Abstract
Purpose
To examine the extent to which changes in the latency of the multifocal visual evoked potential (mfVEP) overlap in patients with glaucoma, recovered optic neuritis/multiple sclerosis (ON/MS), and retinal disease.
Methods
Monocular mfVEPs were obtained for both eyes of all subjects. Latencies and amplitudes of individual mfVEP responses were measured using custom software and expressed relative to a normative group (n = 100). Recordings were obtained from patients with ON/MS (n = 12), glaucoma (n = 50), and retinal disease (n = 15), as well as control subjects (n = 50). All subjects had 24-2 visual fields; patients with retinal disease had multifocal electroretinograms (mfERGs). The patients with retinal disease were examined by a neuro-ophthalmologist to rule out optic nerve disease and, in general, had relatively subtle or unremarkable fundus examinations but abnormal mfERG amplitudes.
Results
There was considerable overlap in the latencies for the patient groups for both monocular and interocular measures of mfVEP latency. This was particularly true for the patients with retinal disease and ON/MS, for whom the range of latencies was almost identical, as was the percentage of points in the field showing significant delays.
Conclusion
The mfVEP delays seen in patients with retinal disease are similar in magnitude and prevalence to those seen in patients with a history of ON/MS. In general, this does not present a problem when using the mfVEP in the clinic. However, a retinal problem can be confused with ON/MS or, in fact, dismissed as functional, especially if the fundus appears normal.
INTRODUCTION
With the multifocal technique, visual evoked potentials (VEPs) can be recorded simultaneously from many regions of the visual field. The multifocal VEP (mfVEP) technique1 has generated considerable interest, especially among those seeking objective measures of glaucomatous damage,2–7 as well as those seeking to detect and follow visual abnormalities secondary to optic neuritis/multiple sclerosis (ON/MS).3,8–11
Recently, the usefulness of the mfVEP technique has been extended with the development of an automated, computerized method of measuring the latency of the local mfVEP responses.12,13 Traditionally, delays in the conventional VEP have been taken as a sign of demyelinization, typically secondary to an attack of ON/MS,14–16 with recent studies8–11 suggesting that the mfVEP is more effective in identifying demyelinating events. However, using the new automated method for measuring mfVEP latency, the results of three recent studies17,18 from the authors’ laboratory raise questions about the simple correspondence between delayed mfVEP responses and ON/MS. In this report, the data from these three studies are reanalyzed and compared.
First, Yang and colleagues (Yang B et al, IOVS Annual Meeting, 2005, Abstract) found that mfVEP latency recovered over time in 10 of the 14 eyes with ON/MS they studied. They did not report how many returned to the normal latency range, although they do show the results for one patient who completely recovered. In any case, if the latency of the mfVEP is recovering in patients with long-term ON/MS, then the simple correspondence between delayed mfVEP responses and ON/MS is open to question.
Second, Rodarte and colleagues17 reported increases in mfVEP latency due to glaucomatous damage. Although these delays were smaller than those found in some conventional VEP studies,19–21 they were as large as 10 ms in some patients. Thus, glaucoma can also produce prolonged VEP latencies in some patients, and again this raises the question of the uniqueness of ON/MS in producing prolonged mfVEPs.
Finally, Chen and colleagues18 found that patients with retinal diseases, which leave the receptors functioning, can show mfVEPs delayed by as much as 25 ms. Surprisingly, these mfVEPs can have relatively normal amplitudes even though local multifocal electroretinograms (mfERGs) are markedly depressed. Again, the possibility exists that the delays seen with retinal disease may be confused with those seen with ON/MS.
To examine the extent to which the prolonged latency of the mfVEP with recovered ON/MS is quantitatively similar to that seen with glaucoma and retinal disease, the results from these studies were analyzed.17,18 The implications for clinical diagnoses are considered in the “Discussion” section.
METHODS
SUBJECTS
Procedures followed the tenets of the Declaration of Helsinki, and the protocol was approved by the Committee of the Institutional Board of Research of Columbia University. Patients with ON/MS (n = 12; age, 39.8 ± 11.9 years), glaucoma (n = 50; age, 58.8 ± 12 years), and retinal diseases (n = 15; age, 66.6 ± 14.6 years) as well as 50 controls (age, 50.4 ± 10.3 years) took part in this study. Fourteen of the 24 eyes of the patients with ON/MS had confirmed acute attacks of ON with MS confirmed on magnetic resonance imaging. All were in the recovery phase and were tested on average 20 months (range, 8 to 63 months) after the acute attack. Of the 50 patients with glaucoma, 25 had high-tension and 25 had normal-tension glaucoma. All had mild to moderate field losses (MD, < −8 dB). Sixty-four eyes of the 50 patients were classified as glaucomatous (affected) based upon abnormal visual fields and typical glaucomatous cupping.
