate (M-6-P) solution at the time of surgery, followed by subconjunctival injections of antibody to connective tissue growth factor at days 2 and 4, and Ilomastat, a broad-spectrum matrix metalloproteinase inhibitor, at days 7, 12, and 20 postoperatively. Bleb survival was again assessed. In the final part of the experiment, blebs treated with either BSS, MMC, or the above sequential multitreatment regimen were examined histologically at 14 days postoperatively in three additional NZW rabbits.
Results
All six individual therapies selected resulted in some improvement of bleb survival compared to BSS control. Blebs treated with the new sequential, multitreatment protocol survived an average of 29 days (regression slope, P < .0001 compared to control), those receiving BSS an average of 17 days, and those treated with MMC (0.4 mg/mL) an average of 36 days. The sequential, multitreatment regimen was significantly superior to any of the six monotherapies for time to zero analysis (flattening) of the bleb (P < .002). Histologic examination of the bleb tissues showed a markedly less epithelial thinning, subepithelial collagen thinning, and goblet cell loss in the multitreatment group, when compared with the MMC blebs.
Conclusions
In a rabbit model of GFS, a sequential, targeted, multitreatment approach prolonged bleb survival compared to BSS controls and decreased bleb tissue morphological changes when compared to those treated with MMC. It is not known whether these findings can be reproduced in humans, and further work is needed to determine an optimum regimen and timing of therapeutic delivery.
INTRODUCTION
Worldwide, it is estimated that 65 million people are affected by glaucoma, which remains a leading cause of blindness.1–5 Primary open-angle glaucoma, the most common cause and presentation of the disease, is estimated to have an incidence of 2.4 million new cases per year.6
There are many risk factors for glaucoma, including intraocular pressure (IOP), older age, black race,7–12 family history, genetic predisposition,13–17 and thin central corneal thickness.18–20 Considering that at present the only treatable risk factor is IOP, the goal of glaucoma therapy is to lower it to safe levels for the optic nerve.21,22 This can be achieved with medical therapy (eye drops or systemic medications), laser surgery, or incisional surgery. Of these options, glaucoma filtering surgery (GFS) has been demonstrated to produce the largest and most sustained decrease in IOP.23–26
There is growing recognition that many patients with glaucoma require low-normal IOPs to prevent progression of visual field loss.23,27–33
GFS is generally performed when medical therapy fails to adequately control IOP. Excessive subconjunctival scarring following GFS is responsible for failure of the surgery in the majority of cases.34–42 There is a huge interest in developing a new drug or treatment modality that would be able to minimize fibrosis and provide better outcome with GFS.
Antimetabolites, predominantly 5-fluorouracil (5-FU) and mitomycin-C (MMC), are commonly used to reduce the formation of scar tissue at the site of GFS.36,42–47 These antimetabolites have been shown to be beneficial in preventing scarring and enhancing the long-term success of GFS, but they are relatively nonspecific and may be associated with an increased incidence of severe and potentially blinding complications.48–61 Some of the factors that mediate the bleb-scarring process have recently been identified, including transforming growth factor β2 (TGF-β2),62,63 the predominant form in the eye, and its downstream mediator connective tissue growth factor (CTGF).64
It is possible to neutralize TGF-β using some agents, including TGF-β antibody CAT-152 (Cambridge Antibody Technology, Cambridge, United Kingdom), specific to the active form of human TGF-β2. Another way to neutralize TGF-β is to block gene expression of a growth factor or its receptor. This can be achieved using antisense oligonucleotide, a sequence of DNA complementary to the gene sequence of the growth factor.62,65 Antisense oligonucleotide binds to TGF-β mRNA and prevents protein synthesis by inhibiting transcription. A clinical trial using a human monoclonal antibody to TGF-β2 reported initial promising results.66
CTGF is a secreted peptide that has been implicated in multiple cellular events, including angiogenesis, skeletogenesis, and wound healing.67 The actions of CTGF have been clearly distinguished from those of TGF-β by showing that CTGF alone does not induce anchorage-independent growth of fibroblasts.68 Gene regulation of CTGF is also a target for antifibrotic therapy.69
A rat model of GFS was recently used to investigate postoperative changes in gene expression in bleb tissues and confirmed highly significant up-regulation of certain growth factors (TGF-β1, 2, 3 and CTGF), various structural proteins, and matrix metalloproteinase enzymes (MMPs) 2, 3, and 9.37 Highest levels of MMPs were expressed during the later part of the wound-healing cycle. Highest levels of TGF-β2 and CTGF were noted at day 5, which is consistent with previous enzyme-linked immunosorbent assay work in the rabbit model.64
MMPs are a family of proteolytic enzymes that are essential in the wound-healing process. In addition to degradation of the extracellular matrix,70,71 these enzymes are also believed to be involved in wound contraction.72–74 Wound healing following GFS is a complex process involving multiple pathways and mediators.37,40,42,75–80 Until now, only broad-spectrum antifibrotic agents or therapies targeting single pathways have been investigated. The purpose of this study was to examine the concept of treating multiple factors at several different time points following GFS, in order to maximally prolong bleb survival while at the same time minimizing the long-term tissue-damaging side effects of the currently used antimetabolites.
METHODS
All animal experiments were conducted in accordance with the ARVO statement governing the use of animals in ophthalmic research and were approved by the University of Florida’s Institutional Animal Care and Use Committee. New Zealand White, male rabbits weighing approximately 2 to 4 lb were used in the study. Surgery was performed on the left eye only of each animal, and the same surgeon performed all surgeries.
ANESTHESIA
All animals were anesthetized using a combination of ketamine (Ketaject; Phoenix Pharmaceuticals, St Joseph, Missouri) (50 mg/kg) and xylazine (Xylaject; Phoenix Pharmaceuticals) (10 mg/kg) administered by intramuscular injection. Additional topical anesthetic in the form of 0.1% proparacaine eye drops (Bausch & Lomb, Tampa, Florida) was also administered.
GLAUCOMA FILTRATION SURGERY
The technique used has been described previously.64 Briefly, the eyelids were retracted using an eyelid speculum. A partial-thickness, corneal traction-suture was placed in the superior cornea and used to rotate the eye inferiorly. A limbus-based conjunctival flap was fashioned in the superior lateral quadrant of the eye, approximately 8 mm from the limbus. The conjunctiva and Tenon’s capsule were undermined by blunt dissection until the limbus was reached. A clear corneal paracentesis tract was made between the 5- and 7-o’clock positions using a Beaver blade (Becton Dickinson & Co, Franklin Lakes, New Jersey) and a viscoelastic material (Healon, 10 mg/mL; Pharmacia & Upjohn) was injected to maintain the anterior chamber.
Next, a scleral tract was fashioned by tunneling a beveled 22-gauge, IV cannula (Insyte; Becton Dickinson Vascular Access, Sandy, Utah) through the sclera, beginning behind the limbus and continuing until the cannula was visible in the anterior chamber. The cannula needle was then withdrawn and the cannula advanced beyond the pupillary margin to prevent iris blockage of the tube. The cannula was trimmed at its scleral end so that it protruded approximately 1 mm from the insertion point and was secured to the sclera using an encircling 10-0 nylon suture (Ethicon Inc, Somerville, New Jersey).
The Tenon’s capsule was closed with a continuous locking suture of 8-0 absorbable suture material (Vicryl; Ethicon Inc, Somerville, New Jersey) attached to a BV needle, and the conjunctiva was closed with a continuous, nonlocked suture of the same material.
A single application of combined neomycin and dexamethasone ointment was instilled at the end of surgery.
EVALUATION OF POTENTIAL TREATMENTS
In part 1 of the experiment, a number of agents that target previously identified mediators of the scarring process were individually evaluated to determine their potential efficacy. Compounds active against CTGF, TGF-β2, and MMPs were selected.
With the exception of MMC, which was given as a single, 5-minute, intraoperative treatment,81,82 the other compounds were injected aseptically into the bleb area at time of surgery and again 5 days postoperatively. Five days was selected as the time point for the second injection based upon the known expression patterns of TGF-β2 and CTGF in rabbit bleb tissues,60 and in the case of the MMP inhibitor, to provide uniformity across treatment groups. This treatment regimen may not be the ideal protocol for each of these individual agents but was selected in order to provide comparable efficacy data.
The antiscarring compounds were prepared as follows:
Control. Physiological saline solution (Santen Pharmaceuticals, Napa, California).
Mitomycin-C. A solution of MMC, 0.4 mg/mL,81, 82 (Novartis, East Hanover, New Jersey) was prepared and applied using a section of sponge (Microsponge; Alcon, Fort Worth, Texas) soaked in the solution.
CTGF and TGF-β2 Antisense. Twenty-mer CTGF and TGF-β2 antisense oligodeoxynucleotides83 with homology to the rabbit sequences were synthesized as previously described.84 Briefly, human, mouse, and rat mRNA genes were analyzed for unique, nonrepetitive, 20-mer nucleotide sequences with high GC contents that would minimize self-hybridization and provide stability of the oligodeoxynucleotide mRNA complex. After testing their ability to reduce mRNA levels using a cell culture screening assay, a CTGF oligodeoxynucleotide sequence and a TGF-β2 oligodeoxynucleotide sequence were selected. The oligonucleotides were suspended in phosphate-buffered saline at a final concentration of 10 μM and filter sterilized through a Millex GP syringe-driven filter unit (Millipore Corp, Bedford, Massachusetts) before injection.
