Abstract
Despite extensive similarities between the genomes of the Streptomyces temperate phages φC31 and φBT1, the attP-int loci are poorly conserved. Here we demonstrate that φBT1 integrates into a different attachment site than φC31. φBT1 attB lies within SCO4848 encoding a 79-amino-acid putative integral membrane protein. Integration vectors based on φBT1 integrase were shown to have a broad host range and are fully compatible with those based on the φC31 attP-int locus.
The attP-int locus from φC31 has been heavily exploited in the construction of versatile, low-copy-number, and convenient vectors for use in a broad range of Streptomyces species (5, 10). Despite their wide use and clear advantages, it has been reported that integration of these vectors into the φC31 attB site can cause detrimental effects on antibiotic production in some strains (2). φC31 integrates intragenically into SCO3798, a highly conserved gene in prokaryotes and eukaryotes but is not essential for the growth of Streptomyces coelicolor in the laboratory (8). Although some phages can regenerate a functional gene after insertion (7), there is no evidence that this is the case with φC31. Furthermore, a vector, pSET152 containing the φC31 attP-int locus, introduced by conjugation from Escherichia coli can integrate into secondary or pseudo-attB sites in both S. coelicolor and Streptomyces lividans (8). The reported reductions in antibiotic synthesis could be caused by insertional mutagenesis into SCO3798 or by integration into one of the pseudo-attB sites or some other factor. Another potential problem with integrating vectors could be the absence of an efficiently recognized attB site in some streptomycete strains. Indeed, Saccharopolyspora erythraea appears to lack a φC31 attB site (P. Leadlay, personal communication). For these reasons and as many workers would like to use two compatible integrating vectors in the same organism, we have investigated the integration site of the Streptomyces phage φBT1, a homoimmune relative of φC31. We demonstrate that φBT1 does indeed integrate into a different attB site in S. coelicolor, and we have constructed novel integrating vectors derived from the φBT1 attP-int locus.
The organization of the φBT1 genome is highly similar to that of φC31, and the majority of gene products are closely related (9). There is evidence, however, of mosaicism between the two genomes where DNA has been inserted and/or deleted in one genome but not in the other, and there are sudden transitions in the level of sequence similarity (9). One of the most noticeable differences is the relatively poor sequence similarity of int and the three genes upstream, genes 26 to 28. φBT1 integrase and gp26 to gp28 exhibit 26% and 10 to 18% identity to their φC31 homologues, respectively. Despite this poor similarity, φBT1 integrase is clearly a member of the large serine recombinase family, as it contains conserved motifs present in other members of this group (12). Furthermore, no significant similarity could be detected between the φC31 attP site and any φBT1 sequence. These observations strongly suggest that φBT1 encodes a site-specific recombination system that has a different specificity from that in φC31 and therefore integrates into a different attB site in the S. coelicolor genome. Southern blots of DNA from an S. coelicolor J1929 φBT1 lysogen (strain J1929 contains ΔpglY conferring sensitivity to φC31 and φBT1 [3]) probed with DNA encoding the φC31 attB site indicated that φC31 attB was intact, suggesting that φBT1 was integrated elsewhere in the genome (data not shown). To test this further and to identify the φBT1 attB site, we performed vectorette PCR (Sigma-Genosys) extending outwards from the φBT1 DNA into the host DNA in an S. coelicolor φBT1 lysogen. This procedure is designed to isolate unknown flanking sequences from a known integrated sequence (1). One product was obtained using S. coelicolor J1929 φBT1 DNA digested with BclI as the original template. The DNA sequence of this product read outwards from the φBT1 genome into a segment of the S. coelicolor genome sequence and finally into the vectorette linker sequence. The S. coelicolor genome sequence matched part of cosmid SC5G8 (4). To confirm the site of φBT1 integration, primers were designed against this region of SC5G8 and used to amplify attB from S. coelicolor J1929 DNA and attL and attR from J1929 φBT1 lysogen DNA. The attB sequence was most similar to the genomic sequence coordinates 5279863 to 5280017 (contained within SC5G8), and the attL and attR sequences indicated that the recombination had occurred between 9 bp of identical sequence between S. coelicolor coordinates 5279923 to 5279915 and φBT1 (Fig. 1). We confirmed that this locus is the major integration site by Southern blotting of genomic DNA isolated from S. coelicolor J1929 containing integration vector pMS81 or pMS82 described below; no bands other than those predicted as a consequence of integration via the attB site were observed (data not shown).
FIG. 1.