The patients with retinal disease were seen by a neuro-ophthalmologist to rule out optic nerve vs retinal disease and generally had relatively subtle or unremarkable findings on fundus examination. The diagnoses included autoimmune retinopathy (3 patients), branch retinal arterial occlusion (3), branch retinal vein occlusion (1), vitamin A deficiency (1), digoxin and/or age-related macular degeneration (1), multiple evanescent white dot syndrome (1), and nonspecific retinal disease (5). None of these patients had a disease such as retinitis pigmentosa, known to affect large numbers of photoreceptors. In addition, the 15 patients included here had abnormal mfERG amplitudes in 25% or more of the central 45°. Twenty-one of their eyes were affected. All subjects had static automated perimetry (program 24-2, Humphrey Zeiss) and mfVEP tests; the patients with retinal disease also had mfERGs. More information about these patients is available in previously published work.17,18
MFVEP RECORDING AND ANALYSIS
The stimulus—a scaled, dartboard display, 44.5° of visual angle in diameter—was produced by VERIS software from EDI (Electro-Diagnostic Imaging, San Mateo, California) and contained 60 sectors, each with 16 checks, eight white (200 cd/m2) and eight black (< 1 cd/m2). Three channels of recording were obtained using gold cup electrodes. Recording electrodes were placed at the inion, 4 cm above the inion, and at two lateral locations up 1 cm and over 4 cm from the inion. By subtracting different combinations of pairs of channels, three additional “derived” channels were obtained. The VEP was recorded with cutoffs set at 3 Hz and 100 Hz (1/2 amplitude; Grass preamplifier P511J, Quincy, Massachusetts). Two 7-minute recordings were obtained for monocular stimulation of each eye. For each eye, the two recordings were averaged and the mfVEP responses extracted with the VERIS 4.x software from EDI. In addition, the mfVEPs were low-pass-filtered (sharp cutoff at 35 Hz) and analyzed off-line with programs written in MATLAB (Mathworks Inc, Natick, Massachusetts). All analyses were performed on the responses from the best channel. Details are available in previously published work.3,7
The timing of all responses meeting a criterion amplitude, based upon the signal-to-noise ratio (SNR), were analyzed. For each individual and each eye, the relative monocular latency and the interocular difference in latency were determined for each of the 60 locations meeting this criterion and compared with norms based upon a group of 100 controls22 using computerized techniques previously described.12,13 For the monocular analysis, only the affected eyes were analyzed. This included all eyes classified as glaucomatous (glaucoma group), eyes with retinal disease (retinal disease group), or an eye that had an acute attack of ON (ON/MS group), as described above. For the interocular analysis, the absolute value of the interocular latency difference was determined. In addition, the amplitude (SNR) of the monocular responses and the percent of these responses that were significantly delayed were also determined.
RESULTS
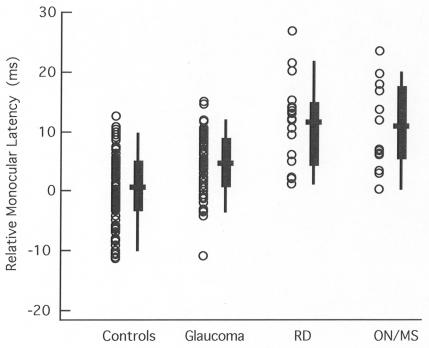
Figure 1 shows the monocular latency data for the control group and for the affected eyes of the three patient groups. Each data point represents the mean relative latency for an individual eye. For the summary whisker plots, the 25% to 75% range and the 5% to 95% range are shown by the box and lines, respectively, with the bold horizontal bar indicating the mean. The retinal disease and ON groups clearly had the longer latencies. However, there is considerable overlap among all groups, with the retinal disease and ON groups showing extremely similar ranges of latencies.
FIGURE 1.
