Abstract
Purpose
To investigate whether age influences optic disc cupping in addition to the effects of intraocular pressure (IOP).
Methods
Population-based study (N = 4,926 at baseline). All measures including fundus photography were done according to standard protocols. Stereoscopic images of the optic disc were graded in masked fashion.
Results
Cup-to-disc ratios (C/D) were directly related to IOP at baseline. Age of 75 years or older was associated with increase in cupping at a given level of IOP, but the age association was attenuated when including refraction in multivariable models. Change in C/D between baseline and 15-year follow-up was also influenced by age such that those 75 years of age or older were at increased risk of cupping. When refraction was included in this model, age was still significant.
Conclusions
People who are at least 75 years of age are at greater risk of developing optic disc cupping, which is associated with greater IOP.
INTRODUCTION
Optic disc cupping is affected by intraocular pressure (IOP) in most studies that have evaluated this relationship.1,2 Increase in optic disc cupping is strongly suggestive of pathologic change that is characteristic of glaucoma.2 Klein and associates3 have shown that IOP is associated with subsequent change in cup-to-disc ratio (C/D) in a general population over a 5-year interval. The prevalence of primary open-angle glaucoma increases with increasing age in many populations studied.4 It was the purpose of the current investigation to examine the relationships of C/D, IOP, and age and to evaluate whether the increase in glaucoma with age might be accounted for by higher IOP at increasing age, independent of other risk factor changes with age (eg, blood pressure level).
METHODS
POPULATION
This study was carried out with data from the Beaver Dam Eye Study. Details of the baseline and follow-up visits have appeared in previous reports.5–8 In brief, a private census of the population of Beaver Dam, Wisconsin, was performed from September 15, 1987, to May 4, 1988, to identify all residents in the city or township of Beaver Dam in the target age range. Of the 5,924 eligible individuals, 4,926 (83%) persons 43 to 86 years of age participated in the baseline examination between March 1, 1988, and September 14, 1990. Ninety-nine percent of the population was white. Study evaluations took place at successive 5-year intervals. Data for this report are from the baseline examination and the 15-year follow-up (2003–2005). There were 2,358 participants at the 15-year examination who had participated at baseline. Four thousand five hundred ninety-four participants had photographs taken that were gradable for optic cup and disc diameters at baseline and had data for IOP. Of these, 4,378 also had refraction measured at baseline. Of the 2,358 persons seen at both visits, 1,985 had C/D graded at both. All data were collected with institutional review board approval in conformity with all federal and state laws, and the study was in adherence to the tenets of the Declaration of Helsinki.
Comparisons of baseline characteristics between participants and nonparticipants at the 15-year follow-up examination appear elsewhere.9 In general, live and deceased nonparticipants at the 15-year follow-up were older, had higher IOPs on average, and had had more medical conditions than participants.9
At each examination, IOPs were measured with a Goldmann applanation tonometer according to a specified protocol.10 An examination of the anterior chamber was performed, and the pupils were pharmacologically dilated if it appeared that there was minimal risk of angle closure. Stereoscopic 30° color fundus photographs centered on the disc (Diabetic Retinopathy Study standard field 1) of each eye were taken. A medical and lifestyle history questionnaire was administered. Refractions were performed using a modification of the Early Treatment of Diabetic Retinopathy Study protocol.11 Refraction was defined as a spherical equivalent.
GRADING
The optic cups and discs were measured using a template of graded circles of specific diameters in inches12 from the stereoscopic photographic pairs centered on the optic disc. Optic disc diameters ranged from .094 to .250 inches, optic cups ranged from 0 to .187 inches, and C/D ranged from 0 to .946 (in the vertical axis).
STATISTICS
Mean IOP, C/D, and change in C/D were computed. The relationship of age, IOP, and C/D was analyzed, with adjustments, using linear regression and analysis of variance. SAS was used for statistical analysis.13
RESULTS
At baseline, mean IOPs and C/D increased significantly with increasing age (for the sake of space and because relationships were similar for right and left eyes, data are presented for right eyes only, Table 1). Refraction also varied with age from −0.79 diopters (D) in those 43 to 54 years of age to 1.23 D in those 75 years of age and older.14 In multivariable analyses for the entire population (n = 4,594 persons with relevant variables), we found that being 75 years of age or older and having higher IOP were significantly associated with C/D (positive slope) adjusting for age and gender (Table 2). However, with additional adjustment for refraction, the age effect was attenuated and no longer statistically significant (Table 2). Blood pressure (systolic or diastolic) was not associated with C/D (data not shown). Despite including refraction in the model, when we examined incident change in C/D over a 15-year interval for the whole surviving population, IOP and being aged 75 years or older (n = 38) were significantly associated with C/D change. In addition, an interaction between age and IOP was significant in its relationship to incident change in C/D (Table 3, Figure). Size of the optic disc at baseline when included in the model in addition to C/D was not a significant factor associated with change in C/D. Cataract surgery in the interval between baseline and the 15-year follow-up was not independently associated with change in C/D.