CTGF and TGF-β2 Antibody. A specific goat anti-human CTGF polyclonal antibody, which recognizes predominantly epitopes on the N-terminal half of the protein, was used at a concentration of 5 μg per 100 μL injection.84 A specific goat anti-porcine TGF-β2 polyclonal antibody (R&D Systems, Minneapolis, Minnesota) was also used at a concentration of 5 μg per 100 μL injection. Both antibodies were diluted in phosphate-buffered saline containing 0.1% bovine serum albumin and filter sterilized through a Millex GP syringe driven filter unit before injection.
d-Mannose-6-Phosphate (D-6-P). This product (Sigma, St Louis, Missouri) was reconstituted in NaCl to a concentration of 200 mmol/L and sterilized through a Millex GP syringe-driven filter unit.
Ilomastat (GM6001). N-[(2R0)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-l-tryptophan methylamide (Chemicon, Temecula, California) was reconstituted from 1 mg/mL (2.5 nmol/L in dimethyl sulfoxide) to 100 μmol/L in NaCl and sterilized through a Millex GP syringe-driven filter unit.72,85–88
To facilitate the postoperative, subconjunctival injection on day 5, animals were anesthetized using a combination of ketamine (50 mg/kg) and xylazine (10 mg/kg) administered by intramuscular injection. Topical anesthestic in the form of 0.1% proparacaine eye drops was also administered. Eyelids were retracted with a speculum, the conjunctivae were tented using a pair of nontoothed Bishop-Harmon forceps, and a 0.1-mL injection was given using a 30-gauge needle attached to a 1-mL syringe.
EVALUATION OF SEQUENTIAL MULTIPLE TREATMENT
In part 2 of the study, the left eyes of six rabbits were injected with a sequential protocol of compounds selected on the basis of the results of part 1 of the experiment. This multitreatment protocol consisted of a single dose of 200 mmol/L M-6-P, which was applied to the bleb tissues during surgery, before the anterior chamber was entered, 0.1 mL of CTGF antibody, which was injected subconjunctivally on days 2 and 4 postoperatively, and 0.1 mL of Ilomastat injected on days 7, 12, and 20 postoperatively. As a negative control, two additional randomly selected rabbits were treated at the same five postoperative time points following surgery using a similar volume of balanced saline solution (BSS). As a positive control, two additional randomly selected rabbits were treated at the time of surgery with a solution of 0.4 mg/mL MMC using a section of soaked Microsponge, applied to the exposed bleb area for 5 minutes.
SURVIVAL ANALYSIS
All glaucoma filtering blebs were evaluated postoperatively each day until failure. This was accomplished by measuring the length and width of the bleb using a caliper and thus calculating its area as previously described.64 All bleb areas were recorded as percentages of their maximum size, to eliminate variation resulting from individual differences in initial bleb area following surgery. Additionally, the anterior chamber of each animal was evaluated daily at the slit lamp for evidence of inflammation, hemorrhage, or shallowing; the eye was stained with fluorescein to look for evidence of corneal epithelial toxicity or bleb leak; and conscious IOPs were measured (average of three consecutive readings) using a handheld tonometer (Tonopen; Mentor, Santa Barbara, California).
STATISTICAL ANALYSIS
To compare the rate of bleb failure of the MMC and sequential multitreatment groups with the control group, a one-way analysis of variance of the time to bleb failure was conducted. The groups were significantly different (F = 250.87, df = 8,31, P < .0001). The mean time to bleb failure of the multitreatment group was 29.2 days. This mean was compared to the mean time to bleb failure for each of the other groups. The bleb survival time in the multitreatment group was significantly greater than the single-therapy groups or BSS control, but significantly less than the MMC positive control (Table 1).
TABLE 1.
BLEB SURVIVAL TIME OF THE SEQUENTIAL MULTITREATMENT GROUP COMPARED TO THE SIX INDIVIDUAL SINGLE-THERAPY GROUPS FROM PART 1 OF THE STUDY, AND TO CONTROL GROUPS
| GROUP | MEAN DIFFERENCE (GROUP MEAN − MULTITREATMENT GROUP MEAN) | SE | TVALUE | PVALUE |
|---|---|---|---|---|
| Negative control (BSS) | −12.2 | 0.54 | −22.8 | <.0001 |
| CTGF antisense | −4.70 | 0.59 | −7.9 | <.0001 |
| CTGF antibody | −3.45 | 0.59 | −5.8 | <.0001 |
| TGF-β2 antisense | −8.45 | 0.59 | −14.2 | <.0001 |
| TGF-β2 antibody | −8.95 | 0.59 | −15.1 | <.0001 |
| M-6-P | −9.95 | 0.59 | −16.8 | <.0001 |
| Ilomastat | −2.20 | 0.65 | −3.4 | .0019 |
| Mitomycin-C | 7.30 | 0.54 | 13.6 | <.0001 |
BSS = balanced saline solution; M-6-P = mannose-6-phosphate; SE = standard error of the mean.
HISTOLOGY
In the part 3 of the experiment, similar GFS was performed on three additional rabbits to generate tissues for histological evaluation. One rabbit received the sequential multitreatment protocol, one received injections of BSS only (negative control), and one received an intraoperative application of MMC (positive control).
One eye from each of the three treatment groups was harvested at day 14 following surgery. In addition, an eye that had undergone no surgery was also examined histologically. The eyes were perfused in situ with 4% formaldehyde for 3 minutes, before being dissected en bloc, fixed in 4% formaldehyde overnight, and transferred to 70% ethanol. The eyes were then processed for paraffin embedding and 4μ- to 6μ-thick sections made. Sections were stained with standard hematoxylin-eosin for cellularity (including fibroblasts), Gomori’s trichrome for collagen density and architecture, periodic acid–Schiff (PAS) for goblet cell identification, and Verhoeff stain for elastic fiber presence. All evaluations were performed by a qualified, ocular histopathologist, masked to treatment group and with the normal, nonoperated eye used as a control standard.
PHOTOGRAPHY
Mounted, stained sections were photographed using bright field illumination at ×40 magnification. Photographs were taken at a constant exposure (430 ms) using a Peltier-cooled Olympus digital camera.
RESULTS
No bleb leaks or corneal epithelial staining was noted during the postoperative period. One of the animals treated with Ilomastat in part 1 of the study and one of the animals receiving multitreatment in part 2 of the study exhibited tube advancement into the anterior chamber (at postoperative days 16 and 10, respectively). Data for these two animals were censored beyond these time points.
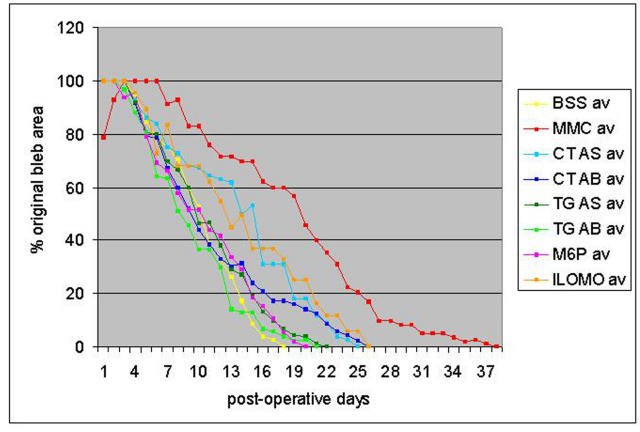
Figure 1 shows the results of treatment using individual agents. In keeping with the author’s previous experience with this rabbit model, the BSS-treated (negative control) blebs survived an average of 17 days and the MMC-treated (positive control) blebs an average of 36 days. The two rabbits that received BSS and the two that received MMC in part 2 of the experiment had a similar time to zero bleb survival as the four in part 1 of the study, and their results have been combined (n = 6 for each). All of the individual experimental therapies, which were given at the time of surgery and at 5 days postoperatively, enhanced bleb survival, with the TGF-β2 antibody and antisense and the M-6-P improving bleb survival a little to approximately 22 days and the CTGF antibody and antisense and Ilomastat improving bleb survival to approximately 26 days.
FIGURE 1.
Bleb survival for individual agents. Bleb area and time to failure were measured in a rabbit model of glaucoma filtering surgery using six different agents in addition to a negative control (balanced saline solution [BSS]) and a positive control (mitomycin-C [MMC], 0.4 mg/mL). The agents studied were d-mannose-6-phosphate (M6P), TGF-β2 antisense (TG AS), TGF-β2 antibody (TG AB), CTGF antisense (CT AS), CTGF antibody (CT AB), and Ilomastat (ILOMO), a broad-spectrum matrix metalloproteinase inhibitor.
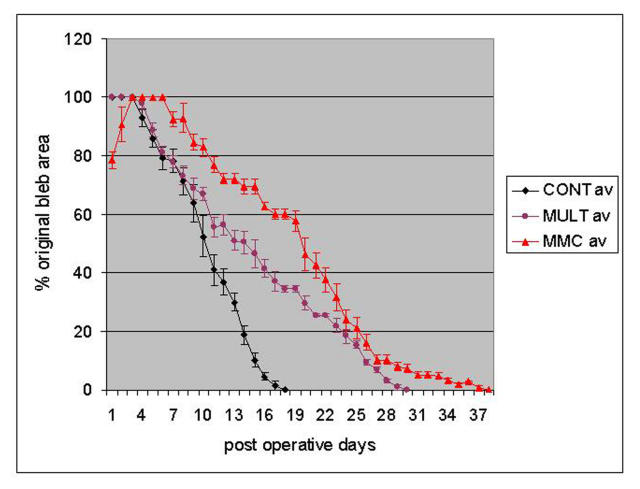
Figure 2 shows the results of treatment using the sequential multitreatment approach. Multiple-treated blebs survived an average of 29 days, those treated with BSS an average of 17 days, and those treated with MMC an average of 36 days. Table 2 shows the results of the (SAS) statistical analysis, indicating that the slopes of the curves for both MMC and multitreatment groups in Figure 2 are significantly different from the control group (P < .0001). The clinical appearance of these blebs is shown in Figure 3.
FIGURE 2.