Average monocular latency of the multifocal visual evoked potential (mfVEP) is shown relative to a normative group for individual eyes (symbols) of the control (n = 100 eyes), glaucoma (n = 64), retinal disease (RD) (n = 21), and optic neuritis/multiple sclerosis (ON/MS) (n = 14) groups. For the patient groups, only the affected eye is included. The box plot shows the 25% to 75% range (box), the 5% to 95% range (vertical line), and the mean of the group (horizontal bar).
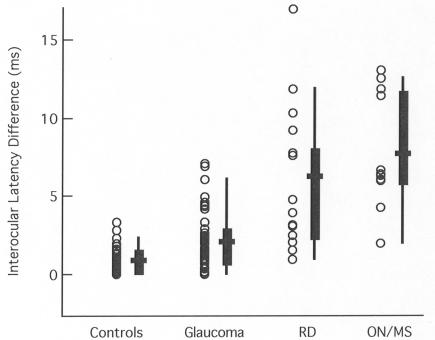
The interocular latency difference between the two eyes is shown in Figure 2. Each point is the absolute difference in interocular latencies for an individual. There is less overlap on the interocular plots than for the monocular data in Figure 1, with more of the individual points for the retinal disease and ON groups falling above the 95% confidence limits for both the controls and the glaucoma patients. However, there is still nearly complete overlap of the data for the retinal disease and ON/MS groups.
FIGURE 2.
The absolute value of the average interocular latency difference of the multifocal visual evoked potential (mfVEP) is shown for individual eyes (symbols) of the control (n = 50 individuals), glaucoma (n = 50), retinal disease (RD) (n = 15), and optic neuritis/multiple sclerosis (ON/MS) (n = 12) groups. The box plot shows the 25% to 75% range (box), the 5% to 95% range (vertical line), and the mean of the group (horizontal bar).
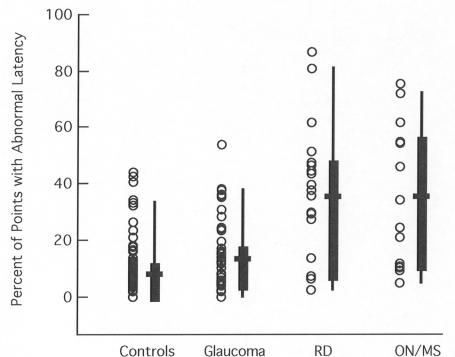
Although the mean latencies of the retinal disease and ON/MS groups overlap, it is possible that the extent of the field showing delayed mfVEPs differs. Figure 3 indicates that this is not the case. The percent of the points showing abnormal latencies is nearly the same for these two groups.
FIGURE 3.
The percent of the points with significantly (5% level) prolonged multifocal visual evoked potential (mfVEP) latencies is shown for individual eyes (symbols) of the control (n = 100 eyes), glaucoma (n = 64), retinal disease (RD) (n = 21), and ON/MS (n = 14) groups. For the patient groups, only the affected eye is included. The box plot shows the 25% to 75% range (box), the 5% to 95% range (vertical line), and the mean of the group (horizontal bar).
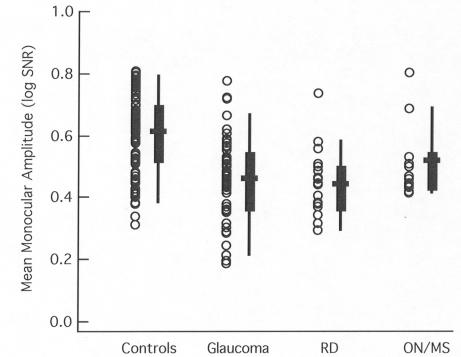
Finally, the monocular amplitudes are smaller than for the controls for all three patient groups, as can be seen in Figure 4. Although the amplitudes tended to be larger for the ON/MS group, again there was considerable overlap among the groups.
FIGURE 4.
The average monocular amplitude (SNR) of the multifocal visual evoked potentials (mfVEP) is shown for individual eyes (symbols) of the control (n = 100 eyes), glaucoma (n = 64), retinal disease (RD) (n = 21), and ON/MS (n = 14) groups. For the patient groups, only the affected eye is included. The box plot shows the 25% to 75% range (box), the 5% to 95% range (vertical line), and the mean of the group (horizontal bar).