TABLE 1.
INTRAOCULAR PRESSURE (IOP), VERTICAL CUP-TO-DISC RATIO (C/D), AND 15-YEAR CHANGE IN C/D BY AGE, RIGHT EYES
| VERTICAL C/D | 15-YEAR CHANGE IN C/D | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AGE (YR) | N | MEAN IOP (MM HG) | SD | N | MEAN | SD | N | MEAN | SD |
| 43–54 | 1507 | 14.95 | 3.10 | 1479 | 0.356 | 0.122 | 965 | 0.012 | 0.097 |
| 55–64 | 1312 | 15.46 | 3.24 | 1284 | 0.357 | 0.132 | 654 | 0.011 | 0.110 |
| 65–74 | 1271 | 15.77 | 3.38 | 1195 | 0.363 | 0.135 | 328 | 0.005 | 0.108 |
| 75+ | 793 | 15.64 | 3.80 | 646 | 0.377 | 0.143 | 38 | 0.016 | 0.122 |
| Total | 4883 | 15.42 | 3.35 | 4604 | 0.361 | 0.132 | 1985 | 0.011 | 0.104 |
| P value for trend | <.001 | .001 | .42 | ||||||
SD = standard deviation.
TABLE 2.
MULTIVARIABLE MODEL OF ASSOCIATION OF INTRAOCULAR PRESSURE (IOP) AND VERTICAL CUP-TO-DISC RATIO, RIGHT EYES AT BASELINE
| AGE/GENDER ADJUSTED | AGE/GENDER/REFRACTION ADJUSTED | |||||
|---|---|---|---|---|---|---|
| VARIABLE | ESTIMATE* | SE | PVALUE | ESTIMATE* | SE | PVALUE |
| Intercept | 0.3170 | 0.0096 | <.001 | 0.3193 | 0.0098 | <.001 |
| Age, 43–54 yr | 0 | 0 | ||||
| 55–64 yr | −0.0003 | 0.0050 | .95 | −0.0058 | 0.0051 | .26 |
| 65–74 yr | 0.0049 | 0.0051 | .34 | −0.0015 | 0.0054 | .79 |
| 75+ yr | 0.0194 | 0.0062 | .002 | 0.0076 | 0.0068 | .27 |
| Sex (men) | 0.0054 | 0.0039 | .17 | 0.0057 | 0.0039 | .15 |
| Refraction, per diopter | 0.0042 | 0.0009 | <.001 | |||
| IOP, per 1 mm Hg | 0.0024 | 0.0006 | <.001 | 0.0025 | 0.0006 | <.001 |
SE = standard error of estimate.
Estimate is the amount of change in cup-to-disc ratio for every unit of change in the variable. For the intercept, this represents the cup-to-disc ratio value setting all other variables equal to zero.
TABLE 3.
MULTIVARIABLE MODEL OF CHANGE IN CUP-TO-DISC RATIO (C/D) BETWEEN BASELINE AND 15-YEAR FOLLOW-UP EXAMINATION, RIGHT EYES
| VARIABLE | ESTIMATE* | SE | PVALUE | GROUPPVALUE |
|---|---|---|---|---|
| Intercept | −0.0340 | 0.0168 | .04 | |
| Sex (men) | 0.0001 | 0.0047 | .98 | |
| Refraction, per diopter | −0.0034 | 0.0011 | .002 | |
| Age, 43–54 yr | 0 | |||
| 55–64 yr | 0.0459 | 0.0259 | .08 | |
| 65–74 yr | −0.0188 | 0.0328 | .57 | |
| 75+ yr | −0.1606 | 0.0724 | .03 | .02 |
| IOP, per 1 mm Hg | 0.0030 | 0.0011 | .008 | |
| IOP × age 43–54 yr | 0 | |||
| IOP × age 55–64 yr | −0.0030 | 0.0017 | .08 | |
| IOP × age 65–74 yr | 0.0008 | 0.0021 | .70 | |
| IOP × age 75+ yr | 0.0106 | 0.0044 | .02 | .01 |
IOP = intraocular pressure; SE = standard error.|
Estimate is the amount of change in the 15-year change in C/D for every unit of change in the variable. For the intercept, this represents the 15-year C/D change value setting when all other variables equal to zero.