Bleb survival for sequential multitreatment (MULT) regimen (d-mannose-6-phosphate at surgery, CTGF antibody injections on days 2 and 4, and Ilomastat on days 7, 12, and 20). Blebs receiving MULT survived an average of 29 days. Those treated with balanced saline solution, the negative control (CONT), survived an average of 17 days, and those treated with mitomycin-C (MMC) survived an average of 36 days.
TABLE 2.
RESULTS OF REGRESSION ANALYSIS OF THE SLOPES OF THE CURVES COMPARING BLEB SURVIVAL FOR THE BALANCED SALINE SOLUTION (BSS) CONTROL, MITOMYCIN-C (MMC), AND SEQUENTIAL MULTITREATMENT (MULTI-RX) GROUPS
| GROUP | INTERCEPT | SLOPE | LOWER CI | UPPER CI | PVALUE FOR DIFFERENT FROM CONTROL |
|---|---|---|---|---|---|
| BSS control | 118.7 | −6.86 | −7.22 | −6.5 | |
| MMC | 110.3 | −3.25 | −3.35 | −3.13 | <.0001 |
| Multi-Rx | 104.3 | −3.67 | −3.84 | −3.5 | <.0001 |
CI = confidence interval.
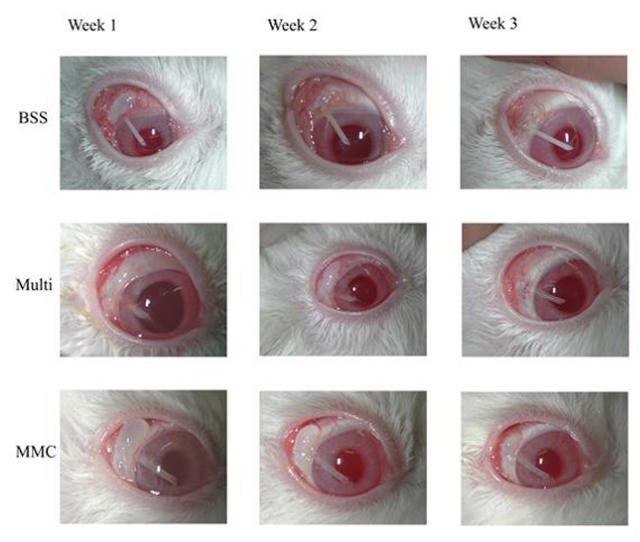
FIGURE 3.
Progression of clinical bleb failure. Representative photographic examples of clinical blebs for balanced saline solution (BSS), the negative control; mitomycin-C (MMC) (0.4 mg/mL), the positive control; and the sequential multitreatment group are shown at 1, 2, and 3 weeks postoperatively.
Comparison of the bleb survival time (time to zero analysis) between the sequential multitreatment group and each of the six individual therapies in part 1 of the study showed a significantly longer bleb survival for the multitreatment group (P < .002) (Table 1).
HISTOLOGY
Nonoperated (Normal Rabbit Conjunctiva)
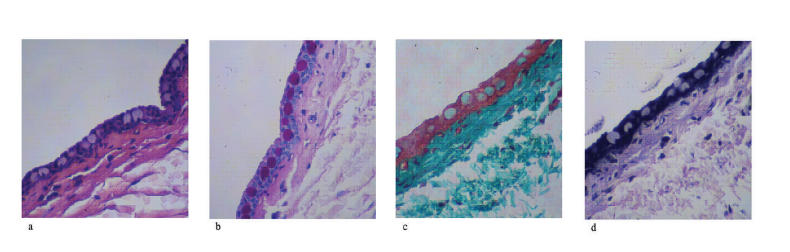
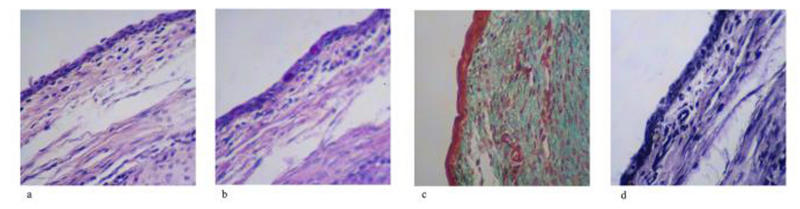
Hematoxylin-eosin staining sections from the nonoperated eye showed a normal, bulbar conjunctiva consisting of columnar epithelium, which was approximately three to five cell layers thick (Figure 4A). PAS staining indicated a relatively high density of epithelial goblet cells, which decreased in density toward the limbus (Figure 4B). Trichrome staining revealed loosely arranged, subepithelial connective tissue consisting of small collagen fibrils, which was more compacted immediately beneath the epithelium (Figure 4C). Verhoeff staining showed the presence of occasional, scattered, fine, elastic fibers in the subepithelial connective tissue (Figure 4D).
FIGURE 4.
Histology of nonoperated (normal) bleb site tissues. Four stains are shown for nonoperated rabbit conjunctival and Tenon’s capsule tissues. A, Hematoxylin-eosin stain for cellularity including fibroblast. B, Periodic acid–Schiff stain for goblet cell identification. C, Gamoris trichrome for collagen density and architecture. D, Verhoeff stain for elastic fiber presence.
BSS-Treated (Negative Control) Eye
Hematoxylin-eosin staining of sections from the negative control showed a thicker, bulbar conjunctiva consisting of a more stratified epithelium. The subepithelial connective tissue contained numerous fibroblasts as well as scattered inflammatory cells, including lymphocytes, macrophages, and occasional neutrophils (Figure 5A). PAS staining indicated the presence of epithelial goblet cells, although fewer than in the unoperated eye (Figure 5B). Trichrome staining revealed significantly more densely packed subepithelial collagen, with less distinction between the immediate subepithelial layer and the rest of the underlying connective tissue (Figure 5C). Verhoeff staining showed an increased density of elastic fibers throughout the connective tissue (which were also thicker), relative to the nonoperated eye (Figure 5D).
FIGURE 5.
Histology of balanced saline solution–treated (negative control) bleb site rabbit conjunctival and Tenon’s capsule tissues. A, Hematoxylin-eosin stain for cellularity, including fibroblast. B, Periodic acid–Schiff stain for goblet cell identification. C, Gamoris trichrome for collagen density and architecture. D, Verhoeff stain for elastic fiber presence.
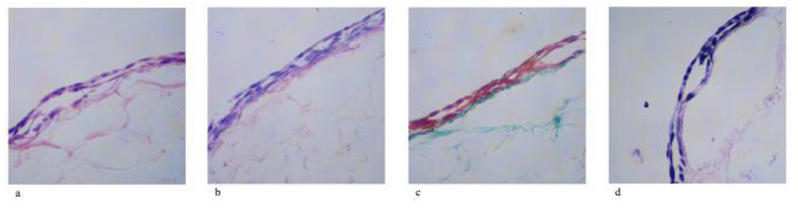
MMC-Treated (Positive Control) Eye
Hematoxylin-eosin staining of sections from the positive control eye showed a bulbar conjunctiva consisting of stratified epithelium, which was sharply demarcated from the adjacent tissues and markedly reduced in thickness, consisting of only a single layer over the major portion of the bleb area. Occasional inflammatory cells (lymphocytes and plasma cells), as well as rare fibroblasts, were noted in the subepithelial connective tissue (Figure 6A). PAS staining indicated an absence of epithelial goblet cells over the bleb area (Figure 6B). Trichrome staining revealed a significant reduction in the density of subepithelial collagen, with no distinction between the immediate subepithelial layer and the rest of the underlying connective tissue but an increase in density at the bleb margins (Figure 6C). Verhoeff staining showed an increased density of elastic fibers immediately beneath the conjunctival epithelium (Figure 6D).
FIGURE 6.
Histology of mitomycin-C–treated (positive control) bleb site rabbit conjunctival and Tenon’s capsule tissues. A, Hematoxylin-eosin stain for cellularity, including fibroblast. B, Periodic acid–Schiff stain for goblet cell identification. C, Gamoris trichrome for collagen density and architecture. D, Verhoeff stain for elastic fiber presence.
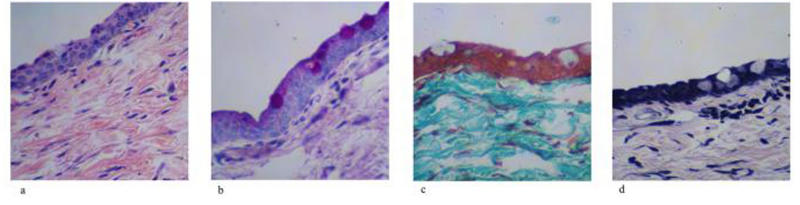
Sequential, Multiple Therapy Eye
Hematoxylin-eosin staining of sections from the multitreated eye showed a bulbar conjunctiva consisting of stratified to columnar epithelium, which was similar in structure and thickness to that of the nonoperated eye. Numerous fibroblasts, as well as occasional inflammatory cells (predominantly lymphocytes and macrophages), were noted in the subepithelial connective tissue (Figure 7A). PAS staining indicated an increased density of goblet cells relative to the positive control (MMC) (Figure 7B). Trichrome staining revealed an even, moderate density of subepithelial collagen, with no distinction between the immediate subepithelial layer and the rest of the underlying connective tissue (Figure 7B). Verhoeff staining showed occasional elastic fibers scattered throughout the connective tissue with a density similar to the nonoperated eye (Figure 7D).
FIGURE 7.
Histology of sequential multitreatment bleb site rabbit conjunctival and Tenon’s capsule tissues. A, Hematoxylin-eosin stain for cellularity, including fibroblast. B, Periodic acid–Schiff stain for goblet cell identification. C, Gamoris trichrome for collagen density and architecture. D, Verhoeff stain for elastic fiber presence
DISCUSSION
It is hypothesized that ocular wound healing, similar to healing in other tissues, occurs in several overlapping phases.89–92 These include hemostasis, inflammation, fibroblast migration, matrix production, and, finally, remodeling and contracture.