Integration of φBT1 in S. coelicolor A3(2). (A) DNA sequences of φBT1 attP and attB sites. Bases that are identical in the attB and attP sites are shown in black type. The imperfect inverted repeats are indicated by arrows. The crossover site occurs within the region shown in black bold type. The GenBank accession number for the complete φBT1 genome is AJ550940. (B) Consequence of φBT1 integration on the predicted protein sequence of SCO4848 containing attB. SCO4848 is the native predicted protein sequence, and SCO4848int is the sequence after φBT1 integration. In SCO4848int, the N-terminal 29 amino acids are predicted from the antisense strand of the int gene, and the site of interruption of SC04848 is shown as a vertical arrowhead. Three possible initiating methionines in SCO4848int are underlined. The amino acids in bold type are predicted by PHDhtm (11) to be membrane-spanning alpha helices. The functional consequences of φBT1 integration on SCO4848 have not been tested experimentally.
An alignment of the φBT1 attB site and the phage attP site indicated that, like the φC31 recombination system, the attB and attP sequences are quite different. The φBT1 attP site contains an imperfect inverted repeat centered around the dinucleotide 5′GT within the 9-bp core sequence (Fig. 1). In other phages or prophages encoding serine integrases, the attP sites also contain imperfect inverted repeats that in φC31, TP901-1, and φRv1 are symmetrical around the 2-bp sequence at which crossover occurs (6, 8).
The φBT1 attB site lies approximately 1 Mb to the right of oriC compared to the φC31 attB site, which is approximately 90 kbp to the left of oriC. The φBT1 attB site lies within SCO4848 coding for a putative integral membrane protein. This protein contains two predicted membrane-spanning alpha helices (Fig. 1). Analysis of the attL sequence indicates that an alternative protein coding sequence, SCO4848int, is generated after φBT1 integration, which still retains an N-terminal putative membrane-spanning alpha helix (Fig. 1). Thus, if SCO4848int is transcribed, an active gene product may produced even after φBT1 integration and which may, therefore, confer a neutral phenotype.
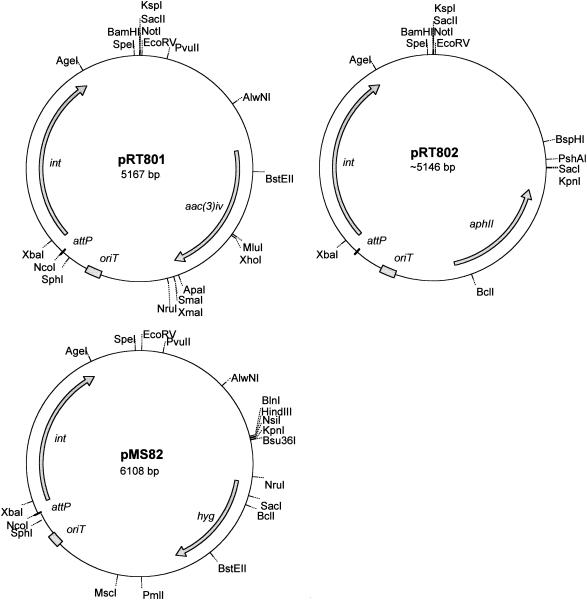
For this reason and in order to provide additional integration vectors, we constructed integrating plasmids similar in design to pSET152 except that they integrate via φBT1 attP-int (3). pRT801 (Fig. 2) was constructed in two steps. PCR was used to amplify the φBT1 int-attP region from phage particles in the presence of primers RT26 (5′ ATT AGC ATG CTG GCG CCG GAC GGG GCT TCA GAC) and RT27 (5′ TAA TGG ATC CGC TCC CTG CCC GCT GTG GTG AC) and Pfu polymerase, using the manufacturer's recommended procedures (Stratagene). The PCR fragment and pGEM7 (Promega) were cut with BamHI and SphI, ligated to form pRT800, and the attP-int region was sequenced. The φBT1 attP-int- containing fragment was then used to replace the φC31 attP-int sequences in pSET152 using the restriction sites BamHI and SphI to form pRT801. The plasmids pRT801 and pSET152 were introduced into the nonmethylating E. coli strain ET12567 (containing pUZ8002, required to provide the transfer functions [10]), and these strains were used as donors in conjugations with S. coelicolor J1929. Comparable numbers of apramycin-resistant transconjugants of S. coelicolor J1929 were obtained with E. coli ET12567(pUZ8001, pRT801) and with ET12567(pUZ8001, pSET152) (Table 1).
FIG. 2.
Integration vectors derived from the φBT1 attP-int locus for use in Streptomyces species.
TABLE 1.