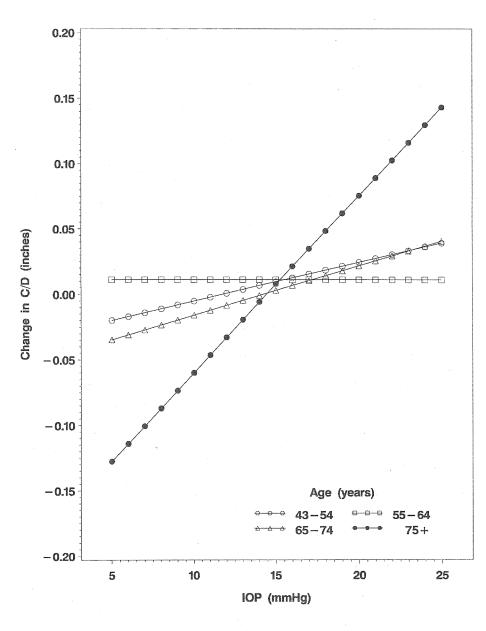
FIGURE.
Estimated linear equation for the relationship of intraocular pressure (IOP) to changes in cup-to-disc ratio (C/D) over 15 years by age-groups, adjusted for age and gender.
DISCUSSION
Intraocular pressure is currently thought of as the most important risk factor for open-angle glaucoma,15–17 and probably the most apparent morphologic sign of this is its effect on optic disc cupping as reflected in a larger C/D. We have found that age affects this relationship such that the slope of change in C/D with IOP is steeper in persons 75 years of age or older than it is for younger age-groups.
Refraction has been previously associated with glaucoma18–21 and IOP,22,23 and the C/D in myopic eyes is greater than in eyes that are not myopic.24,25 It has been suggested that the higher C/D in eyes with high myopia may predispose the nerve fibers to damage by IOP.26–28 Refraction attenuated the importance of the modifying influence of age in the association of IOP with optic disc cupping in the prevalence analyses. However, the prospective analyses, including both refraction and age (75 years or greater) at baseline, indicated that both influence the association of baseline IOP to change in optic disc cupping. Thus, these data are compatible with the interpretation that there may be increased vulnerability of the optic nerve to a given level of IOP at older ages. We infer that since we cannot intervene on age, nor can we alter the globe size and morphology in myopia, our only therapeutic approach at present is to lower IOP. Therefore, it may be important to monitor IOP in persons 75 years of age or older, especially those with relatively high IOPs.
One limitation of our study is the loss to follow-up over the interval. This may have influenced our findings, and the direction of that influence would be hard to predict. Also, we have just one measure of IOP at each visit. In addition, we did not have a measure of corneal thickness or corneal “stiffness,” which may have an effect on IOP, although it is uncertain as to whether either would influence the relationship of IOP to change in C/D.
Another caveat to generalizing our results is that these data are from a population of Northern European descent. Whether similar associations are likely to be found in other racial or ethnic groups is not known. This would be important because the greatest health burden of changing optic cupping as a surrogate for glaucoma is found in those of sub-Saharan African descent.4 Studies such as ours should be replicated in persons with this background.
Optic disc cupping is associated with IOP. Change in cupping (C/D) in relation to IOP is especially apparent in those 75 years of age or older, and the reasons for this are unknown. These persons may benefit in regard to risk of glaucoma from surveillance of IOP.
PEER DISCUSSION
DR HUGH R. TAYLOR
This is another interesting paper from the authors’ important population-based study at Beaver Dam. Epidemiologic studies can give us information about the distribution of disease and they can also give us information about the causes and associated factors of disease.
Dr Klein’s work suggests that older people with higher intraocular pressure are more likely to have a disc change, which is an increase in optic disc cupping, than younger people who have these higher pressures, or than older people who have lower pressures. From this work she reaffirms the recommendation that people over 75 years of age “may benefit” in regard to risk of glaucoma from surveillance of intraocular pressure.