After hemostasis, an inflammatory phase occurs, characterized by the influx of neutrophils and monocytes, followed by lymphocytes and macrophages and the release of inflammatory mediators and growth factors.93–97 Angiogenesis and fibroblast migration then occur,93–95 followed by extracellular matrix deposition, fibroblast-mediated wound contracture, and tissue remodeling.34,37,38,72,76,89,97–104
Scarring can be reduced and the long-term success of GFS enhanced by treatment with 5-FU44,105–110 and MMC.75,111–115 The action of these compounds, however, is relatively nonspecific, and their use may be associated with an increased incidence of adverse effects, including hypotony-maculopathy, bleb leaks, bleb infections, and endophthalmitis.48–61 Primarily because of the long-term side effects of the currently used antimetabolite agents, there has been interest in identifying other approaches to preventing the formation of scar tissue following GFS.
In the initial part of this study, agents were selected for pilot investigation that could potentially target mediators of the various phases noted above. Antibody to TGF-β2, antisense to TGF-β2, antibody to CTGF, antisense to CTGF, M-6-P, and a broad-spectrum MMP inhibitor (Ilomastat) were individually tested. All six compounds prolonged bleb survival in the rabbit model compared to BSS control, although none were as effective as MMC (0.4 mg/mL). Overall, antibody and antisense to CTGF and the MMP inhibitor Ilomastat appeared to be slightly more effective than the other three agents and resulted in a similar length of bleb survival of 25 to 26 days.
Many fields in medicine, most notably the use of chemotherapy in cancer, have found that therapies targeting more than one area are required to decrease toxicity and maximize efficacy of therapy.116–124 In ocular wound healing, topical corticosteroids125–128 have been used to decrease early postoperative inflammation for many years, and either single antimetabolite agents129–140 or, more recently, antibody to TGF-β262,63,66,141 has been used to further modify the wound-healing response. A rat model of GFS was recently developed, and using microarray analysis, changes in the expression of numerous genes postoperatively were demonstrated. Some, such as the growth factors TGF-β2 and CTGF, reached maximal levels at day 5 following surgery and then fell, whereas others, such as the MMPs, were stable early on and then rose and remained high in the later stages of bleb scarring.37
Following surgery (or wounding), it is theorized that TGF-β is released in two phases, initially predominantly from degranulating platelets and later from attracted activated inflammatory cells, mainly monocytes and macrophages.142 TGF-β is released from platelets in its latent form, and activation of latent TGF-β occurs by cleavage of part of the molecule.143 The latent TGF-β requires binding to the large M-6-P/insulin-like growth factor-II (IGF-II) receptor for this activation to occur.144–147 TGF-β activation in cultured endothelial and smooth muscle cells can be disrupted by the addition of M-6-P or by antibodies directed against the M-6-P/IGF-II receptor in a dose-dependent manner.147
The production of TGF-β by inflammatory cells is thought to contribute to the postoperative peak noted around days 5 to 7 following GFS in both rabbit and rat models.37,64 Neutralizing antibodies have the ability to bind to and inhibit the action of target antigens. Cambridge Antibody Technology (CAT, Cambridge, United Kingdom) has produced a human monoclonal antibody that is specific to human TGF-β2 (CAT-152).148–151 After modified glaucoma surgery in 48 rabbits, seven postoperative subconjunctival injections of CAT-152 (1 mg/mL) significantly improved surgical outcome and reduced subconjunctival scarring compared with 5-FU (50 mg/mL) or no treatment. Median bleb survival was 23.5 days in the CAT-152 group, 20 days with 5-FU, and 16 days for the control treatment group.141 A phase I/II two-center study of this antibody in glaucoma filtration surgery has shown no significant side effects or inflammatory reaction and possibly some benefit compared with placebo in a masked randomized clinical study.152
A human phase I/II clinical trial examining CAT-152 in primary glaucoma filtration surgery showed improved efficacy compared to no antifibrosis treatment,66 but a recent report of the 1-year result of the first European phase II/III study (CAT-152-0102) with 344 patients did not support this.153
CTGF has been shown to be a downstream mediator of TGF-β.69,84,154–159 CTGF acts as a mitogen in fibroblast cell cultures and up-regulates components of the extracellular matrix, such as collagen, integrin, and fibronectin.75,99,111,160–162 In a rabbit model of GFS, injected exogenous CTGF was shown to increase the rate of bleb failure.64 Additionally, in a rat model, CTGF showed the highest percentage of up-regulation following GFS.37 The addition of CTGF antisense oligonucleotides (ASOs) or CTGF-neutralizing antibody blocked more than 85% of the increased collagen synthesis induced by TGF-β1 in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. However, neither ASOs nor neutralizing antibody totally suppressed the effect of TGF-β.163
Reorganization of fibrillar collagen and other matrix proteins, together with contraction, occurs in the later stages of wound healing.102,164 This function is mediated by a family of structurally related proteolytic enzymes, the MMPs.165–170 Their function is to degrade extracellular matrix, and more than 20 different MMPs have been identified. Most MMPs are not expressed in normal tissues but are transcribed in response to stimuli such as inflammatory cytokines and growth factors by multiple cell types, including macrophages, fibroblasts, and neutrophils.171–175 Using gene array analysis in the rat model, a more than fivefold increase in the expression of MMPs 2, 3, and 9 following GFS was observed, and these were maximally expressed in the later phase of wound healing, between days 5 and 12 postoperatively.37 Scott and coworkers73 showed that collagen contraction can be inhibited using MMP inhibitors.
Ilomastat is a broad-spectrum MMP inhibitor that has shown activity in a number of biologic systems, including animal wound-healing models.72,176 Wong and coworkers88 have shown an improved duration of bleb survival in the rabbit model of GFS using Ilomastat (GM6001) alone, but in these experiments 10 subconjunctival injections of Ilomastat were given. As noted by the investigators, for many patients, this intensity of postoperative care may be difficult to achieve in clinical practice.
In a subsequent experiment using Ilomastat, the same investigators showed that Ilomastat successfully prolongs bleb survival, giving 15 injections during the study period. Blebs started to fail, on average, 12 days after the last subconjunctival injection, with total failure occurring at 46.2 days.177
In the first part of the study reported here, Ilomastat was delivered only twice, on postoperative days 0 and 5, and although it was better than BSS control, it was not as effective as the multitherapy regimen at this reduced dosage. The sequential regimen in this study was based on the hypothesis that the healing process for glaucoma blebs involves multiple pathways and processes. Hemostasis was achieved at surgery, and corticosteroids were given at the end of surgery to decrease inflammation during the initial phase of wound healing. Based on the data above, a sequential protocol was devised and designed to target existing latent TGF-β in the initial phase following surgery by using M-6-P.178–180 Just before its peak expression, CTGF was then targeted using CTGF antibody, as this was shown to be slightly more effective for prolonging bleb function than the TGF-β2 antibody or antisense. Finally, Ilomastat was used to reduce MMP activity in the later part of wound healing, during the peak of the tissue remodeling phase. As shown in Figure 2, this sequential multiple therapy protocol enhanced bleb survival significantly beyond that of any of the individual therapies to over 29 days, using a total of five postoperative subconjunctival injections. The MMC (0.4 mg/mL) blebs survived longer, to a mean of 36 days, but of key importance, they clinically appeared more avascular initially and histologically showed significant thinning and alteration of the conjunctival tissue morphology.
CONCLUSIONS
An ideal therapy to prolong bleb survival and improve long-term surgical outcomes following GFS should be both safe and specific. The conventional approach in the search for alternatives to the nonspecific antimetabolites currently in use has focused on the identification and treatment of individual mediators of the bleb scarring process. The process of bleb failure is complex, involving multiple families of mediators, including inflammatory mediators, growth factors, structural proteins, and MMPs.88,148,181–191
This study has demonstrated that the sequential use of multiple agents to target different modulators of wound healing prolongs bleb survival in this aggressively scarring animal model of GFS and histologically appears to result in less thinning and other changes in the bleb tissues. To the author’s knowledge, this is the first study investigating a multiple sequential treatment approach to enhancing bleb survival in an animal model of GFS. Future studies to determine the most effective combination of agents and to optimize the timing and method of their delivery may improve efficacy further while maintaining the histological integrity of the tissues.
ACKNOWLEDGMENTS
I would like to acknowledge D.W. Esson, DVM, Department of Ophthalmology, E.M. Sampson, MS, Institute for Wound Research, D. Samuelson, PhD, College of Veterinary Medicine, and G.S. Schultz, PhD, Institute for Wound Research, University of Florida, Gainesville, for their help in the scientific content of these studies. I would also like to acknowledge Gary Stevens, PhD, Jonathan Shuster, PhD, and Linda Young, PhD, Department of Biostatistics, University of Florida, Gainesville, for their assistance with the statistical analysis of the data.
REFERENCES
- 1.Roodhooft JM. Leading causes of blindness worldwide. Bull Soc Belge Ophtalmol. 2002;283:19–25. [PubMed] [Google Scholar]
- 2.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzarello L, Abiose A, Ffytche T, et al. VISION 2020: The Right to Sight: A global initiative to eliminate avoidable blindness. Arch Ophthalmol. 2004;122:615–620. doi: 10.1001/archopht.122.4.615. [DOI] [PubMed] [Google Scholar]
- 4.Thylefors B, Negrel A. The global impact of glaucoma. Bull World Health Org. 1994;72:323–326. [PMC free article] [PubMed] [Google Scholar]
- 5.Foster A, Johnson G. Magnitude and causes of blindness in the developing world. Int Ophthalmol. 1990;14:135–140. doi: 10.1007/BF00158310. [DOI] [PubMed] [Google Scholar]
- 6.Basic and Clinical Sciences Course. Section 10, Glaucoma. San Francisco, California: American Academy of Ophthalmology; 2001–2002.