DISCUSSION
Delays in the mfVEP have been taken as an indication of local demyelinization.8–11 To be useful in the clinic, it is important to determine the extent to which other disease processes can result in comparable delays in the mfVEP. Thus, this study examined the extent to which mfVEP latency changes overlap in patients with glaucoma, recovered ON/MS, and retinal disease. Because delays in both conventional and mfVEPs have been associated with ON/MS, the authors were particularly interested in the recent findings that showed, on one hand, that the mfVEP can recover in latency and, on the other hand, that glaucoma, and in particular retinal disease, can result in prolonged mfVEP latencies. In fact, the results here indicate that the eyes with retinal disease and ON/MS had the same range of monocular and interocular latencies and the same range of percent of points in the field showing abnormal latency.
In general, these results do not pose a problem for the use of the mfVEP as a diagnostic tool. First, glaucoma is easy to distinguish from retinal disease and ON/MS. In the case of glaucoma, the amplitude of the mfVEP is used clinically to confirm field defects in patients for whom the results of static automated perimetry are difficult to interpret, not as a diagnostic tool.7 Similarly, retinal disease can be distinguished from ON/MS based upon a variety of clinical criteria. However, many of the retinal patients included here had normal fundus examinations.18 Thus, in principle, retinal disease could be confused with ON in a patient with a normal-appearing fundus and a markedly delayed mfVEP. Figure 5 illustrates this point.
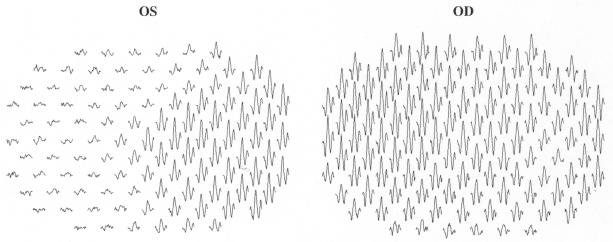
FIGURE 5.
The multifocal electroretinograms from a patient initially thought to have optic neuritis/multiple sclerosis.
The mfERGs in Figure 5 are from a 35-year-old woman who complained of seeing gray in her left eye. She had no pain on eye movement. Because of her history and the presence of a relative afferent pupillary defect in the affected eye, she was initially thought to have ON/MS. Delayed mfVEP responses were consistent with this diagnosis. However, the mfERGs clearly indicated that her problem was retinal, and a diagnosis of MEWDS was made.
In summary, the mfVEP delays seen in some patients with retinal disease are similar in magnitude, and in prevalence across the visual field, to those seen in patients with a history of ON/MS. In general, this does not present a problem when using the mfVEP in the clinic. However, a retinal problem can be confused with ON/MS or, in fact, dismissed as functional/nonorganic,18 especially if the fundus has a normal appearance.
PEER DISCUSSION
DR ALFREDO A. SADUN
Dr Donald Hood is well known as one of the developers and seminal investigators of the multifocal visual evoked potential. This is an exciting technology that is currently being applied to a variety of ophthalmologic and neuro-ophthalmologic diseases. In the present work Dr. Hood describes the use of this exciting technology in discriminating and understanding optic nerve and retinal diseases.
The mfVEP has latency delays that overlap considerably in the various groups studied. Generally speaking, the mfVEP in glaucoma looked pretty close to that seen in controls, and that seen in retinal disease looked not unlike optic neuritis. In short this technique by itself could not discriminate between optic neuritis of the sort associated with multiple sclerosis and retinal disease. The authors wisely suggest that other assessments are needed to make such a distinction. There remain a few technical points and questions.
The patients who had optic neuritis/MS were younger than the other groups. Since we know that VEP latencies get longer with age, could this be a minor confound?
In patients who had latency delays, how did this correlate with their specific visual field losses? It might be interesting to look at specific mfVEP plots illustrating the most dramatic recordings.
What does an increase in mfVEP latency mean physiologically? The usual explanation is that delayed conduction is due to demyelination. This makes sense and is certainly largely true. Axons missing myelin are no longer capable of saltatory regenerative conduction and the axon potential must travel a slower continuous route. Demyelination also means that the greater number of sodium channels required increases the axon’s metabolic needs and produces a longer refractory period. However this is not the whole story. Neurons, including retinal ganglion cells, have electrical cable properties. This means that the branching structures of a neuron’s dendrites, as well as the distribution of voltage-gated channels, integrates the input coming to each neuron. This integration is both “temporal”--that is the summation of stimuli in rapid succession, and “spatial”--that is the aggregation of excitatory and inhibitory inputs from separate branches. Delayed latencies can occur as a result of stimulus impoverishment to the retinal ganglion cell (for example from loss of photoreceptors and/or bipolar cells). Recovery of latency delays can be due to the effects of remodeling of the dendrites and this effect on the electrical cable properties.