Dr Klein has looked at the association of two factors; intraocular pressure and optic disc cupping. Although longitudinal studies have much strength, they also have some weaknesses. The Beaver Dam study was started over 18 years ago and the study methodology was designed nearly 20 years ago. Since then some of our measurement techniques have changed. For example, we have learned that variation in corneal pachymetry can make a significant difference in intraocular pressure measurement and for precise pressure measurements one needs to adjust for corneal thickness. The differences are usually small, but can be up to several millimetres of mercury.
Similarly, we have learned the importance of looking at the cup: disc ratio not as an absolute value, but in terms of the overall disc diameter. A large cup in a large disc has a different significance than a large cup in a small disc. Dr Klein and colleagues have measured disc diameters, although they have not presented these data.
Neither or these shortcomings are of major significance for a general analysis, but both take on a new significance in analyses looking at very small differences. In her paper, Dr Klein found very small differences; so that over a 15 year period an increase of 1mm Hg of IOP gave just a 0.002 increase to disc ratio. Although statistically significant this is 20 to 40 times smaller than is clinically detectable, and it is over a very long time, 15 years. The clinical significance of this statistical association is not clear.
A second area of consideration is the composition of the population of people actually being analysed, “the denominator” - the number that epidemiologists and statisticians dwell on. We know that people with glaucoma will have an increase in cup diameter with time if there is slow progression. Those with higher pressures are those who are less well controlled and therefore more likely to progress. The question is therefore, have those people with glaucoma been excluded from Dr Klein’s study group, the denominator? If not they probably should be.
The whole reason for concern about disc cupping and intraocular pressure is to make sure that we detect those people who may have developed glaucoma and that we make sure they are not overlooked. For this reason the denominator needs to include all those people who are at risk of developing glaucoma and should not include those in whom glaucoma has already been diagnosed.
A final point relates to the age range of the cohort. Data from our studies have shown that the risk of developing glaucoma continues until people reach their eighties when it seems that all those who are going to develop glaucoma will have done so. In the end 9% of people will develop glaucoma. It could be interesting to explore whether the risk of developing cupping would be similar in those aged 75 to 80 and those aged 80 to 90 and 90 and above.
As Dr Klein says, the bottom line is that we need to be careful when we examine people, for signs of glaucoma, especially the elderly.
DR JERRY SEBAG
The question of the pathogenesis of glaucomatous optic neuropathy has long been of interest. The two schools of thought, one that is it is neurogenic and the other that it is vasculogenic in etiology, may be addressed by your data set, given that these are the same patients that we heard about earlier today in Dr. Ron Klein’s presentation who had retinopathy and a higher incidence of systemic hypertension during the 15-year study. Could you analyze that data set and compare it to this, meaning do the people who have retinopathy and went on to develop hypertension, have a higher progression, or a greater progression of disc cupping in your study?
DR GEORGE L. SPAETH
In your graph the change in intraocular pressure over time actually peaked and then went down. So, the highest pressure is not in the oldest group. The cupping, in contrast, went almost flat and then went up rapidly, while the intraocular pressure was falling. How do you explain that? What percentage of your patients, in your eldest group, had received cataract extraction? Estimating cup to disc ratio in phakic patients and aphakic patients is very different. In the phakic patient the change in color, caused by the lens opacity, frequently makes the cup look much smaller. But, when your remove the cataract, the cup looks much bigger. Perhaps the apparent larger cup in the oldest group is merely an artifact related to the fact that they were probably more pseudo-phakic patients in that group. Also, I believe the neuroretinal rim becomes narrower with age just as a part of “normal” aging.
DR BARBARA E. KLEIN
With regard to Dr. Taylor’s comments, I do not mean to imply that the change in cupping that we find across the population itself is clinically significant. In fact, all across the 15-year follow-up for the entire age range, the change in cupping was small. Presumably, that is weighted by the slightly higher change in the older age group, which is not meant to suggest clinical significance, only to suggest that it may be an important clue to what’s going on in older people. It is meant to say “monitor people who are of this age group” as glaucoma may be more common in older people. We have not eliminated persons with glaucoma because we are interested in the population perspective. We view the phenomenon of increasing optic cup diameters with increasing IOP to be the most important mechanism in nerve fiber loss even before achieving the arbitrary diagnostic criteria for glaucoma.
Dr. Sebag asked about the influence of hypertension. We did not dichotomize or categorize the blood pressure level, but we did include blood pressure as a continuous variable. In that regard, there did not appear to be an affect of blood pressure on this relationship.
With respect to Dr. Spaeth’s comments, we found that cataract surgery had no effect on our findings.