- 7.Miller RD, Barber JC. Trabeculectomy in black patients. Ophthalmic Surg. 1981;12:46–50. [PubMed] [Google Scholar]
- 8.Berson D, Zauberman H, Landau L, et al. Filtering operations in Africans. Am J Ophthalmol. 1969;67:395–398. doi: 10.1016/0002-9394(69)92054-6. [DOI] [PubMed] [Google Scholar]
- 9.Scott IU, Greenfield DS, Schiffman J, et al. Outcomes of primary trabeculectomy with the use of adjunctive mitomycin. Arch Ophthalmol. 1998;116:286–291. doi: 10.1001/archopht.116.3.286. [DOI] [PubMed] [Google Scholar]
- 10.Shin DH, Hughes BA, Song MS, et al. Primary glaucoma triple procedure with or without adjunctive mitomycin. Prognostic factors for filtration failure. Ophthalmology. 1996;103:1925–1933. doi: 10.1016/s0161-6420(96)30406-5. [DOI] [PubMed] [Google Scholar]
- 11.Merritt JC. Filtering procedures in American blacks. Ophthalmic Surg. 1980;11:91–94. [PubMed] [Google Scholar]
- 12.Iliff CE. Surgical control of glaucoma in the negro. Am J Ophthalmol. 1944;27:731–738. [Google Scholar]
- 13.Polansky JR. Current perspectives on the TIGR/MYOC gene (Myocilin) and glaucoma. Ophthalmol Clin North Am. 2003;16:515–527. v–vi. doi: 10.1016/s0896-1549(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 14.Weisschuh N, Schiefer U. Progress in the genetics of glaucoma. Dev Ophthalmol. 2003;37:83–93. doi: 10.1159/000072040. [DOI] [PubMed] [Google Scholar]
- 15.Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 16.Vasconcellos JP, Melo MB, Costa VP, et al. Novel mutation in the MYOC gene in primary open glaucoma patients. J Med Genet. 2000;37:301–303. doi: 10.1136/jmg.37.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarfarazi M, Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol Clin North Am. 2003;16:529–541. doi: 10.1016/s0896-1549(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 18.Herndon LW, Weizer JS, Stinnett SS. Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol. 2004;122:17–21. doi: 10.1001/archopht.122.1.17. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 20.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 21.Palmberg P. Evidence-based target pressures: how to choose and achieve them. Int Ophthalmol Clin. 2004;44:1–14. doi: 10.1097/00004397-200404420-00003. [DOI] [PubMed] [Google Scholar]
- 22.Palmberg P. How clinical trial results are changing our thinking about target pressures. Curr Opin Ophthalmol. 2002;13:85–88. doi: 10.1097/00055735-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Migdal C, Gregory W, Hitchings RA. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101:1651–1657. doi: 10.1016/s0161-6420(94)31120-1. [DOI] [PubMed] [Google Scholar]
- 24.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 25.Feiner L, Plitz-Seymour JR. Collaborative Initial Glaucoma Treatment Study: A summary of results to date. Curr Opin Ophthalmol. 2003;14:106–111. doi: 10.1097/00055735-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Jay J. Rational choice of therapy in primary open angle glaucoma. Eye. 1992;6:243–247. doi: 10.1038/eye.1992.47. [DOI] [PubMed] [Google Scholar]
- 27.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 28.Shirakashi M, Iwata K, Sawaguchi S, et al. Intraocular pressure-dependent progression of visual field loss in advanced primary open-angle glaucoma: a 15-year follow-up. Ophthalmologica. 1993;207:1–5. doi: 10.1159/000310397. [DOI] [PubMed] [Google Scholar]
- 29.Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 30.Shigeeda T, Tomidokoro A, Araie M, et al. Long-term follow-up of visual field progression after trabeculectomy in progressive normal-tension glaucoma. Ophthalmology. 2002;109:766–770. doi: 10.1016/s0161-6420(01)01009-0. [DOI] [PubMed] [Google Scholar]
- 31.Wilson MR, Kosoko O, Cowan CL, Jr, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am J Ophthalmol. 2002;134:399–405. doi: 10.1016/s0002-9394(02)01585-4. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg I. Relationship between intraocular pressure and preservation of visual field in glaucoma. Surv Ophthalmol. 2003;48 (Suppl 1):S3–7. doi: 10.1016/s0039-6257(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 33.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 34.Addicks EM, Quigley A, Green WR, et al. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983;101:795–798. doi: 10.1001/archopht.1983.01040010795021. [DOI] [PubMed] [Google Scholar]
- 35.Hitchings RA, Grierson I. Clinico pathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc UK. 1983;103:84–88. [PubMed] [Google Scholar]
- 36.Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48:314–346. doi: 10.1016/s0039-6257(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 37.Esson DW, Popp MP, Liu L, et al. Microarray analysis of the failure of filtering “blebs” in a rat model of glaucoma filtering surgery. Invest Ophthalmol Vis Sci. 2004;45:4450–4462. doi: 10.1167/iovs.04-0375. [DOI] [PubMed] [Google Scholar]
- 38.Cordeiro MF, Chang L, Lim KS, et al. Modulating conjunctival wound healing. Eye. 2000;14 (Pt 3B):536–547. doi: 10.1038/eye.2000.141. [DOI] [PubMed] [Google Scholar]
- 39.Chang MR, Cheng Q, Lee DA. Basic science and clinical aspects of wound healing in glaucoma filtering surgery. J Ocul Pharmacol Ther. 1998;14:75–95. doi: 10.1089/jop.1998.14.75. [DOI] [PubMed] [Google Scholar]
- 40.Skuta GL, Parrish RK., 2nd Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32:149–170. doi: 10.1016/0039-6257(87)90091-9. [DOI] [PubMed] [Google Scholar]
- 41.Grierson I, Joseph J, Miller M, et al. Wound repair: the fibroblast and the inhibition of scar formation. Eye. 1988;2 (Pt 2):135–148. doi: 10.1038/eye.1988.27. [DOI] [PubMed] [Google Scholar]
- 42.Tahery MM, Lee DA. Review: pharmacologic control of wound healing in glaucoma filtration surgery. J Ocul Pharmacol. 1989;5:155–179. doi: 10.1089/jop.1989.5.155. [DOI] [PubMed] [Google Scholar]
- 43.Skuta GL. Antifibrotic agents in glaucoma filtering surgery. Int Ophthalmol Clin. 1993;33:165–182. doi: 10.1097/00004397-199303340-00014. [DOI] [PubMed] [Google Scholar]
- 44.Rockwood EJ, Parrish RK, 2nd, Heuer DK, et al. Glaucoma filtering surgery with 5-fluorouracil. Ophthalmology. 1987;94:1071–1078. doi: 10.1016/s0161-6420(87)33321-4. [DOI] [PubMed] [Google Scholar]
- 45.Akarsu C, Onol M, Hasanreisoglu B. Postoperative 5-fluorouracil versus intraoperative mitomycin C in high-risk glaucoma filtering surgery: extended follow up. Clin Exp Ophthalmol. 2003;31:199–205. doi: 10.1046/j.1442-9071.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- 46.Membrey WL, Poinoosawmy DP, Bunce C, et al. Glaucoma surgery with or without adjunctive antiproliferatives in normal tension glaucoma: 1 intraocular pressure control and complications. Br J Ophthalmol. 2000;84:586–590. doi: 10.1136/bjo.84.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon PS, Singh K. Update on antifibrotic use in glaucoma surgery, including use in trabeculectomy and glaucoma drainage implants and combined cataract and glaucoma surgery. Curr Opin Ophthalmol. 2004;15:141–146. doi: 10.1097/00055735-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Stamper RL, McMenemy MG, Lieberman MF. Hypotonous maculopathy after trabeculectomy with subconjunctival 5-flourouracil. Am J Ophthalmol. 1992;114:544–553. doi: 10.1016/s0002-9394(14)74481-2. [DOI] [PubMed] [Google Scholar]
- 49.Jampel HD, Pasquale LR, Dibernardo C. Hypotony maculopathy following trabeculectomy with mitomycin-C (letter) Arch Ophthalmol. 1992;110:1049–1050. doi: 10.1001/archopht.1992.01080200029011. [DOI] [PubMed] [Google Scholar]
- 50.Greenfield DS, Liebmann JM, Jee J, et al. Late onset bleb leaks after glaucoma filtering surgery. Arch Ophthalmol. 1998;16:443–447. doi: 10.1001/archopht.116.4.443. [DOI] [PubMed] [Google Scholar]
- 51.Mietz H, Addicks K, Bloch W, et al. Long-term intraocular toxic effects on topical mitomycin-C in rabbits. J Glaucoma. 1996;5:325–333. [PubMed] [Google Scholar]
- 52.Wolner B, Liebmann JM, Sassani JW, et al. Late bleb-related endophthalmitis after trabeculectomy with adjunctive 5-flourouracil. Ophthalmology. 1991;98:1053–1060. doi: 10.1016/s0161-6420(91)32177-8. [DOI] [PubMed] [Google Scholar]