Numbers of apramycin-resistant transconjugants obtained with different E. coli donors and Streptomyces recipients
| E. coli donor | Streptomyces recipient | No. of apramycin- resistant transconjugants/ spore |
|---|---|---|
| ET12567(pUZ8002, pSET152) | J1929 | 5.0 × 10−3 |
| ET12567(pUZ8002, pRT801) | J1929 | 3.5 × 10−3 |
| S17-1(pSET152) | TK24 | 5.0 × 10−4 |
| S17-1(pSET152) | TK24(pRT802) | 9.4 × 10−4 |
To ensure that the φBT1 attP-int-based vectors were compatible with pSET152 and to assay the frequency of integration into the S. lividans genome, pRT802 was constructed containing the aphII gene from Tn5 to replace the apramycin resistance marker (Fig. 2). pRT802 was constructed by inserting a 1,327-bp fragment from pNRT4 (encoding the aphII gene from Tn5 and kindly provided by P. Herron, University of Swansea) cut with HindIII, blunt ended with Klenow fragment, and then cut with SacI and inserted into pRT801 cut with NruI and SacI. It should be noted that during the construction of pRT802, an approximately 600-bp deletion was introduced in the oriT region (S. Ward, personal communication). pRT802 and pSET152 were introduced separately into S. lividans TK24 by conjugation from E. coli S17-1(pRT802) or S17-1(pSET152), and similar numbers of kanamycin- and apramycin-resistant colonies were obtained (data not shown). S. lividans TK24 or TK24(pRT802) was then used as the recipient for the introduction of pSET152 from E. coli S17-1(pSET152), and similar numbers of apramycin-resistant colonies were obtained in both recipients (Table 1). Thus, the φBT1 attP-int-containing vectors are transferred efficiently to both S. coelicolor and S. lividans, and integration of pSET152 is not inhibited by the presence of pRT802.
The broad host range of pRT801 was demonstrated by conjugation into other streptomycetes using standard methods (8). Apramycin-resistant transconjugants were recovered for many of the Streptomyces strains investigated, including S. avermitilis, S. cinnamonensis, S. fradiae, S. lincolnensis, S. nogolater, S. roseosporus, and S. venezuelae. To complete the suite of φBT1 int/attP vectors, we constructed hygromycin-resistant derivatives of pRT801, pMS81, and pMS82 (Fig. 2). These plasmids were constructed by ligation of a HpaI fragment encoding hygromycin resistance (derived from Tn5099-10) (13) with the 4,415-bp Ecl136II fragment from pRT801 and differ only in the orientation in which the fragment encoding hygromycin resistance has inserted. The hygromycin resistance marker in S. coelicolor J1929(pMS81) and J1929(pMS82) was confirmed to be stable after two rounds of sporulation in the absence of selection (data not shown). We believe that all of these vectors will become a useful addition to the genetic toolbox of the streptomycete researcher.
Acknowledgments
We are grateful to Paul Herron and to Pat Solenberg for providing materials.
This study was funded by the BBSRC.
REFERENCES
- 1.Allen, M. J., A. Collick, and A. J. Jefferies. 1994. Use of vectorette and subvectorette PCR to isolate transgene flanking DNA. PCR Methods Appl. 4:71-75. [DOI] [PubMed] [Google Scholar]
- 2.Baltz, R. H. 1998. Genetic manipulation of antibiotic-producing Streptomyces. Trends Microbiol. 6:76-83. [DOI] [PubMed] [Google Scholar]
- 3.Bedford, D. J., C. Laity, and M. J. Buttner. 1995. Two genes involved in the phase-variable φC31 resistance mechanism of Streptomyces coelicolor A3(2). J. Bacteriol. 177:4681-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 5.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 6.Breuner, A., L. Brondsted, and K. Hammer. 2001. Resolvase-like recombination performed by the TP901-1 integrase. Microbiology 147:2051-2063. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, A., S. J. Schneider, and B. Song. 1992. Lambdoid phages as elements of bacterial genomes (integrase/phage21/Escherichia coli K-12/icd gene). Genetica 86:259-267. [DOI] [PubMed] [Google Scholar]
- 8.Combes, P., R. Till, S. Bee, and M. C. M. Smith. 2002. The Streptomyces genome contains multiple pseudo-attB sites for the φC31-encoded site-specific recombination system. J. Bacteriol. 184:5746-5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory, M. A. 2000. Characterisation and evolution of homoimmune Streptomyces phages. Ph.D. dissertation. University of Nottingham, Nottingham, United Kingdom.
- 10.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 11.Rost, B., R. Casadio, P. Fariselli, and C. Sander. 1995. Transmembrane helices predicted at 95% accuracy. Protein Sci. 4:521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith, M. C. M., and H. M. Thorpe. 2002. Diversity in the serine recombinases. Mol. Microbiol. 44:299-307. [DOI] [PubMed] [Google Scholar]
- 13.Solenberg, P. J., and R. H. Baltz. 1994. Hypertransposing derivatives of the streptomycete insertion sequence IS493. Gene 147:47-54. [DOI] [PubMed] [Google Scholar]