REFERENCES
- 1.Leske MC. The epidemiology of open-angle glaucoma: a review. Am J Epidemiol. 1983;118:166–191. doi: 10.1093/oxfordjournals.aje.a113626. [DOI] [PubMed] [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 3.Klein BE, Klein R, Jensen SC. Changes in the optic disc over a five-year interval: the Beaver Dam Eye Study. Curr Eye Res. 1997;16:738–740. doi: 10.1076/ceyr.16.7.738.5062. [DOI] [PubMed] [Google Scholar]
- 4.The Eye Disease Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134:1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Linton KL, et al. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BEK, Lee KE, et al. Changes in visual acuity in a population over a 15-year period. The Beaver Dam Eye Study. Am J Ophthalmol. 2006 doi: 10.1016/j.ajo.2006.06.015. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–2228. [PubMed] [Google Scholar]
- 11.Diabetic Retinopathy Study Research Group. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21:210–226. [PubMed] [Google Scholar]
- 12.Klein BE, Magli YL, Richie KA, et al. Quantitation of optic disc cupping. Ophthalmology. 1985;92:1654–1656. doi: 10.1016/s0161-6420(85)34085-x. [DOI] [PubMed] [Google Scholar]
- 13.SAS Institute Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute Inc; 1999.
- 14.Wang Q, Klein BE, Klein R, et al. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994;35:4344–4347. [PubMed] [Google Scholar]
- 15.Leske MC, Connell AM, Wu SY, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. Arch Ophthalmol. 2001;119:89–95. [PubMed] [Google Scholar]
- 16.Armaly MF. Ocular pressure and visual fields. A ten-year follow-up study. Arch Ophthalmol. 1969;81:25–40. doi: 10.1001/archopht.1969.00990010027005. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MR, Martone JF. Epidemiology of chronic open-angle glaucoma and ocular hypertension. In: Ritch R, Shields MB, Krupin T, eds. The Glaucomas: A Multi-Volume Reference. St Louis: Mosby-Year Book; 1996:753–768.
- 18.Wong TY, Klein BE, Klein R, et al. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. 2003;110:211–217. doi: 10.1016/s0161-6420(02)01260-5. [DOI] [PubMed] [Google Scholar]
- 19.Podos SM, Becker B, Morton WR. High myopia and primary open-angle glaucoma. Am J Ophthalmol. 1966;62:1038–1043. [PubMed] [Google Scholar]
- 20.Mastropasqua L, Lobefalo L, Mancini A, et al. Prevalence of myopia in open angle glaucoma. Eur J Ophthalmol. 1992;2:33–35. doi: 10.1177/112067219200200108. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell P, Hourihan F, Sandbach J, et al. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 22.David R, Zangwill LM, Tessler Z, et al. The correlation between intraocular pressure and refractive status. Arch Ophthalmol. 1985;103:1812–1815. doi: 10.1001/archopht.1985.01050120046017. [DOI] [PubMed] [Google Scholar]
- 23.Daubs JG, Crick RP. Effect of refractive error on the risk of ocular hypertension and open angle glaucoma. Trans Ophthalmol Soc U K. 1981;101:121–126. [PubMed] [Google Scholar]
- 24.Tomlinson A, Phillips CI. Ratio of optic cup to optic disc. In relation to axial length of eyeball and refraction. Br J Ophthalmol. 1969;53:765–768. doi: 10.1136/bjo.53.11.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahane M, Bartov E. Axial length and scleral thickness effect on susceptibility to glaucomatous damage: a theoretical model implementing Laplace’s law. Ophthalmic Res. 1992;24:280–284. doi: 10.1159/000267179. [DOI] [PubMed] [Google Scholar]
- 26.Chihara E, Sawada A. Atypical nerve fiber layer defects in high myopes with high-tension glaucoma. Arch Ophthalmol. 1990;108:228–232. doi: 10.1001/archopht.1990.01070040080035. [DOI] [PubMed] [Google Scholar]
- 27.Jonas JB, Dichtl A. Optic disc morphology in myopic primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1997;235:627–633. doi: 10.1007/BF00946938. [DOI] [PubMed] [Google Scholar]
- 28.Jonas JB, Gusek GC, Naumann GO. Optic disk morphometry in high myopia. Graefes Arch Clin Exp Ophthalmol. 1988;226:587–590. doi: 10.1007/BF02169209. [DOI] [PubMed] [Google Scholar]