- 53.Waheed S, Liebmann JM, Greenfield DS, et al. Recurrent bleb infections. Br J Ophthalmol. 1998;82:926–929. doi: 10.1136/bjo.82.8.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mac I, Soltau JB. Glaucoma-filtering bleb infections. Curr Opin Ophthalmol. 2003;14:91–94. doi: 10.1097/00055735-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Belyea DA, Dan JA, Stamper RL, et al. Late onset of sequential multifocal bleb leaks after glaucoma filtration surgery with 5-fluorouracil and mitomycin C. Am J Ophthalmol. 1997;124:40–45. doi: 10.1016/s0002-9394(14)71642-3. [DOI] [PubMed] [Google Scholar]
- 56.Parrish R, Minckler D. Late endophthalmitis: filtering surgery time-bomb? Ophthalmology. 1996;103:1167–1168. doi: 10.1016/s0161-6420(96)30527-7. [DOI] [PubMed] [Google Scholar]
- 57.Higginbotham EJ, Stevens RK, Musch DC, et al. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology. 1996;103:650–656. doi: 10.1016/s0161-6420(96)30639-8. [DOI] [PubMed] [Google Scholar]
- 58.Jampel HD, Quigley HA, Kerrigan-Baumrind LA, et al. Glaucoma surgical outcomes study group. Risk factors for late-onset infection following glaucoma filtration surgery. Arch Ophthalmol. 2001;119:1001–1008. doi: 10.1001/archopht.119.7.1001. [DOI] [PubMed] [Google Scholar]
- 59.Mietz H, Jacobi PC, Krieglstein GK. Postoperative application of mitomycin for trabeculectomies. Arch Ophthalmol. 2000;118:1341–1348. doi: 10.1001/archopht.118.10.1341. [DOI] [PubMed] [Google Scholar]
- 60.Kangas TA, Greenfield DS, Flynn HW, et al. Delayed onset endophthalmitis associated with conjunctival filtering blebs. Ophthalmology. 1997;104:746–752. doi: 10.1016/s0161-6420(97)30238-3. [DOI] [PubMed] [Google Scholar]
- 61.Soltau JB, Rothman RF, Bidenz DL, et al. Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol. 2000;118:338–342. doi: 10.1001/archopht.118.3.338. [DOI] [PubMed] [Google Scholar]
- 62.Cordeiro MF, Mead A, Ali RR, et al. Novel antisense oligonucleotides targeting TGF-beta inhibit in-vivo scarring and improve surgical outcome. Gene Ther. 2003;10:59–71. doi: 10.1038/sj.gt.3301865. [DOI] [PubMed] [Google Scholar]
- 63.Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci. 1999;40:2225–2234. [PubMed] [Google Scholar]
- 64.Esson DW, Neelakantan A, Iyer SA, et al. Expression of connective tissue growth factor after glaucoma filtration surgery in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45:485–491. doi: 10.1167/iovs.03-0485. [DOI] [PubMed] [Google Scholar]
- 65.Cordeiro MF, Balasubramanian L, Ali RR, et al. Effect and localization of a TGF-β1 antisense oligonucleotide in conjunctival scarring—a potential new anti-scarring strategy in glaucoma surgery [abstract] Invest Ophthalmol Vis Sci. 2000;41:S744. [Google Scholar]
- 66.Siriwardena D, Khaw PT, King AJ, et al. Hyman anti-transforming growth factor beta(2) monoclonal antibody—a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology. 2002;109:427–431. doi: 10.1016/s0161-6420(01)00997-6. [DOI] [PubMed] [Google Scholar]
- 67.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kothapalli D, Frazier KS, Welply A, et al. Transforming growth factor beta induces anchorage-independent growth of NRK fibroblasts via a connective tissue growth factor dependent signaling pathway. Cell Growth Differ. 1997;8:61–68. [PubMed] [Google Scholar]
- 69.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–82. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 70.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 71.Mauch C, Krieg T, Bauer EA. Role of the extracellular matrix in the degradation of connective tissue. Arch Dermatol Res. 1994;287:107–114. doi: 10.1007/BF00370728. [DOI] [PubMed] [Google Scholar]
- 72.Daniels JT, Cambrey AD, Occleston NL, et al. Matrix metalloproteinase inhibition modulate fibroblast-mediated matrix contraction and collagen production in-vitro. Invest Ophthalmol Vis Sci. 2003;44:1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- 73.Scott KA, Wood EJ, Karran EH. A matrix metalloproteinase inhibitor which prevents fibroblast-mediated collagen lattice contraction. FEBS Lett. 1998;441:137–140. doi: 10.1016/s0014-5793(98)01542-7. [DOI] [PubMed] [Google Scholar]
- 74.Sheridan CM, Occleston NL, Hiscott P, et al. Matrix metalloproteinases: a role in the contraction of vitreo-retinal scar tissue. Am J Pathol. 2001;159:1555–1566. doi: 10.1016/S0002-9440(10)62540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pelton RW, Hogan BL, Miller DA, Moses HL. Differential expression of genes encoding TGFs beta 1, beta 2, and beta 3 during murine palate formation. Dev Biol. 1990;141:456–460. doi: 10.1016/0012-1606(90)90401-4. [DOI] [PubMed] [Google Scholar]
- 76.Chang L, Crowston JG, Cordeiro MF, et al. The role of the immune system in conjunctival wound healing after glaucoma surgery. Surv Ophthalmol. 2000;45:49–68. doi: 10.1016/s0039-6257(00)00135-1. [DOI] [PubMed] [Google Scholar]
- 77.Khaw PT, Chang L, Wong TT, et al. Modulation of wound healing after glaucoma surgery. Curr Opin Ophthalmol. 2001;12:143–8. doi: 10.1097/00055735-200104000-00011. [erratum in: Curr Opin Ophthalmol 2001;12:235] [DOI] [PubMed] [Google Scholar]
- 78.Cordeiro MF, Siriwardena D, Chang L, Khaw PT. Wound healing modulation after glaucoma surgery. Curr Opin Ophthalmol. 2000;11:121–126. doi: 10.1097/00055735-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 79.Atreides SP, Skuta GL, Reynolds AC. Wound healing modulation in glaucoma filtering surgery. Int Ophthalmol Clin. 2004;44:61–106. doi: 10.1097/00004397-200404420-00007. [DOI] [PubMed] [Google Scholar]
- 80.Costa VP, Spaeth GL, Eiferman RA, Orengo-Nania S. Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:152–170. [PubMed] [Google Scholar]
- 81.Jampel HD. Effect of brief exposure to mitomycin C on viability and proliferation of cultured human Tenon’s capsule fibroblasts. Ophthalmology. 1992;99:1471–1476. doi: 10.1016/s0161-6420(92)31781-6. [DOI] [PubMed] [Google Scholar]
- 82.Khaw PT, Sherwood MB, MacKay SL, et al. Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon’s capsule fibroblasts. Arch Ophthalmol. 1992;110:1150–1154. doi: 10.1001/archopht.1992.01080200130040. [DOI] [PubMed] [Google Scholar]
- 83.Uchio K, Graham M, Dean NM, et al. Down-regulation of connective tissue growth factor and type I collagen mRNA expression by connective tissue growth factor antisense oligonucleotide during experimental liver fibrosis. Wound Repair Regen. 2004;12:60–66. doi: 10.1111/j.1067-1927.2004.012112.x. [DOI] [PubMed] [Google Scholar]
- 84.Moussad EE, Brigstock DR. Connective tissue growth factor: What’s in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 85.Galardy RE, Cassabonne ME, Giese C, et al. Low molecular weight inhibitors in corneal ulceration. Ann N Y Acad Sci. 1994;732:315–323. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 86.Levy DE, Lapierre F, Liang W, et al. Matrix metalloproteinase inhibitors: a structure-activity study. J Med Chem. 1998;41:199–223. doi: 10.1021/jm970494j. [DOI] [PubMed] [Google Scholar]
- 87.Daniels JT, Schultz GS, Blalock TD, et al. Medication of transforming growth factor-beta(1)-stimulated matrix contraction by fibroblasts: a role for connective tissue growth factor in contractile scarring. Am J Pathol. 2003;163:2043–2052. doi: 10.1016/s0002-9440(10)63562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong TT, Mead AL, Khaw PT. Matrix metalloproteinase inhibition modulates post-operative scarring after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2003;44:1097–1103. doi: 10.1167/iovs.02-0366. [DOI] [PubMed] [Google Scholar]
- 89.Clark R, Henson P. The Molecular and Cellular Biology of Wound Repair New York: Plenum Press; 1988.
- 90.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 91.Singer A, Clark R. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 92.Desjardins DC, Parrish RK, II, Folberg R, et al. Wound healing after filtering surgery in owl monkeys. Arch Ophthalmol. 1986;104:1835–1839. doi: 10.1001/archopht.1986.01050240109050. [DOI] [PubMed] [Google Scholar]
- 93.Desjardins DC, Parrish RK, II, Folberg R, et al. Wound healing after filtering surgery in owl monkeys. Arch Ophthalmol. 1986;104:1835–1839. doi: 10.1001/archopht.1986.01050240109050. [DOI] [PubMed] [Google Scholar]
- 94.Dvorak HF. Tumors: wounds that do not heal; similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 95.Albert DM, Jakobiec FA. New surgical techniques in glaucoma management. In: Principles and Practice of Ophthalmology 2nd ed. Philadelphia: WB Saunders;2000;3024–3030.