In patients with optic neuritis, subsequent improvement in symptoms, recovery of vision, and improvement in the mfVEP latency delay can therefore be attributed to remyelination, to myelin remodeling that reduces the number of nodes of Ranvier, to local increases in sodium channels, and changes in the dendrite cable properties, most particularly the elaboration of new dendrites and the profusion and placement of new synapses.
The mfVEP and mfERG work of Hood and colleagues brings us closer to understanding the complexities of neuronal processing, particularly involving retinal ganglion cells.
DR ALLAN J.FLACH
What were the 18 retinal diseases? In particular, the ones that were not visible within an ophthalmoscopic examination?
DR GEORGE L. SPAETH
We’ve been working with this same methodology and studying glaucoma patients with high pressures and low pressures with both distance and white on white perimetry. These were patients who had marked pressure lowering, from pressure’s about 30 to 40 range, and then falling to the 20’s or lower. The patient’s VEP will quite routinely showed a significant improvement but this did not show up on the white on white perimetry. How would you explain that?
DR ARTHUR JAMPOLSKY
"In all sensory inputs, there is a physiologic asymmetry. In pathology, the defect in the poorer eye is exaggerated under simultaneous bilateral stimulation. The question is, have you measured any of these parameters under simultaneous bilateral stimulation and have you found any exaggeration in the asymmetry findings?"
DR DONALD C. HOOD
To answer Dr Jampolsky’s question, we have tried binocular stimulation but not with patients.
Dr Sadun asked whether I was concerned about the age difference between the optic neuritis and the control groups, because the optic neuritis patients were 10 years younger. The answer is no, for two reasons. First, the comparisons we are making are within eye comparisons and this does not seem to be age-dependent. Second, more generally, age is not a large factor in the case of the latency of the multi-focal VEPs, as we have already published. In the monocular case, it’s about 1.3 milliseconds per decade and in the interocular case, it is about 0.1 milliseconds per decade.
Concerning latency changes and visual field defects in glaucoma, there is a very weak correlation (r2=.04). In the optic neuritis patients, all had recovered fields, so you would not expect a good correlation. However, if you go back to the acute phase, wherever you have delays in the recovery phase, you have field defects in the acute phase. So you the correlation would be better between field loss in the acute phase and latency in the recovered phase. The retina patients are more complicated because you do not find, nor do we expect to find, delays in all retina patients. To see delays, the patients must have functioning retinas, but it is as if the gain has been turned down. If you have a retina in which you have destroyed large parts of the receptors you would not expect responses in those areas. So, overall, the correlation does not look good. But if you only look at the patients that have delays, then there’s a very strong positive correlation with the worst field having the longer delays. This is true for the ERG as well as the VEP.
Regarding the mechanism, we do not think we are looking at remodeling, although that’s a possibility. It’s more likely that the denuded axon is recovering sodium channels or the sodium pump is adjusting. There could be changes at the synapse as well.
Dr Flach asked about the other retinal diseases in the patients. They had a variety of problems. Five of them were autoimmune problems and five were non-specified and possibly also autoimmune. And there was also MEWS and branch retinal artery occlusion.
With regard to Dr Spaeth’s question, we published a paper on IOVS about two years ago about the conditions under which one would expect the fields and the VEPs to differ. We find, to a first approximation, a remarkable agreement between field changes and VEPs. We have a paper in the Archives of Ophthalmology on the quantitative relationship. There are conditions under which the VEPs pick up differences that the visual field misses, and there are conditions where the reverse is true. Before I concluded that the IOP lowering showed up in the VEP but not in the field, I would want to know more about the signal size and where these defects were, because these are some of the factors that determine whether one technique is more sensitive than the other.
ACKNOWLEDGMENT
We thank Dr John Mitchell for referring the patient whose records are shown in Figure 5.