- 96.Seetner A, Morin JD. Healing of trabeculectomies in rabbits. Can J Ophthalmol. 1979;14:121–125. [PubMed] [Google Scholar]
- 97.Cordeiro MF, Chang L, Lim KS, et al. Modulating conjunctival wound healing. Eye. 2000;14:536–547. doi: 10.1038/eye.2000.141. [DOI] [PubMed] [Google Scholar]
- 98.Cordeiro M, Reichel M, Gay J, et al. TGF-1, -2 and -3 in vivo: effects on normal and mitomycin-C modulated conjunctival scarring. Invest Ophthalmol Vis Sci. 1999:1975–1982. [PubMed] [Google Scholar]
- 99.Cordeiro M, Bhattacharya S, Schultz G, Khaw PT. TGF-beta1, -beta2 and -beta3 in vitro: biphasic effects on Tenon’s fibroblast contraction, proliferation and migration. Invest Ophthalmol Vis Sci. 2000;41:756–763. [PubMed] [Google Scholar]
- 100.Connor T, Roberts A, Sporn M, et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83:1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jampel H, Roche N, Stark W, et al. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9:963–969. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- 102.Miller MH, Grierson I, Unger WI, Hitchings RA. Wound healing in an animal model of glaucoma fistulizing surgery in the rabbit. Ophthalmic Surg. 1989;20:350–357. [PubMed] [Google Scholar]
- 103.Reichel MB, Cordeiro MF, Alexander RA, Cree IA, et al. New model of conjunctival scarring in the mouse eye. Br J Ophthalmol. 1998;82:1072–1077. doi: 10.1136/bjo.82.9.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jampel HD, McGuigan LJ, Dunkelberger GR. Cellular proliferation after experimental glaucoma filtering surgery. Arch Ophthalmol. 1988;106:89–94. doi: 10.1001/archopht.1988.01060130095036. [DOI] [PubMed] [Google Scholar]
- 105.The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol. 1996;121:349–366. doi: 10.1016/s0002-9394(14)70431-3. [DOI] [PubMed] [Google Scholar]
- 106.Heuer DK, Parrish RK, II, Gressel MG, et al. 5-Fluorouracil and glaucoma filtering surgery: II. A pilot study. Ophthalmology. 1984;91:384–393. doi: 10.1016/s0161-6420(84)34291-9. [DOI] [PubMed] [Google Scholar]
- 107.Heuer DK, Parrish RK, II, Gressel MG, et al. 5-Fluorouracil and glaucoma filtering surgery: III. Intermediate follow-up of a pilot study. Ophthalmology. 1986;93:1537–1546. doi: 10.1016/s0161-6420(86)33542-5. [DOI] [PubMed] [Google Scholar]
- 108.Rothman RF, Liebmann JM, Ritch R. Low-dose 5-fluorouracil trabeculectomy as initial surgery in uncomplicated glaucoma: long-term follow-up. Ophthalmology. 2000;107:1184–1190. doi: 10.1016/s0161-6420(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 109.Goldenfeld M, Krupin T, Ruderman JM, et al. 5-Fluorouracil in initial trabeculectomy: a prospective, randomized, multi-center study. Ophthalmology. 1994;101:1024–1029. doi: 10.1016/s0161-6420(94)31223-1. [DOI] [PubMed] [Google Scholar]
- 110.Ruderman JM, Welch DB, Smith MF, et al. A randomized study of 5-fluorouracil and filtration surgery. Am J Ophthalmol. 1987;104:218–224. doi: 10.1016/0002-9394(87)90407-7. [DOI] [PubMed] [Google Scholar]
- 111.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beckers HJ, Kinders KC, Webers CA. Five-year results of trabeculectomy with mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2003;241:106–110. doi: 10.1007/s00417-002-0621-5. [DOI] [PubMed] [Google Scholar]
- 113.Cheung JC, Wright MM, Murali S, et al. Intermediate-term outcome of variable dose mitomycin C filtering surgery. Ophthalmology. 1997;104:143–149. doi: 10.1016/s0161-6420(97)30347-9. [DOI] [PubMed] [Google Scholar]
- 114.Perkins TW, Gangnon R, Ladd W, et al. Trabeculectomy with mitomycin C: intermediate-term results. J Glaucoma. 1998;7:230–236. [PubMed] [Google Scholar]
- 115.Bindlish R, Condon GP, Schlosser JD, et al. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002;109:1336–1341. doi: 10.1016/s0161-6420(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 116.Gonzalez Vela JL, Sanchez Guillen JM, Trevino Aguirre SA, et al. Efectiveness of 5-fluoruracil and vinorelbine in patients who had received multi-treatments for metastatic breast cancer. Clin Transl Oncol. 2005;7:441–446. doi: 10.1007/BF02716594. [DOI] [PubMed] [Google Scholar]
- 117.Smith TR, Sunshine A, Stark SR, et al. Sumatriptan and naproxen sodium for the acute treatment of migraine. Headache. 2005;45:983–991. doi: 10.1111/j.1526-4610.2005.05178.x. [DOI] [PubMed] [Google Scholar]
- 118.Youdim MB, Buccafusco JJ. CNS Targets for multi-functional drugs in the treatment of Alzheimer’s and Parkinson’s diseases. J Neural Transm. 2005;112:519–537. doi: 10.1007/s00702-004-0214-z. [DOI] [PubMed] [Google Scholar]
- 119.Youdim MB, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci. 2005;26:27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 120.Daub H, Specht K, Ullrich A. Strategies to overcome resistance to targeted protein kinase inhibitors. Nat Rev Drug Discov. 2004;3:1001–1010. doi: 10.1038/nrd1579. [DOI] [PubMed] [Google Scholar]
- 121.Litz J, Sakuntala Warshamana-Greene G, Sulanke G, et al. The multi-targeted kinase inhibitor SU5416 inhibits small cell lung cancer growth and angiogenesis, in part by blocking Kit-mediated VEGF expression. Lung Cancer. 2004;46:283–291. doi: 10.1016/j.lungcan.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 122.Leese MP, Newman SP, Purohit A, et al. 2-Alkylsulfanyl estrogen derivatives: synthesis of a novel class of multi-targeted anti-tumour agents. Bioorg Med Chem Lett. 2004;14:3135–3138. doi: 10.1016/j.bmcl.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 123.Borkow G, Lapidot A. Multi-targeting the entrance door to block HIV-1. Curr Drug Targets Infect Disord. 2005;5:3–15. doi: 10.2174/1568005053174645. [DOI] [PubMed] [Google Scholar]
- 124.Latham V, Stebbing J, Mandalia S, et al. Adherence to trizivir and tenofovir as a simplified salvage regimen is associated with suppression of viraemia and a decreased cholesterol. J Antimicrob Chemother. 2005;56:186–189. doi: 10.1093/jac/dki170. [DOI] [PubMed] [Google Scholar]
- 125.Starita RJ, Fellman RL, Spaeth GL, et al. Short- and long-term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology. 1985;92:938–946. doi: 10.1016/s0161-6420(85)33931-3. [DOI] [PubMed] [Google Scholar]
- 126.Araujo SV, Spaeth GL, Roth SM, Starita RJ. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology. 1995;102:1753–1759. doi: 10.1016/s0161-6420(95)30797-x. [DOI] [PubMed] [Google Scholar]
- 127.Parrillo JE, Fauci AS. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- 128.Cupps TR, Fauci AS. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 129.Oshima T, Kurosaka D, Kato K, et al. Tranilast inhibits cell proliferation and collagen synthesis by rabbit corneal and Tenon’s capsule fibroblasts. Curr Eye Res. 2000;20:283–286. [PubMed] [Google Scholar]
- 130.Chihara E, Dong J, Ochiai H, et al. Effects of tranilast on filtering blebs: a pilot study. J Glaucoma. 2002;11:127–133. doi: 10.1097/00061198-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 131.Akman A, Bilezikci B, Kucukerdonmez C, et al. Suramin modulates wound healing of rabbit conjunctiva after trabeculectomy: comparison with mitomycin C. Curr Eye Res. 2003;26:37–43. doi: 10.1076/ceyr.26.1.37.14248. [DOI] [PubMed] [Google Scholar]
- 132.Mietz H, Chevez-Barrios P, Feldman RM, et al. Suramin inhibits wound healing following filtering procedures for glaucoma. Br J Ophthalmol. 1998;82:816–820. doi: 10.1136/bjo.82.7.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mietz H, Krieglstein GK. Suramin to enhance glaucoma filtering procedures: a clinical comparison with mitomycin. Ophthalmic Surg Lasers. 2001;32:358–369. [PubMed] [Google Scholar]
- 134.Gillies MC, Brooks AM, Young S, et al. A randomized phase II trial of interferon alpha2b versus 5-fluorouracil after trabeculectomy. Aust N Z J Ophthalmol. 1999;27:37–44. doi: 10.1046/j.1440-1606.1999.00165.x. [DOI] [PubMed] [Google Scholar]
- 135.Cristofanilli M, Pescosolido N, Risuleo G, et al. A murine cell culture model for post-trabeculectomy anfibrotic treatment: induction of apoptosis by cyclosporine. Acta Ophthalmol Scand. 2001;79:309–312. doi: 10.1034/j.1600-0420.2001.790321.x. [DOI] [PubMed] [Google Scholar]
- 136.Nuzzi R, Cerruti A, Finazzo C. Cyclosporine C: a study of wound-healing modulation after trabeculectomy in rabbit. Acta Ophthalmol Scand Suppl. 1998;227:48–49. doi: 10.1111/j.1600-0420.1998.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 137.Turacli ME, Gunduz K, Aktan G, et al. Topical cyclosporine as a possible new antimetabolite in trabeculectomy. Ophthalmic Surg Lasers. 1996;27:438–444. [PubMed] [Google Scholar]
- 138.Turacli E, Gunduz K, Aktan G, et al. A comparative clinical trial of mitomycin C and cyclosporine A in trabeculectomy. Eur J Ophthalmol. 1996;6:398–401. doi: 10.1177/112067219600600410. [DOI] [PubMed] [Google Scholar]
- 139.Grisanti S, Szurman P, Warga M, et al. Decorin modulates wound healing in experimental glaucoma filtration surgery: a pilot study. Invest Ophthalmol Vis Sci. 2005;46:191–196. doi: 10.1167/iovs.04-0902. [DOI] [PubMed] [Google Scholar]
- 140.Mietz H, Chevez-Barrios P, Lieberman MW, et al. Decorin and suramin inhibit ocular fibroblast collagen production. Graefes Arch Clin Exp Ophthalmol. 1997;235:399–403. doi: 10.1007/BF00937291. [DOI] [PubMed] [Google Scholar]
- 141.Mead AL, Wong TT, Cordeiro MF, et al. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma sugery. Invest Ophthalmol Vis Sci. 2003;44:3394–3401. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- 142.Blakytny R, Ludlow A, Martin GE, et al. Latent TGF beta-1 activation by platelets. J Cell Physiol. 2004;1991:67–76. doi: 10.1002/jcp.10454. [DOI] [PubMed] [Google Scholar]
- 143.Khalil N. TGF beta: from latent to active. Microbes Infect. 1999;1:1255–1263. doi: 10.1016/s1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 144.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weiner JA, Chen A, Davis BH. Platelet-derived growth factor is a principal inductive factor modulating mannose 6-phosphate/insulin-like growth factor-II receptor gene expression via a distal E-box in activated hepatic stellate cells. Biochem J. 2000;345(Pt 2):225–231. [PMC free article] [PubMed] [Google Scholar]
- 146.Ghahary A, Tredget EE, Shen Q. Insulin-like growth factor II/mannose-6 phosphate receptors facilitate the matrix effects of latent transforming growth factor β1 released from genetically modified keratinocytes in a fibroblast/keratinocyte coculture system. J Cell Physiol. 1999;180:61–70. doi: 10.1002/(SICI)1097-4652(199907)180:1<61::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 147.Ghahary A, Tredget EE, Shen Q, et al. Mannose 6 phosphate/IGF-II receptors mediate the effects of IGF-1 induced latent transforming growth factor beta 1 on expression of type 1 collagen and collagenase in dermal fibroblasts. Growth Factors. 2000;17:167–176. doi: 10.3109/08977190009001066. [DOI] [PubMed] [Google Scholar]
- 148.Cordeiro MF. Beyond mitomycin: TGF-beta and wound healing. Prog Retin Eye Res. 2002;21:75–89. doi: 10.1016/s1350-9462(01)00021-0. [DOI] [PubMed] [Google Scholar]
- 149.Cordeiro MF, Chang L, Lim KS, et al. Modulating conjunctival wound healing. Eye. 2000;14(Pt 3B):536–547. doi: 10.1038/eye.2000.141. [DOI] [PubMed] [Google Scholar]
- 150.Cordeiro MF. Technology evaluation: lerdelimumab, Cambridge Antibody Technology. Curr Opin Mol Ther. 2003;5:199–203. [PubMed] [Google Scholar]
- 151.Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci. 1999;40:2225–2234. [PubMed] [Google Scholar]
- 152.Siriwardena D, Khaw PT, Donaldson ML, et al. A randomised placebo controlled trial of human anti-TGFβ2 monoclonal antibody (CAT-152): a new modulator of wound healing following trabeculectomy [abstract] Invest Ophthalmol Vis Sci. 2000;41:S744. [Google Scholar]
- 153.Cambridge Antibody Technology announces preliminary results of TrabioR phase II/III clinical trial. (Press release) Cambridge, England: Cambridge Antibody Technology, November 9, 2004.
- 154.Duncan MR, Frazier KS, Abramson S, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down regulation by camp. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 155.Ihn H. Pathogenesis of fibrosis: role of TGF-beta abd CTGF. Curr Opin Rheumatol. 2002;14:681–685. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 156.Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;11;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- 157.Daniels JT, Schultz GS, Blalock TD, et al. Mediation of transforming growth factor-beta(1)-stimulated matrix contraction by fibroblasts: a role for connective tissue growth factor in contractile scarring. Am J Pathol. 2003;163:2043–2052. doi: 10.1016/s0002-9440(10)63562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4:136–142. doi: 10.1007/s11926-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 159.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 160.Parekh T, Saxena B, Reibman J, et al. Neutrophil chemotaxis in response to TGF-beta isoforms (TGF-beta 1, TGF-beta 2, TGF-beta-3) is mediated by fibronectin. J Immunol. 1994;152:2456–2466. [PubMed] [Google Scholar]
- 161.Pena RA, Jerdan JA, Glaser BM. Effects of TGF-beta and TGF-beta neutralizing antibodies on fibroblast-induced collagen gel contraction: implications for proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1994;35:2804–2808. [PubMed] [Google Scholar]
- 162.Frazier K, Williams S, Kothapalli D, et al. Stimulation of fibroblast cell growth, matrix production and granulation of tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 163.Blalock TD, Duncan MR, Varela JC, et al. Connective tissue growth factor expression and action in human corneal fibrolast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- 164.Berry DP, Harding KG, Stanton MR, et al. Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr Surg. 1998;102:124–131. doi: 10.1097/00006534-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 165.Daniels JT, Cambrey AD, Occleston NL, et al. Matrix metalloproteinase inhibition modulates fibrolast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44:1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- 166.Ravanti L, Kahari V-M. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- 167.Wong TTL, Sethi C, Daniels JT, et al. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47:239–256. doi: 10.1016/s0039-6257(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 168.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 169.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 170.Steffensen B, Hakkinen L, Larjava H. Proteolytic events of wound-healing—coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 171.St-Pierre Y, Van Themsche C, Esteve PO. Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies. Curr Drug Targets Inflamm Allergy. 2003;2:206–215. doi: 10.2174/1568010033484133. [DOI] [PubMed] [Google Scholar]
- 172.Van den Steen PE, Dubois B, Nelissen I, et al. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 173.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling as well as pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 174.Mietz H, Esser JM, Welsandt G, et al. Latanoprost stimulates secretion of matrix metalloproteinases in tenon fibroblasts both in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003;44:5182–5188. doi: 10.1167/iovs.02-0462. [DOI] [PubMed] [Google Scholar]
- 175.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 176.Galardy RE, Cassabonne ME, Giese C, et al. Low molecular weight inhibitors in corneal ulceration. Ann N Y Acad Sci. 1994;732:315–323. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 177.Wong TT, Mead AL, Khaw PT. Prolonged antiscarring effects of ilomastat and MMC after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2005;46:2018–2022. doi: 10.1167/iovs.04-0820. [DOI] [PubMed] [Google Scholar]
- 178.Purchio AF, Cooper JA, Brunner AM, et al. Identification of mannose 6-phosphate in two asparagine-linked sugar chains of recombinant transforming growth factor-beta 1 precursor. J Biol Chem. 1988;263:14211–14215. [PubMed] [Google Scholar]
- 179.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.DeBleser PJ, Jannes P, Van Buul-Offers SC, et al. Insulin like growth factor-II/mannose 6-phosphate receptor is expressed on CC14-exposed rat fat-storing cells and facilitates activation of latent transforming growth factor-beta in cocultures with sinusoidal endothelial cells. Hepatology. 1995;21:1429–1437. doi: 10.1002/hep.1840210529. [DOI] [PubMed] [Google Scholar]
- 181.Cunliffe IA, Richardson PS, Rees RC, Rennie IG. Effect of TNF, IL-1, and IL-6 on the proliferation of human Tenon’s capsule fibroblasts in tissue culture. Br J Ophthalmol. 1995;79:590–595. doi: 10.1136/bjo.79.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Denk PO, Roth-Eichhorn S, Gressner AM, Knorr M. Effect of cytokines on regulation of the production of transforming growth factor beta-1 in cultured human Tenon’s capsule fibroblasts. Eur J Ophthalmol. 2000;10:110–115. doi: 10.1177/112067210001000203. [DOI] [PubMed] [Google Scholar]
- 183.Klenkler B, Sheardown H. Growth factors in the anterior segment: role in tissue maintenance, wound healing and ocular pathology. Exp Eye Res. 2004;79:677–688. doi: 10.1016/j.exer.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 184.Chang L, Siriwardena D, Wilkins MR, et al. In vivo production of interferon beta by human Tenon’s fibroblasts; a possible mediator for the development of chronic conjunctival inflammation. Br J Ophthalmol. 2002;86:611–615. doi: 10.1136/bjo.86.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-beta1, -beta2, and -beta3 in vitro: biphasic effects on Tenon’s fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci. 2000;41:756–763. [PubMed] [Google Scholar]
- 186.Tripathi RC, Li J, Chalam KV, Tripathi BJ. Expression of growth factor mRNAs by human Tenon’s capsule fibroblasts. Exp Eye Res. 1996;63:339–346. doi: 10.1006/exer.1996.0123. [DOI] [PubMed] [Google Scholar]
- 187.Ellis DG, Cheng Q, Lee DA. The effects of growth factors on Tenon’s capsule fibroblasts in serum-free culture. Curr Eye Res. 1996;15:27–35. doi: 10.3109/02713689609017608. [DOI] [PubMed] [Google Scholar]
- 188.Gillies MC, Su T. Cytokines, fibrosis and the failure of glaucoma filtration surgery. Aust N Z J Ophthalmol. 1991;19:299–304. doi: 10.1111/j.1442-9071.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 189.Denk PO, Hoppe J, Hoppe V, Knorr M. Effect of growth factors on the activation of human Tenon’s capsule fibroblasts. Curr Eye Res. 2003;27:35–44. doi: 10.1076/ceyr.27.2.35.15456. [DOI] [PubMed] [Google Scholar]
- 190.Wong TT, Mead AL, Khaw PT. Prolonged antiscarring effects of ilomastat and MMC after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2005;46:2018–2022. doi: 10.1167/iovs.04-0820. [DOI] [PubMed] [Google Scholar]
- 191.Liu CJ, Huang YL, Chiu AW, Ju JP. Transcript expression of matrix metalloproteinases in the conjunctiva following glaucoma filtration surgery in rabbits. Ophthalmic Res. 2004;36:114–119. doi: 10.1159/000076891. [DOI] [PubMed] [Google Scholar]