REFERENCES
- 1.Baseler HA, Sutter EE, Klein SA, et al. The topography of visual evoked response properties across the visual field. Electroenceph Clin Neurophysiol. 1994;90:65–81. doi: 10.1016/0013-4694(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 2.Klistorner AI, Graham SL, Grigg JR, et al. Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci. 1998;39:937– 950. [PubMed] [Google Scholar]
- 3.Hood DC, Zhang X, Greenstein VC, et al. An interocular comparison of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci. 2000;41:1580–1587. [PubMed] [Google Scholar]
- 4.Hasegawa S, Abe H. Mapping of glaucomatous visual field defects by multifocal VEPs. Invest Ophthalmol Vis Sci. 2001;42:3341–3348. [PubMed] [Google Scholar]
- 5.Goldberg I, Graham SL, Klistorner AI. Multifocal objective perimetry in the detection of glaucomatous field loss. Am J Ophthalmol. 2002;133:29–39. doi: 10.1016/s0002-9394(01)01294-6. [DOI] [PubMed] [Google Scholar]
- 6.Fortune B, Goh K, Demirel S, et al. Detection of glaucomatous field loss using Multifocal VEP. In: Perimetry Update 2002/2003 The Hague, Netherlands: Kugler; 2004:251–260.
- 7.Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22:201–251. doi: 10.1016/s1350-9462(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 8.Hood DC, Odel JG, Zhang X. Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci. 2000;41:4032–4038. [PubMed] [Google Scholar]
- 9.Kardon, RH, Givre SJ, Wall M, et al. Comparison of threshold and multifocal-VEP perimetry in recovered optic neuritis. In: Wall M, Mills RP, eds: Perimetry Update 2000/2001: Proceedings of the XVIIth International Perimetric Society Meeting New York: Kugler:19–28.
- 10.Ruseckaite R, Maddess T, Danta G, et al. Sparse multifocal stimuli for the detection of multiple sclerosis. Ann Neurol. 2005;57:904–913. doi: 10.1002/ana.20504. [DOI] [PubMed] [Google Scholar]
- 11.Hood DC. Electrophysiologic imaging of retinal and optic nerve damage: the multifocal technique. Ophthalmol Clin North Am. 2004;17:69–88. doi: 10.1016/S0896-1549(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 12.Hood DC, Zhang X, Rodarte C, et al. Determining abnormal interocular latencies of multifocal visual evoked potentials. Doc Ophthalmol. 2004;109:177–187. doi: 10.1007/s10633-004-5511-1. [DOI] [PubMed] [Google Scholar]
- 13.Hood DC, Ohri N, Yang EB, et al. Determining abnormal latencies of the multifocal visual evoked potentials: a monocular analysis. Doc Ophthalmol. 2004;109:189–199. doi: 10.1007/s10633-004-5512-0. [DOI] [PubMed] [Google Scholar]
- 14.Halliday AM, McDonald EI, Mushin J. Visual evoked response in diagnosis of multiple sclerosis. Br Med J. 1973;1:661–664. doi: 10.1136/bmj.4.5893.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederiksen JL, Petrera J. Serial visual evoked potentials in 90 untreated patients with acute optic neuritis. Surv Ophthalmol. 1999;44(Suppl 1):S54–62. doi: 10.1016/s0039-6257(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 16.Holder GE. Multiple sclerosis. In: Heckenlively JR, Arden GB, eds. Principles and Practice of Clinical Electrophysiology of Vision. St Louis: Mosby Year Book; 1991:797–805.
- 17.Rodarte C, Hood DC, Yang EB, et al. The effects of glaucoma on the latency of the multifocal visual evoked potential. Br J Ophthalmol. 2006 doi: 10.1136/bjo.2006.095158. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood DC, Odel JG, et al. The effects of retinal abnormalities on the multifocal visual evoked potential. Invest Chen JY Ophthalmol Vis Sci. 2006 doi: 10.1167/iovs.06-0242. In press. [DOI] [PubMed] [Google Scholar]
- 19.Atkin A, Bodis-Wollner I, Podos SM, et al. Flicker threshold and pattern VEP latency in ocular hypertension and glaucoma. Invest Ophthalmol Vis Sci. 1983;24:1524–1528. [PubMed] [Google Scholar]
- 20.Towle VL, Moskowitz A, Sokol S, et al. The visual evoked potential in glaucoma and ocular hypertension: effects of check size, field size, and stimulation rate. Invest Ophthalmol Vis Sci. 1983;24:175–183. [PubMed] [Google Scholar]
- 21.Parisi V, Miglior S, Manni G, et al. Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology. 2006;113:216–228. doi: 10.1016/j.ophtha.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Fortune B, Zhang X, Hood DC, et al. Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol. 2004;109:87–100. doi: 10.1007/s10633-004-3300-5. [DOI] [PubMed] [Google Scholar]